Abstract
4-vinylcyclohexene (VCH) is bioactivated by hepatic CYP 2A and 2B to a monoepoxide (VCM), and subsequently to an ovotoxic diepoxide metabolite (VCD). Studies suggest that the ovary can directly bioactivate VCH via CYP 2E1. The current study was designed to evaluate the role of ovarian CYP 2E1 in VCM-induced ovotoxicity. Postnatal day 4 B6C3F1, and CYP 2E1 wild-type (+/+) and null (-/-) mouse ovaries were cultured (15d) with VCD (30μM), 1,2-VCM (125-1000μM), or vehicle. 28d female CYP 2E1 +/+ and -/- mice were dosed daily (15d; ip) with VCH, 1,2-VCM, VCD or vehicle. Following culture or in vivo dosing, ovaries were histologically evaluated. In culture, VCD decreased (p < 0.05) primordial and primary follicles in ovaries from all three groups of mice. 1,2-VCM decreased (p < 0.05) primordial follicles in B6C3F1 and CYP 2E1 +/+ ovaries, but not in CYP 2E1 -/- ovaries in culture. 1,2-VCM did not affect primary follicles in any group of mouse ovaries. Conversely, following in vivo dosing, primordial and primary follicles were reduced (p<0.05) by VCD and VCM in CYP2E1 +/+ and -/-, and by VCH in +/+ mice. The data demonstrate that, whereas, in vitro ovarian bioactivation of VCM requires CYP 2E1 enzyme, in vivo CYP 2E1 plays a minimal role. Thus, the findings support that hepatic metabolism dominates the contribution made by the ovary in bioactivation of VCM to its ovotoxic metabolite, VCD. This study also demonstrates the use of a novel ovarian culture system to evaluate ovary-specific metabolism of xenobiotics.
Keywords: 4-vinylcyclohexene monoepoxide, 4-vinylcyclohexene diepoxide, CYP 2E1, Ovary
Introduction
Chemicals that cause depletion of ovarian follicles are of toxicological concern because the mammalian ovary contains a finite number of oocyte-containing follicles. This finite pool cannot be regenerated, and thus, its depletion can result in premature ovarian failure (early menopause in women; Hooser et al., 1994; Mayer et al., 2002; 2004). 4-vinylcyclohexene (VCH) is a colorless and odorless chemical used in the manufacture of flame retardants, insecticides, plasticizers, and antioxidants. Furthermore, VCH is formed as a byproduct in the processing of butadiene. Exposure to this occupational chemical results in a loss of small pre-antral (primordial and primary) follicles in the mouse ovary (NTP, 1986; Smith et al., 1990a).
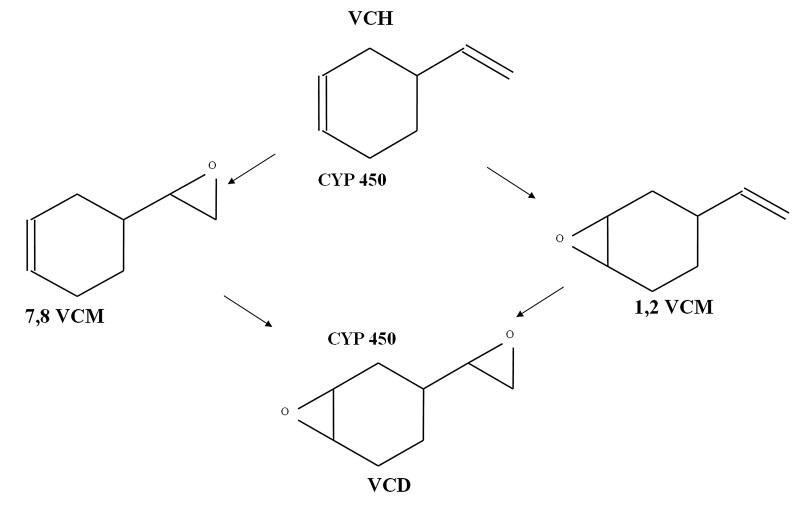
VCH-induced ovarian toxicity has been attributed to bioactivation of this compound to a diepoxide metabolite (VCD), which has been shown in rats to accelerate the normal rate of atresia (apoptosis; Springer et al., 1996a, b; Hu et al., 2001a, b). VCH can be bioactivated to either a 1,2 or 7,8-monoepoxide (VCM) and subsequently to VCD via cytochrome P450 (CYP 450) enzymes (Fig. 1). Structure activity studies suggest that bioactivation to the diepoxide is necessary for VCH-induced ovarian toxicity. Pre-antral follicle loss was not observed in B6C3F1 mice dosed with a number of VCH and VCM analogues such as ethylcyclohexene and vinylcyclohexane that could not be metabolized to a diepoxide (Doerr et al., 1995). Additionally, following in vivo dosing, VCD induced follicle loss at lower concentrations than did VCH or VCM (Smith et al., 1990b). In that study 1,2-VCM and 7,8-VCM were equally effective at causing follicle loss in B6C3F1 mice.
Figure 1. Proposed scheme for hepatic bioactivation of VCH.
The parent compound, VCH, is bioactivated by cytochrome P450 (CYP 450) to form either 1,2 or 7,8-monoepoxide metabolites. These intermediate metabolites further undergo bioactivation by CYP 450 enzymes to form the ultimate ovotoxic diepoxide metabolite (VCD).
Bioactivation of VCH to the ovarian toxicant VCD has been characterized in the liver. VCH treatment in mice caused an increase in hepatic CYP 450 enzyme isoforms CYP 2A and CYP 2B immunoreactive protein levels. Furthermore, in mice, hepatic microsomal CYP 2A and CYP 2B enzyme activities were induced following VCH and VCM exposure (Doerr-Stevens et al., 1999; Fontaine et al., 2001a). Following treatment of mice with VCH, liver microsomal protein expression of CYP 2E1 enzyme was increased; however, catalytic activity of CYP 2E1 did not change. Moreover, following VCH and VCM incubations, there was no difference in the formation of VCM or VCD, respectively, between hepatic microsomes from CYP 2E1 wild-type and null mice. From these observations, it was concluded that in liver the CYP 2E1 isoform is not required for the conversion of VCH to VCM or VCM to VCD (Fontaine et al., 2001b). Taken together, these findings support that in liver CYP 2A and CYP 2B are the major CYP 450 isoforms responsible for VCH and VCM bioactivation. Furthermore, following VCH dosing, circulating levels of VCM and VCD were detected in the blood of female B6C3F1 mice (Doerr-Stevens et al., 1999; Smith et al., 1990a). Thus, it appears the liver is the major site of bioactivation of VCH to VCD and circulating epoxides reach the ovary to cause follicle destruction.
Even though the liver is likely to be the major site of bioactivation of VCH, studies by Cannady et al. (2003) suggest that the mouse ovary also has the capacity to bioactivate VCH. In vivo dosing with VCH increased levels of mRNA encoding Cyp 2B in ovarian small pre-antral follicles (those targeted by VCD). Additionally, in vivo dosing with VCD increased mRNA encoding Cyp 2A and Cyp 2E1 in those same follicles. Although CYP 2A, CYP 2B and CYP 2E1 protein expression was detected in all ovarian compartments only CYP 2B and CYP 2E1 enzymes were shown to be catalytically active in the B6C3F1 mouse ovary. Further, unlike liver, following in vivo exposure to VCH, CYP 2E1 catalytic activity in the ovary was increased, while CYP 2B activity did not change. Collectively, these studies suggest a potential role for ovarian CYP 2E1 in the metabolism of VCH and VCM to the ovotoxic metabolite VCD. CYP 2E1 enzyme is involved in the metabolism of many small molecules including benzene, styrene and carbon tetrachloride (Guengerich et al., 1991). In addition, CYP 2E1 enzyme was shown to metabolize the VCH homologue, 1,3-butadiene, as well as 1,3-butadiene monoepoxide to the diepoxide (Duescher and Elfarra, 1994). Therefore, CYP 2E1 is a likely candidate for VCH/VCM metabolism in the ovary.
This study was designed to more directly evaluate the role of ovarian CYP 2E1 enzyme in ovotoxicity caused by the proximate-toxicant, VCM, using a neonatal mouse whole ovary culture system, where the metabolic role of the liver would not be involved (Devine et al., 2002a, 2004). The hypothesis is that ovarian CYP 2E1 enzyme can participate in bioactivation of VCM to the ovotoxicant VCD.
Materials and Methods
Reagents
VCH (racemic mixture; purity 99%), 1,2-VCM (mixture of isomers; purity 98%), bovine serum albumin (BSA), ascorbic acid (Vitamin C), transferrin, VCD (mixture of isomers; purity 97%), and sesame oil were purchased from Sigma Chemical Co. (St Louis, MO). Dulbecco’s Modified Eagle Medium: nutrient mixture F-12 (Ham) 1X (DMEM/Ham’s F12), Albumax, penicillin/streptomycin (5000U/ml, 5000μg/ml, respectively), and Hanks’ Balanced Salt Solution (without CaCl2, MgCl2, or MgSO4) were purchased from Gibco Inc. (Invitrogen Corporation, Grand Island, NY). Millicell-CM filter inserts were purchased from Millipore (Bedford, MA), and 48 well cell culture plates were obtained from Corning Inc. (Corning, NY).
Animals
CYP 2E1 wild-type (+/+; 129S1/SvImJ background strain; 2 females, 2 males) and null (-/-; 2 females, 2 males) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred at the University of Arizona Animal Care Facility. Late gestation day pregnant C57BL/6 females (B6C3F1 litters) were purchased from Harlan Laboratories (Indianapolis, IN). All animals were housed in plastic cages and maintained in a controlled environment (22 ± 2°C; 12h light/12h dark cycles). The animals were provided standard diet with ad libidum access to food and water. 129S1/SvImJ mice breeding pairs (+/+ or -/-) were housed one male and one female per cage. At weaning (21d) the pups were housed 4 per cage by sex. Pregnant C57BL/6 females were housed one per cage. All animal experiments were approved by the University of Arizona’s Institutional Animal Care and Use Committee.
In vitro ovarian cultures
On postnatal day (PND) 4 female B6C3F1, CYP 2E1 wild-type (+/+), and CYP 2E1 null (-/-) mice were killed by CO2 inhalation followed by decapitation. Each ovary was removed, oviduct and excess tissue was trimmed, and the ovary was placed on a piece of Millicell-CM filter membrane floating on 250μl of DMEM/Ham’s F12 medium containing 1mg/ml BSA, 1mg/ml Albumax, 50μg/ml ascorbic acid, 5U/ml penicillin/5μg/ml streptomycin, and 27.5μg/ml transferrin in one well of a 48 well plate previously equilibrated to 37°C. Using fine forceps, a drop of medium was placed to cover the top of the ovary to prevent drying. 30μM VCD or 1,2-VCM (125, 250, 500, 750, or 1000μM) diluted in medium was added to each well. NOTE: Because 1,2-VCM and 7,8-VCM produced similar levels of follicle loss in B6C3F1 mice in vivo (Smith et al., 1990b), and previous studies have shown 7,8-VCM to be a minor metabolite of VCH in hepatic microsomes (Fontaine et al., 2001c), only 1,2-VCM was used in these studies to reduce numbers of animals used. Due to the chemical property of VCH (vapor pressure = 10.2mmHg @ 25°C), the appropriate capacity to study VCH in culture was not available. No treatment was added to media in control wells. Plates containing ovaries were cultured at 37°C and 5% CO2 in air for 15d. Media were removed and fresh media and treatment were added every 2d.
In vivo animal dosing
Age day 28 (28d) female offspring of both CYP 2E1 wild-type (+/+) and CYP 2E1 null (-/-) mice were dosed daily (15d; ip) with sesame oil (vehicle control) or sesame oil containing VCH (7.4 mmol/kg/day), 1,2-VCM (2.74mmol/kg/day), or VCD (0.57 mmol/kg/day). Previously it was determined that those levels of each chemical provided equivalent degrees of ovotoxicity in small pre-antral follicles (primordial and primary) in B6C3F1 female mice following thirty days of daily ip dosing (Smith et al., 1990b). Animals were killed by CO2 inhalation 4h following the final dose.
Histological evaluation
After 15 days of culture in vitro treated ovaries (5 ovaries/treatment) were fixed in Bouin’s fixative for 1.5h, transferred to 70% ethanol, embedded in paraffin, serially sectioned (5μm thick), and every 6th section was mounted. At 4h following the final in vivo dose, animals (5/group) were euthanized, ovaries removed, and oviduct and excess fat trimmed. Ovaries from In vivo treated mice (one ovary per animal) were placed in Bouin’s fixative for 2h, transferred to 70% ethanol, embedded in paraffin, serially sectioned (5μm thick), and every 20th section was mounted. All ovarian sections were stained with hematoxylin and eosin. Healthy follicle populations containing oocytes were classified and counted in every 12th section (in vitro group) and in every 20th section (in vivo group). In previous neonatal rat ovarian cultures, unhealthy follicles displayed intense eosinophilic staining of oocytes and pyknosis of granulosa cells (Devine et al., 2002b). Unlike the ovary in vivo, in which phagocytic cells in the circulating blood can remove cellular debris, cellular remnants remain in ovaries incubated in vitro. Therefore, in this study only healthy follicles were classified and counted. Follicles were classified as described by Flaws et al. (1994), which was adapted from Pedersen and Peters (1968). Briefly, primordial follicles contain the oocyte surrounded by a single layer of squamous-shaped granulosa cells; primary follicles contain the oocyte surrounded by a single layer of cuboidal-shaped granulosa cells; secondary follicles contain the oocyte surrounded by multiple layers of granulosa cells and a theca cell layer surrounding the granulosa cells; and antral follicles are identified by the fluid filled cavity (antrum) within the follicle. NOTE: Follicle populations in the PND4 cultured ovary in this system are mostly primordial and primary, secondary follicles are rarely observed, and antral follicles are not seen. Thus, the potential effect of VCD on larger follicle populations could not be evaluated
Statistical analysis
Comparisons were made using one-way ANOVA. When significant differences were detected, individual groups were compared with the Fisher’s protected least significant difference (PLSD) multiple range test. The assigned level of significance for all tests was p < 0.05.
Results
Strain dependence on VCD-induced follicle loss
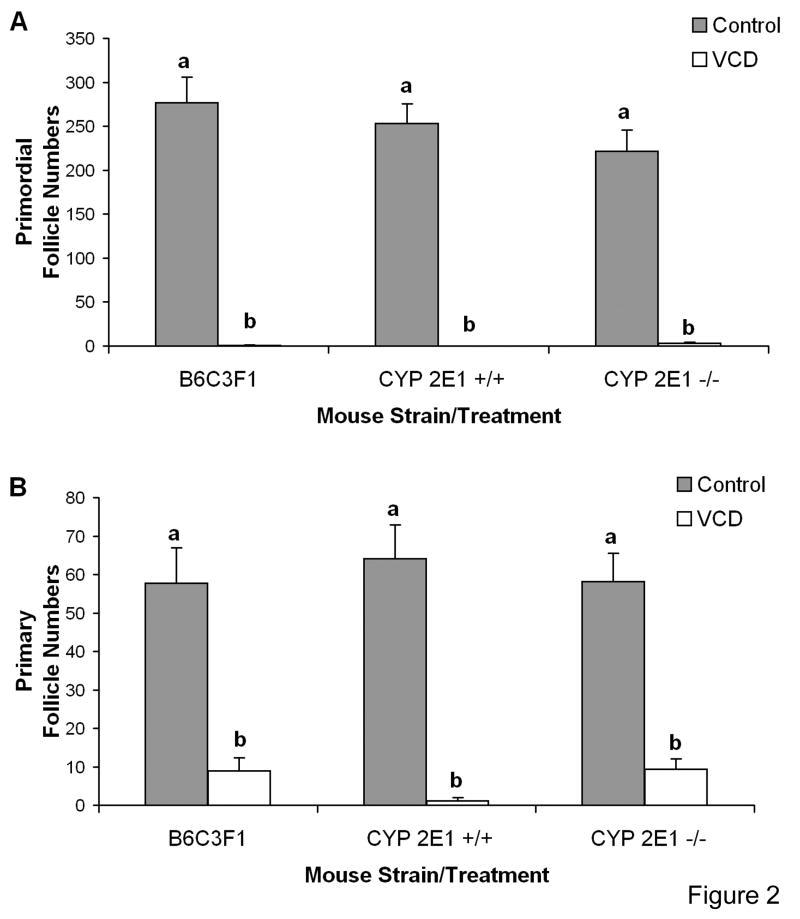
To demonstrate the ovotoxic effects of VCD in a neonatal mouse whole ovary culture system, follicle loss was evaluated in PND4 B6C3F1, and CYP 2E1 +/+ and -/- mouse ovaries cultured with 30μM VCD (Fig. 2). Relative to control medium, incubation with VCD for 15 days depleted (p < 0.05) primordial follicles. However, 30μM VCD did not deplete all healthy primary follicles. Further, there was a non-significant trend for greater primary follicle loss in CYP2E1 +/+ as compared with CYP 2E1 -/- and B6C3F1 ovaries.
Figure 2. VCD - induced ovotoxicity in various strains of mouse ovaries in culture.
Ovaries from PND4 B6C3F1, CYP 2E1 +/+, or CYP 2E1 -/- mice were cultured with medium control or 30μM VCD for 15d. Following incubation, ovaries were collected, and processed for histological evaluation as described in materials and methods. Healthy (A) primordial, and (B) primary follicles were classified and counted. Values are mean ± SE total follicles counted/ovary, n=5; different letters differ (p<0.05) from one another within each follicle type.
Strain dependence on VCM-induced follicle loss
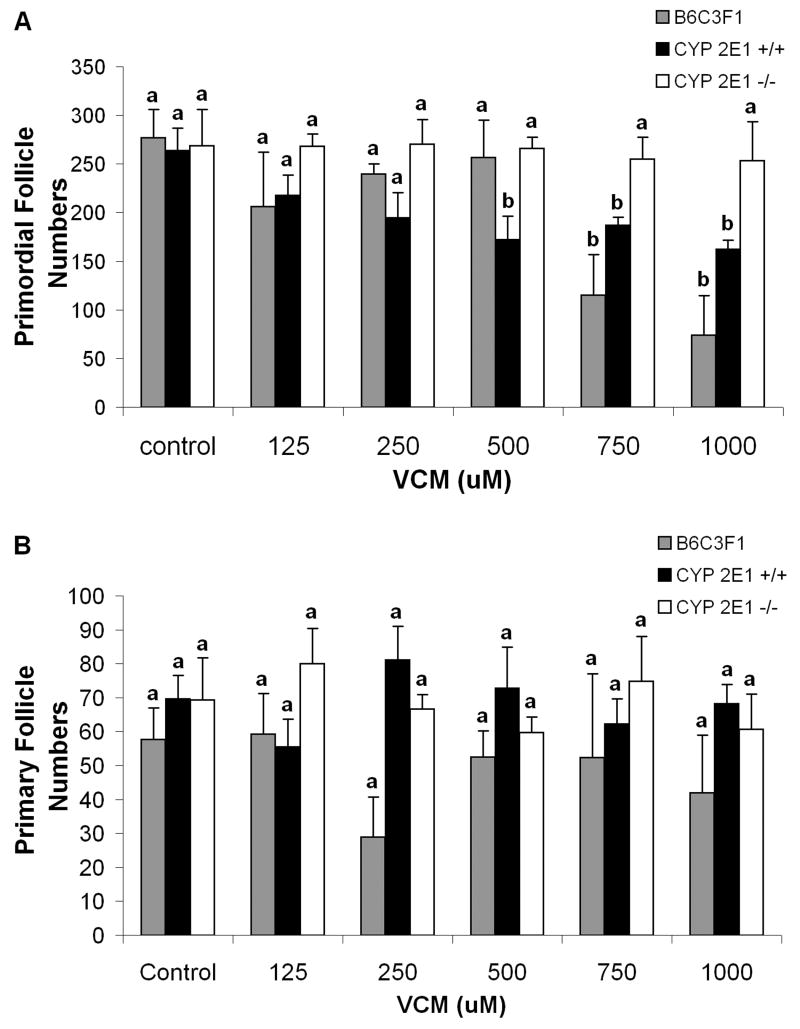
To investigate the potential of ovarian CYP 2E1 for bioactivation of 1,2-VCM in the absence of hepatic tissue, follicle loss was evaluated in PND4 B6C3F1, and CYP 2E1 +/+ and -/- mouse ovaries cultured with increasing concentrations of 1,2-VCM (Fig. 3). Relative to control, primordial follicles were reduced (p < 0.05) in B6C3F1 ovaries incubated with 750 and 1000μM 1,2-VCM. However, primordial follicle loss (p < 0.05) was seen in CYP 2E1 +/+ ovaries at a lower concentration (≥ 500μM). Conversely, primordial follicle numbers in CYP 2E1 -/- mouse ovaries were not affected by incubation with 1,2-VCM incubation at any concentration (Fig. 3A). Primary follicles were not affected by 1,2-VCM at any concentration in any group (Fig. 3B).
Figure 3. Involvement of CYP 2E1 in 1,2-VCM - induced ovotoxicity in ovarian cultures.
Ovaries from PND4 B6C3F1, CYP 2E1 +/+ or -/- mice were cultured with medium control or 1,2-VCM (125, 250, 500, 750, or 1000 μM) for 15d. Following incubation, ovaries were collected, and processed for histological evaluation as described in materials and methods. Healthy (A) primordial, and (B) primary follicles were classified and counted. Values are mean ± SE total follicles counted/ovary, n=5; different letters differ (p<0.05) from one another within each follicle type.
Effect of VCM and VCD on ovarian morphology in CYP 2E1 +/+ and -/- ovaries
Fig. 4 shows the effect of 15 days incubation with VCD (30μM) and 1,2-VCM (1000μM) on morphology of ovaries from CYP 2E1 +/+ and -/- mice. Compared to control, following incubation with 1000μM 1,2-VCM there were more unhealthy primordial and primary follicles in the CYP 2E1 +/+ ovary (Fig. 4A vs. Fig. 4C) as characterized by intense eosin staining in oocytes. However, there was no difference in the morphological appearance of CYP 2E1 -/- ovaries incubated with control medium or 1,2-VCM (Fig. 4B vs. Fig. 4D).
Figure 4. Effect of in vitro VCM and VCD exposure on ovarian morphology.
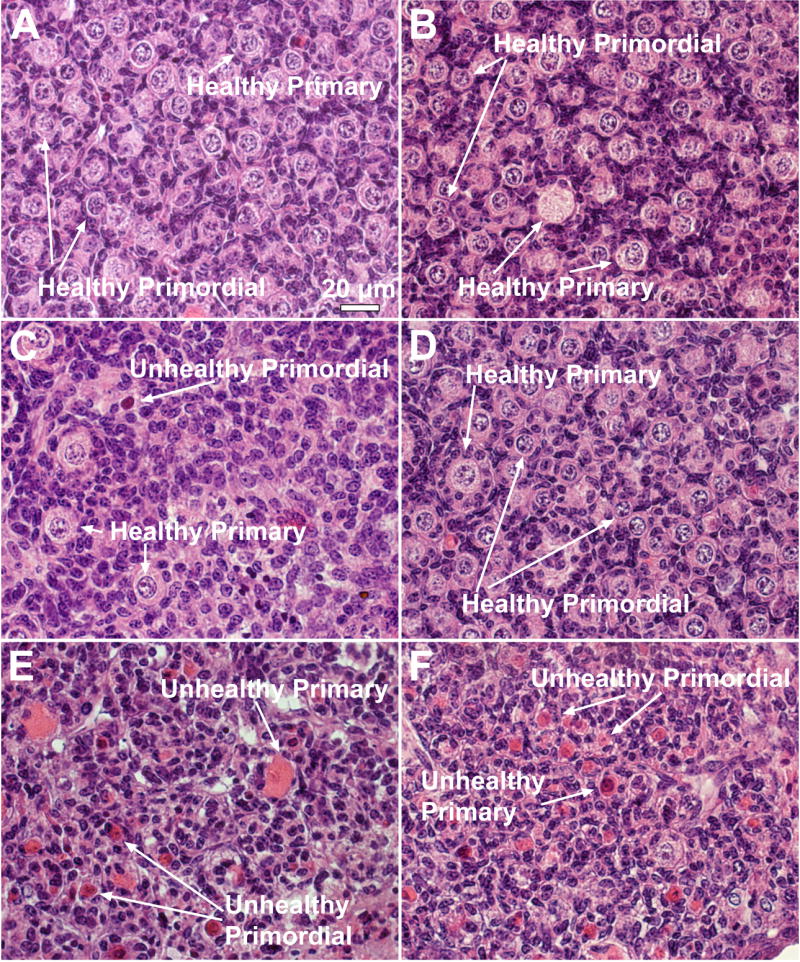
Ovaries from PND4 CYP 2E1 +/+ (A,C and E) and -/- (B,D and F) mice were cultured with control medium (A and B), 1,2-VCM (1000 μM; C and D), or 30μM VCD (E and F) for 15d. Following incubation, ovaries were collected, and processed for histological evaluation as described in materials and methods. All images were captured with a 40X objective lens. Bar represents 20μm.
Following incubation with VCD, all primordial and numerous primary follicles appeared unhealthy. This follicle damage was equivalent between CYP 2E1 +/+ and -/- incubations (Fig. 4E and F) compared to that of vehicle control (Fig. 4A and B). Furthermore, in those ovaries incubated with VCD, follicles have shrunk and contain predominantly follicular debris compared to those ovaries in control incubations.
Effect of VCH, VCM, VCD dosing on follicle loss in CYP 2E1 +/+ and -/- mice
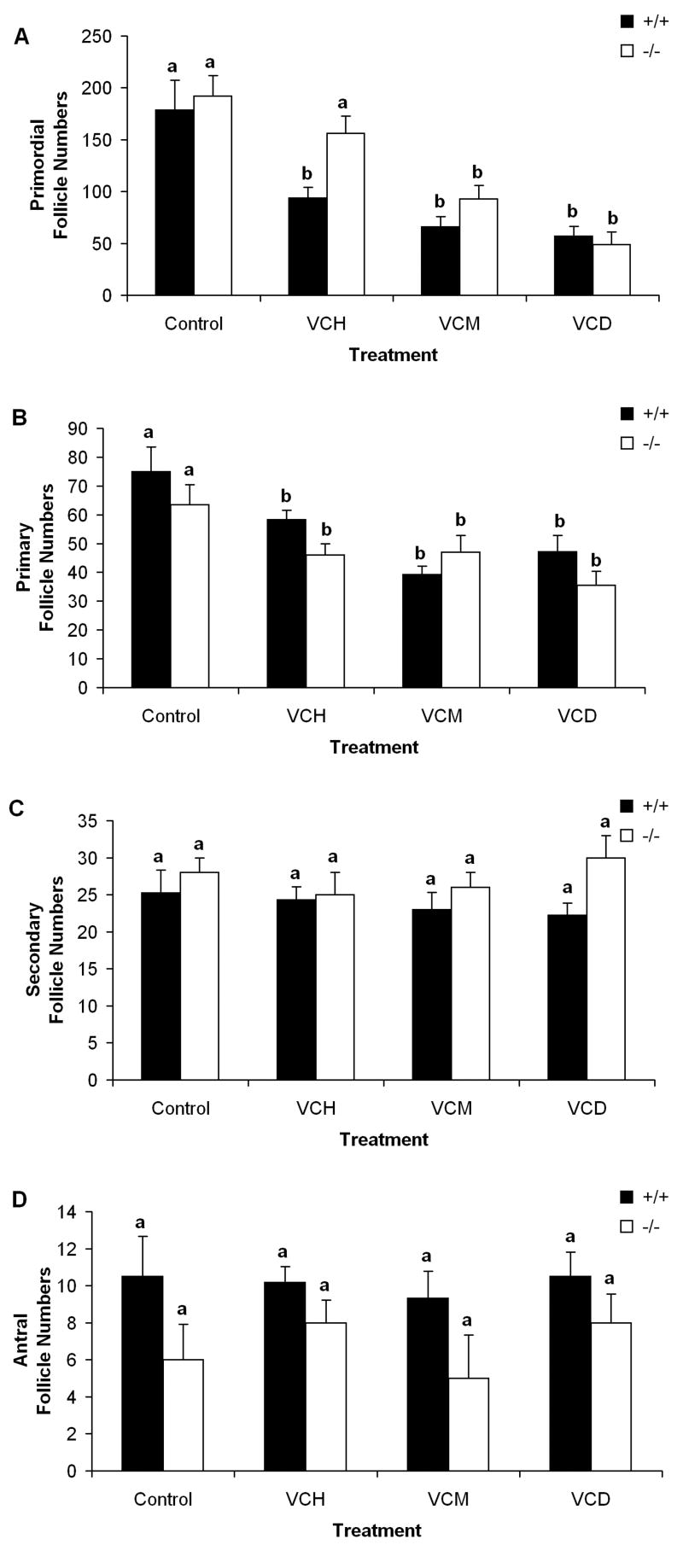
To estimate the significance of ovarian bioactivation of the chemicals in the whole animal, ovarian follicle loss was evaluated in CYP 2E1 +/+ and -/- mice following repeated in vivo exposure to VCH (7.4mmol/kg/day), 1,2-VCM (2.74 mmol/kg/day), VCD (0.57 mmol/kg/day), or vehicle control (15 daily doses, Fig. 5). Compared to vehicle control, primordial follicles (VCD target population) were significantly reduced (p < 0.05) in ovaries from CYP 2E1 +/+ and -/- mice following VCD exposure. 1,2-VCM exposure also resulted in a significant loss (p < 0.05) of primordial follicles in CYP 2E1 +/+ and -/- mouse ovaries. VCH exposure resulted in a significant loss (p < 0.05) of primordial follicles in CYP 2E1 wild-type (+/+) mice, however, VCH exposure did not decrease (p = 0.09) primordial follicles in CYP 2E1 null (-/-) mice (Fig. 5A).
Figure 5. Involvement of CYP 2E1 in VCH – induced ovotoxicity.
Female CYP 2E1 +/+ and -/- mice were treated with repeated daily doses (i.p.) of sesame oil (control), VCH (7.4mmol/kg/d), 1,2-VCM (2.74mmol/kg/d), or VCD (0.57mmol/kg/d) for 15d. 4h following the final dose ovaries were collected, and processed for histological evaluation as described in materials and methods. Healthy (A) primordial, (B) primary, (C) secondary, and (D) antral follicles were classified and counted. Values are mean ± SE total follicles counted/ovary, n=5; different letters differ (p<0.05) from one another within each follicle type.
Primary follicles (VCD target population) were reduced (p < 0.05) in CYP 2E1 +/+ and -/- mouse ovaries with VCD, VCM, and VCH dosing compared to vehicle controls (Fig. 5B).
Secondary and antral follicle populations (VCD non-target populations) were not affected by VCD, VCM, or VCH exposure in either CYP 2E1 +/+ or -/- mice (Fig. 5C and D).
Discussion
A female is born with a finite number of ovarian follicles and once destroyed, no more can be generated. Therefore, exposure to chemicals that target ovarian follicles can impair reproductive function and cause pre-mature menopause in women. Some chemicals such as VCH/VCM require bioactivation to an ovotoxic metabolite to induce follicle loss. Previous studies indicate metabolism of VCH to VCM and then to VCD is primarily catalyzed in liver by CYP 2B and 2A isoforms, whereas, CYP 2E1 may play a role in ovarian bioactivation of VCH/VCM (Doerr-Stevens et al., 1999; Fontaine et al., 2001a, b; Cannady et al., 2003). Because CYP 2E1 is involved in the bioactivation of several small molecules, it could be a candidate for ovarian metabolism of VCH/VCM (Guengerich et al., 1991). Thus, ovarian bioactivation of ovotoxicants could potentiate toxicity induced by hepatic bioactivation at the target organ level.
The current study was designed to further investigate a potential role of ovarian CYP 2E1 in bioactivation of VCM with ovotoxicity as an end point utilizing a novel ovarian culture approach, where contributions of hepatic metabolism have been eliminated. In a previous report, VCD caused loss of primordial and primary follicles in cultures of neonatal rat ovaries using this system (Devine et al., 2002a, 2004). However, ovotoxicity caused by VCD in cultured ovaries from mice has not been studied. Thus, the bioactive form, VCD, was used to validate the use of this culture system.
Agreeing with previous in vivo studies, incubation of ovaries from neonatal B6C3F1 mice with VCD decreased healthy small pre-antral (primordial and primary) follicles (NTP, 1989; Chhabra et al., 1990; Kao et al., 1999; Smith et al., 1990a). Those same follicles were also decreased by VCD in ovaries cultured from CYP2E1 +/+ and -/- mice. These data are also in agreement with previous in vitro studies in neonatal rat ovaries (Devine et al., 2004). Interestingly, unlike mouse ovaries in which healthy primordial follicles were depleted after incubation with 30 μM VCD, not all primordial follicles were targeted in the rat study. This reduced sensitivity of rats to VCD as compared with mice has also been observed in vivo and might be due to enhanced detoxification capacity relative to mice (Smith et al., 1990b; Keller et al., 1997; Kao et al., 1999). Additionally, there was a non-significant trend for cultured ovaries from CYP2E1 +/+ mice to be more susceptible than those from CYP2E1 -/- or B6C3F1 mice.
To estimate the role of CYP 2E1 in ovarian bioactivation of 1,2-VCM, ovotoxicity it was incubated in vitro with ovaries from mice that do or do not express CYP2E1. 1,2-VCM induced primordial follicle loss only in ovaries expressing CYP 2E1 enzymes [B6C3F1 and CYP 2E1+/+ mice] and not in CYP 2E1 -/- mouse ovaries. The greater susceptibility of CYP2E1-expressing ovaries to VCM was apparent with morphological evaluation. Furthermore, ovotoxicity in CYP 2E1+/+ mouse ovaries occurred at a lower (p<0.05) concentration of VCM than in B6C3F1 ovaries. Collectively, these data support indirectly that 1) in the absence of hepatic tissue the ovary can bioactivate VCM to the ovotoxic form, 2) ovarian CYP 2E1 is required for ovarian conversion of VCM to VCD, and 3) there may be strain differences in the ovarian response to VCM.
In the current in vitro study with mice VCD and VCM had less effect on primary follicles than on primordial follicles. This follicle type difference in susceptibility was also seen in the in vitro study with rats (Devine et al., 2004), These observations demonstrate that relative to primordial follicles, primary follicles are less sensitive to VCD-induced ovotoxicity.
There was a markedly reduced sensitivity of cultured ovaries to VCM as compared to VCD. This could result from the direct ovotoxicity of 1,2-VCM which is much less reactive than VCD (Doerr et al., 1995). More likely this reflects the metabolic capacity of the ovary. CYP2E1 formation of VCD from 1,2-VCM probably occurs at rates which allow for efficient detoxification of VCD by microsomal epoxide hydrolase. In addition, 1,2-VCM may also serve as a substrate for mEH, which, in effect, would reduce the formation of VCD (Keller et al. 1997). Cannady et al. (2002) showed that the B6C3F1 mouse ovary expresses catalytically active mEH, which can be induced following in vivo VCH and VCD dosing. CYP2E1 +/+ ovaries were more susceptible to VCM (p<0.05) and VCD (non-significant trend) when compared with CYP2E1 -/- and B6C3F1 ovaries. Because the mouse ovary expresses high levels of mEH, the observed strain differences could result from greater expression of mEH in B6C3F1 (less susceptible) as compared with CYP2E1 +/+ mice. This possibility is currently under investigation. Furthermore, the epoxide of 1,2-VCM can also be detoxified by conjugation with glutathione. Preliminary results demonstrate that a 1,2-VCM glutathione conjugate is formed in cultured mouse ovaries incubated with 1,2-VCM (Rajapaksa et al., unpublished data). Thus, inefficient ovarian bioactivation to VCD and/or efficient detoxification of 1,2-VCM by mEH and/or glutathione conjugation could account for the need to use 1,2-VCM at relatively high concentrations in order to observe ovotoxicity.
An in vivo dosing study was conducted to assess the potential role of CYP2E1 in the presence of hepatic metabolism. Dosing with VCH/VCM/VCD resulted in a similar degree of ovotoxicity between CYP 2E1 +/+ and -/- mice, at concentrations that caused a comparable degree of ovotoxicity in B6C3F1 mice dosed with these three compounds (Smith et al., 1990a). Compared with the apparent inability of VCM to cause follicle loss in ovaries cultured from CYP2E1 -/- mice, these findings suggest that ovarian CYP 2E1 is involved in VCM-induced ovotoxicity; however, in the whole animal it is not required to form VCD. This would indicate that in vivo there is a predominant contribution to bioactivation from the liver.
In the present studies, in vitro incubations were with ovaries from neonatal mice, whereas, in vivo dosing was in older animals (age d28). It has been reported that in rat liver CYP2E1 expression increases with age in older animals (age 3-8 months; Wauthrie et al., 2004). Based on that report, one would expect a greater difference in susceptibility between d28 CYP2E1 +/+ and -/- mice than in neonatal mice. However, unlike neonatal mouse ovaries, those from d28 old mice showed a similar susceptibility to VCM between CYP2E1 +/+ and -/- mice. Therefore, the difference in susceptibility between the ovary culture and in vivo dosing experiments is more likely due to the absence of hepatic contributions in isolated ovaries rather than a difference in CYP2E1 expression as a result of age of mice between the two systems.
The results presented here suggest that in vivo ovarian bioactivation of VCH/VCM to VCD likely plays little if any role in converting VCM to the ovotoxic metabolite, VCD. Based on the previous and present studies regarding the role of CYP2E1 in bioactivation of VCH/VCM to the bioactive metabolite, this isoform likely plays a physiologically unimportant role when the liver is involved. However, it cannot be ruled out that CYP2E1 may be important in competing with other CYP450s for metabolism of other chemicals, or in individuals in which other isoforms are poorly expressed.
In the greater sense, these results suggest that ovarian follicles can be targeted by bioactive compounds (resulting from hepatic metabolism) as well as locally formed ovotoxicants (resulting from ovarian metabolism). Thus, metabolic capabilities in target organs may serve to concentrate/potentiate localized toxic effects. However, in addition to bioactivation, target organ metabolism may also contribute to localized detoxification of xenobiotic chemicals. For example, VCD can be converted to an inactive tetrol metabolite in isolated rat ovarian follicles (Flaws et al., 1994b) and the mouse ovary expresses high levels of mEH (Cannady et al., 2002). Thus, ovarian metabolism may play an important protective role in attenuating the effects of ovotoxic metabolites to which the ovary becomes exposed as a result of hepatic bioactivation.
In summary, the results presented here suggest that, in the physiological setting, hepatic bioactivation of VCH/VCM predominates over metabolic contributions from the ovary. However, ovarian CYP2E1 may provide a level of fine-tuning at the target organ level. These studies also demonstrate the usefulness of a novel ovarian culture system to distinguish between hepatic and ovary-specific metabolism of xenobiotics. A better understanding of the precise role of the ovary in modulating ovarian metabolites can contribute to a greater appreciation of the impact of environmental exposures on reproductive function in women.
Acknowledgments
This work was supported by NIH grant ES09246 and Center Grant 06694. Authors wish to thank Andrea Grantham for histological processing of ovarian tissue, and Douglas Cromey for assistance with imaging.
Footnotes
Conflict of Interest Statement There are no conflicts of interest that inappropriately influence the above work submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of microsomal expoxide hydrolase in follicles isolated from mouse ovaries. Toxicol Sci. 2002;68:24–31. doi: 10.1093/toxsci/68.1.24. [DOI] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of cytochrome P450 2E1, 2A, and 2B in the mouse ovary: The effect of 4-vinylcyclohexene and its diepoxide metabolite. Toxicol Sci. 2003;73:423–430. doi: 10.1093/toxsci/kfg077. [DOI] [PubMed] [Google Scholar]
- Chhabra RS, Elwell MR, Peters A. Toxicity of 4-vinyl-1-cyclohexene diepoxide after 13 weeks of dermal and oral exposure in rats and mice. Fundam Appl Toxicol. 1990;14:745–751. doi: 10.1016/0272-0590(90)90299-y. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Skinner MK, Hoyer PB. Characterization of a rat in vitro ovarian culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol Appl Pharmacol. 2002a;184:107–115. [PubMed] [Google Scholar]
- Devine PJ, Rajapaksa KS, Hoyer PB. In vitro ovarian tissue and organ culture: a review. Front Biosci. 2002b;1:d1979–1989. doi: 10.2741/devine. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Hoyer PB. Initiation of delayed ovotoxicity by in vitro and in vivo exposure of rat ovaries to 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2004;19:71–77. doi: 10.1016/j.reprotox.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Doerr JK, Hooser SB, Smith BJ, Sipes IG. Ovarian toxicity of 4-vinylcyclohexene and related olefins in B6C3F1 mice: role of diepoxides. Chem Res Toxicol. 1995;8:963–969. doi: 10.1021/tx00049a010. [DOI] [PubMed] [Google Scholar]
- Doerr-Stevens JK, Liu J, Stevens GJ, Kraner JC, Fontaine SM, Halpert JR, Sipes IG. Induction of cytochrome P450 enzymes after repeated exposure to 4-vinylcyclohexene in B6C3F1 mice. Drug Metab Dispos. 1999;27:281–287. [PubMed] [Google Scholar]
- Duescher RJ, Elfarra AA. Human liver microsomes are efficient catalysts of 1,3-butadine oxidation: evidence for major roles by cytochromes P450 2A6 and 2E1. Arch Biochem Biophys. 1994;311:342–349. doi: 10.1006/abbi.1994.1246. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994a;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Salyers KL, Sipes IG, Hoyer PB. Reduced ability of rat preantral ovarian follicles to metabolize 4-vinylcyclohexne diepoxide in vitro. Toxicol Appl Pharmacol. 1994b;126:286–294. doi: 10.1006/taap.1994.1118. [DOI] [PubMed] [Google Scholar]
- Fontaine SM, Hoyer PB, Halpert JR, Sipes IG. Role of induction of specific hepatic cytochrome P450 isoforms in epoxidation of 4-vinylcyclohexene. Drug Metab Dispos. 2001a;29:1236–1242. [PubMed] [Google Scholar]
- Fontaine SM, Hoyer PB, Sipes IG. Evaluation of hepatic cytochrome P4502E1 in the species-dependent bioactivation of 4-vinylcyclohexene. Life Sci. 2001b;69:923–934. doi: 10.1016/s0024-3205(01)01170-5. [DOI] [PubMed] [Google Scholar]
- Fontaine SM, Mash EA, Hoyer PB, Sipes IG. Stereochemical aspects of vinylcyclohexene bioactivation in rodent hepatic microsomes and purified human cytochrome P450 enzyme systems. Drug Metab Dispos. 2001c;29:179–184. [PubMed] [Google Scholar]
- Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991;4:168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Hooser SB, Douds DP, DeMerell DG, Hoyer PB, Sipes IG. Long-term ovarian and gonadotropin changes in mice exposed to 4-vinylcyclohexene. Reprod Toxicol. 1994;8:315–323. doi: 10.1016/0890-6238(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian P, Thompson KE, Sipes IG, Hoyer PB. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod. 2001a;65:87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian P, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001b;65:1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- Kao SW, Sipes IG, Hoyer PB. Early effects of ovotoxicity induced by 4-vinylcyclohexnene diepoxide in rats and mice. Reprod Toxicol. 1999;13:67–75. doi: 10.1016/s0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Keller DA, Carpenter SC, Cagen SZ, Reitman FA. In vitro metabolism of 4-vinylcyclohexne in rat and mouse liver, lung, and ovary. Toxicol Appl Pharmacol. 1997;144:36–44. doi: 10.1006/taap.1996.8098. [DOI] [PubMed] [Google Scholar]
- Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2002;16:775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;17:130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (NTP) Toxicology and carcinogenesis studies of 4-vinylcyclohexhene in F344/N rats and B6C3F1 mice. U.S. Department of Health and Human Services, Public Health Services, National Institutes of Health; Research Triangle Park, NC: 1986. Technical Report 303. [Google Scholar]
- National Toxicology Program (NTP) Toxicology and carcinogenesis studies of 4-vinyl-1-cyclohexhene diepoxide in F344/N rats and B6C3F1 mice. U.S. Department of Health and Human Services, Public Health Services, National Institutes of Health, National Toxicology Program; Research Triangle Park, NC: 1989. Technical Report 362. [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Carter DE, Sipes IG. Comparison of the disposition and in vitro metabolism of 4-vinylcyclohexene in the female mouse and rat. Toxicol Appl Pharmacol. 1990a;105:364–371. doi: 10.1016/0041-008x(90)90140-p. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Mattison DR, Sipes IG. The role of epoxidation in 4- vinylcyclohexene-induced ovarian toxicity. Toxicol Appl Pharmacol. 1990b;105:372–381. doi: 10.1016/0041-008x(90)90141-g. [DOI] [PubMed] [Google Scholar]
- Springer LN, Tilly JL, Sipes IG, Hoyer PB. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexne diepoxide-induced ovotoxicity in the rat. Toxicol Appl Pharmacol. 1996a;139:402–410. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclehexene diepoxide induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996b;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Wauthier V, Verbeeck RK, Bac Calderon P. Age-related changes in the protein and mRNA levels of CYP2E1 and CYP3A isoforms as well as in their hepatic activites in Wistar rats. What role for oxidative stress? Acrh Toxicol. 2004;78:131–138. doi: 10.1007/s00204-003-0526-z. [DOI] [PubMed] [Google Scholar]