Abstract
Background:
The hygiene hypothesis contends that fewer opportunities for infection have led to increases in the prevalences of asthma and other allergic diseases.
Objective:
This study evaluated the association between asthma, wheeze, and hay fever and antibodies to 2 oral bacteria associated with periodontal disease.
Methods:
Data were obtained from the Third National Health and Nutrition Examination Survey. Serum levels of IgG antibodies to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis were quantified by enzyme-linked immunoassays in 9385 subjects age 12 years and older. The outcomes were current asthma, wheeze, and hay fever. Odds ratios (ORs) representing a 1–log-unit increase in IgG concentrations were estimated with logistic regression. ORs were adjusted for 8 confounders and weighted to represent the US population.
Results:
For each disease outcome, geometric mean antibody concentrations were higher in persons without the disease outcome than with the disease outcome. For a 1–log-unit increase in P gingivalis antibody concentration, adjusted ORs were 0.41 (95% CI, 0.20-0.87) for asthma, 0.43 (0.23-0.78) for wheeze, and 0.45 (0.23-0.93) for hay fever. For A actinomycetemcomitans, those ORs were 0.56 (0.19-1.72), 0.39 (0.17-0.86), and 0.48 (0.23-1.03), respectively.
Conclusion:
Consistent with the hygiene hypothesis, higher concentrations of IgG antibodies to P gingivalis were significantly associated with lower prevalences of asthma, wheeze, and hay fever, and higher concentrations of IgG antibodies to A actinomycetemcomitans were significantly associated with a lower prevalence of wheeze.
Clinical implications:
Colonization of the oral cavity by bacteria and other microbes might play a protective role in the etiology of allergic disease.
Keywords: Asthma, wheeze, hay fever, hygiene hypothesis, periodontal disease, epidemiology, survey, antibodies, oral pathogens, Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis
In the United States, the prevalence of self-reported, current asthma increased 74% between the years 1980 and 1996.1 In addition, data from the second and third National Health and Nutrition Examination Surveys (NHANES) suggest that the prevalence of allergic sensitization to common allergens also increased during those years.2 One of the leading explanations for increases in allergy and asthma rates in the United States and other industrialized countries is the hygiene hypothesis. The hypothesis contends that infections and microbial exposures, which are thought to be less prevalent now, “might essentially immunize against the development of asthma and allergic and autoimmune diseases.”3 The origin of the hypothesis is usually credited to D. P. Strachan and his 1989 article, “Hay fever, hygiene, and household size.”4 Observing that family size and number of older children in the household were inversely associated with current hay fever among a large British cohort, Strachan stated that such findings could be explained “if allergic diseases were prevented by infection in early childhood, transmitted by unhygienic contact by older siblings, or acquired prenatally from a mother infected by contact with her older children.”4 Strachan went on to state that “later infection or reinfection by younger siblings might confer additional protection against hay fever.”4 Strachan speculated that smaller family sizes and higher standards of personal cleanliness have reduced the opportunities for infections, resulting in more widespread atopic disease.4 Several years later, a plausible biological mechanism—that infection shifts the balance of cytokine release away from a pattern responsible for IgE-mediated allergy—emerged.5
Although investigations of the hygiene hypothesis have not always provided consistent results, reports of inverse associations between allergic disease and various infections and exposures to farms, pets, endotoxin, and day care have provided support for the hypothesis.3,5,6 As an extension of the hypothesis, researchers began examining the role of gastrointestinal microflora in the etiology of allergy and asthma. Several investigations have shown that the composition of gut microflora, which can be altered by antibiotics, diet, and infant feeding regimens, differs between individuals with and without allergy.7 In addition, the supplementation of the diet with beneficial bacteria, known as probiotics, has shown promise in the prevention of atopic dermatitis.8
Similar to the gastrointestinal tract, the oral cavity harbors hundreds of species of microorganisms that colonize after birth and are influenced by environmental and host factors.9 Because oral bacteria have been associated with local and systemic inflammation and with diseases outside the oral cavity, such as cardiovascular disease,10-15 we hypothesized that oral bacteria may play a role in the etiology of allergic diseases. In 2002, a supplemental NHANES III dataset containing IgG antibody concentrations to 2 oral bacteria measured in stored serum samples was released. The 2 bacteria, Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis, are part of the normal oral flora; however, their proportions relative to other microflora are higher in persons with periodontal disease.9 Periodontal disease is considered one of the most common chronic inflammatory disorders in adults, although it also occurs in children.9 The objective of this study was to investigate the associations between IgG antibodies to those 2 oral bacteria and subject-reported asthma, wheeze, and hay fever.
METHODS
Data
Data were obtained from NHANES III, in which a complex survey design was used to sample the civilian, noninstitutionalized population of the United States. The NHANES III, conducted from 1988 to 1994, was designed so that its first 3 years (phase 1), its last 3 years (phase 2), and the entire 6 years were each national probability samples. Over the 6 years, 31,311 individuals age 2 months to 90 years were administered questionnaires and given medical examinations. Details of the NHANES III design may be found elsewhere.16
Oral pathogens
In the absence of direct measurements of the oral pathogens, serum IgG antibody concentrations were used as a marker of exposure. Stored sera from phase 2 subjects age 12 years and older were analyzed with enzyme-linked immunoassays for IgG antibodies to the oral pathogens A actinomycetemcomitans and P gingivalis. IgG antibody concentrations for A actinomycetemcomitans and P gingivalis were reported for 9372 and 9371 subjects, respectively. All IgG concentrations reported in this article are in ELISA units (EU). In a recently published article, Dye et al,13 using the oral pathogen data from NHANES III, defined elevated levels of IgG antibodies to A actinomycetemcomitans and P gingivalis as concentrations greater than 156 EU and 168 EU, respectively. They determined those cut points by selecting the concentration at the 90th percentile among the population without periodontal disease, after excluding the highest and lowest 1% of the IgG distribution.
Disease outcomes
Information on asthma, hay fever, and wheeze was obtained by questionnaire, with parents or guardians providing information for child subjects. Patients with asthma were individuals who answered in the affirmative to the questions, “Has a doctor ever told you that you had asthma?” and “Do you still have asthma?” Likewise, patients with hay fever were those who answered in the affirmative to the questions, “Has a doctor ever told you that you had hay fever?” and “Do you still have hay fever?”
Patients with wheezing were persons who answered in the affirmative to the questions, “Have you had wheezing or whistling in your chest at any time in the past 12 months?” and “Apart from when you have a cold, does your chest ever sound wheezy or whistling?” The second question was asked of all participants regardless of their answers to the first, and the second question was not framed by a time period. Thus, an affirmative answer to both questions does not necessarily mean that the wheezing in the past 12 months was apart from a cold.
Although allergy skin testing to 10 common indoor allergens was performed in NHANES III, allergy skin test positivity is not presented in this article as a primary outcome because the subpopulations for allergy skin testing (all subjects age 6-19 years and a random half-sample of subjects age 20-59 years) and oral pathogen measurements (age 12-90 years in Phase 2 only) had very little overlap, and each subpopulation has its own weighting variables. Of the 10,863 subjects with allergy skin test data and the 9385 subjects with oral pathogen data, only 3702 subjects had data on both. In an exploratory analysis, associations between IgG concentrations and allergy skin test positivity were tested, and those results were reported under the heading Additional analyses. Details of the allergy skin test procedures and the definitions of a positive test used in this analysis may be found elsewhere.2 Briefly, an allergen-specific skin test result was considered positive if the difference in wheal diameters between the allergen-specific test and negative control was at least 3 mm. Allergy skin test positivity was defined as a positive test result to at least 1 of the 10 allergens.
Statistical analyses
Geometric mean antibody concentrations by disease status and by levels of the covariates were reported. Overall differences in those means were tested in unadjusted linear regression models with antibody concentrations logarithmically (base 10) transformed. Differences in prevalences of disease by elevated versus nonelevated IgG antibody concentrations were tested with χ2 statistics. Odds ratios (ORs) for the associations between antibody concentrations and each disease outcome were estimated with logistic regression. ORs were adjusted for confounding by all variables listed in Table I. Income-related variables were not used in the analysis because a significant number of subjects (n = 749) had missing values for family income. In addition, inhaled corticosteroid use was not included as a potential confounder in the primary analysis because only 98 subjects reported inhaled corticosteroid use in the past month, and there were subjects who reported taking prescription medicines but did not answer some or all of the questions about the prescription medicines.17 Differences in adjusted ORs by age, sex, and race were tested by the addition of 2-way interaction terms. For the assessment of interaction by race, individuals categorized as “other” were excluded from the analysis.
TABLE I.
Geometric mean IgG antibody concentrations (EU) to A actinomycetemcomitans and P gingivalis distributed by subject characteristics
|
A actinomycetemcomitans |
P gingivalis |
|||||
|---|---|---|---|---|---|---|
| Variable | N | Geometric mean (SE) | P | N | Geometric mean (SE) | P |
| Total | 9372 | 80.3 (1.08) | 9371 | 81.7 (1.29) | ||
| Age | <.001 | |||||
| 12-29 y | 3172 | 75.0 (1.00) | 3171 | 72.7 (1.31) | ||
| 30-49 y | 2831 | 80.7 (1.63) | 2831 | 83.4 (1.79) | ||
| 50-90 y | 3369 | 85.6 (1.27) | <.001 | 3369 | 89.9 (1.50) | |
| Sex | .631 | |||||
| Male | 4103 | 79.7 (1.18) | 4103 | 82.0 (1.52) | ||
| Female | 5269 | 80.8 (1.18) | .289 | 5268 | 81.3 (1.38) | |
| Race-ethnicity | <.001 | |||||
| Non-Hispanic white | 3423 | 75.2 (0.96) | 3423 | 76.0 (1.09) | ||
| Non-Hispanic black | 2925 | 91.4 (1.45) | 2925 | 105.5 (1.98) | ||
| Mexican American | 2561 | 92.7 (1.41) | 2560 | 93.0 (1.44) | ||
| Other | 463 | 106.2 (3.83) | <.001 | 463 | 98.3 (2.90) | |
| Education of family reference | <.001 | |||||
| <12th Grade | 3781 | 84.9 (1.06) | 3780 | 87.1 (1.22) | ||
| 12th Grade | 2799 | 80.2 (1.41) | 2799 | 82.5 (1.81) | ||
| >12th Grade | 2728 | 77.7 (1.65) | .003 | 2728 | 78.2 (1.77) | |
| Census region | .597 | |||||
| Northeast | 1284 | 82.6 (3.01) | 1284 | 81.1 (1.51) | ||
| Midwest | 1839 | 78.3 (2.27) | 1839 | 79.9 (3.95) | ||
| South | 4507 | 80.2 (1.22) | 4507 | 84.0 (1.92) | ||
| West | 1742 | 80.5 (2.19) | .719 | 1741 | 80.5 (2.24) | |
| Urbanization | .001 | |||||
| Metro areas >1 million | 4545 | 83.7 (1.66) | 4544 | 86.1 (1.48) | ||
| All other areas | 4827 | 77.1 (1.05) | .001 | 4827 | 77.7 (1.58) | |
| Serum cotinine (ng/mL) | <.001 | |||||
| 0.035-0.100 | 2667 | 81.1 (1.81) | 2667 | 80.9 (1.92) | ||
| 0.100-10.00 | 4138 | 82.5 (1.36) | 4137 | 84.4 (1.53) | ||
| 10.00-1080.0 | 2361 | 75.8 (1.08) | <.001 | 2361 | 78.2 (1.00) | |
| Body mass index | <.001 | |||||
| 11.2-18.4 (Underweight) | 397 | 78.6 (2.92) | 397 | 75.4 (2.20) | ||
| 18.5-24.9 (Normal weight) | 3606 | 78.5 (1.06) | 3605 | 77.6 (1.32) | ||
| 25.0-29.9 (Overweight) | 3016 | 81.0 (1.21) | 3016 | 84.6 (1.24) | ||
| 30.0-79.6 (Obese) | 2332 | 82.9 (1.93) | .007 | 2332 | 87.1 (2.22) | |
With the exception of the allergy skin test ORs, all reported statistics other than numbers of subjects were weighted with the Phase 2 variable WTPFHX2 to represent the civilian, noninstitutionalized population of the United States. SEs, with the exception noted, were adjusted for the survey design using SUDAAN statistical software (Release 9.0; Research Triangle Institute, Research Triangle Park, NC) and the phase 2 survey design variables SDPSTRA2 and SDPPSU2. Statistical significance was set at P ≤ .05.
RESULTS
Distribution of IgG antibodies
Fig 1 shows the distributions of the A actinomycetemcomitans and P gingivalis IgG antibody concentrations in the US population age 12 years and older. The minimum and maximum concentrations were 46 and 1994 for A actinomycetemcomitans and 40 and 22,885 for P gingivalis. A notable portion of the population had values that could be considered statistically extreme: for each pathogen, 11% of the population had antibody concentrations more than 1.5 interquartile ranges above the 75th percentile, and 7% had concentrations more than 3 interquartile ranges above the 75th percentile.
FIG 1.
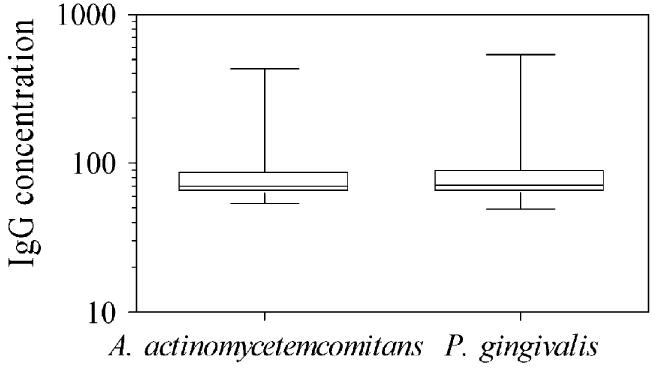
Box plots (1st, 25th, 50th, 75th, and 99th percentiles) showing the distributions of IgG antibody concentrations (EU) to A actinomycetemcomitans and P gingivalis in the US population age 12 years and older.
The geometric mean IgG antibody concentrations among the total population were 80.3 (SE, 1.03) for A actinomycetemcomitans and 81.7 (1.29) for P gingivalis. Table I shows the geometric mean antibody concentrations for the subject characteristics. For both pathogens, geometric mean concentrations differed significantly by age, race, education, urbanization, serum cotinine, and body mass index, but not by sex or census region.
Associations with asthma, wheeze, and hay fever
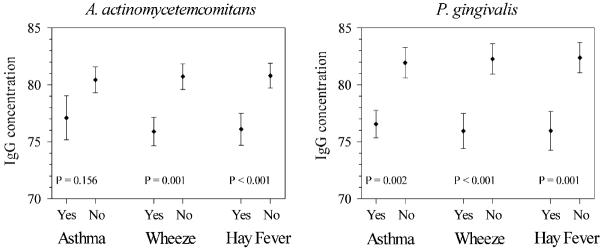
The prevalences of asthma, wheeze, and hay fever in the US population age 12 years and older were 5.2% (SE, 0.34), 9.6% (0.61), and 10.8% (0.61), respectively. Fig 2 shows the geometric mean IgG antibody concentrations for each pathogen among individuals with and without asthma, wheeze, and hay fever. For each pathogen, persons without the disease outcome had higher geometric mean IgG concentrations, although the difference in means for A actinomycetemcomitans and asthma was not statistically significant.
FIG 2.
Geometric mean (±SE) IgG antibody concentrations (EU) to A actinomycetemcomitans and P gingivalis distributed by asthma, wheeze, and hay fever (yes versus no).
Table II shows the unadjusted and adjusted ORs for the associations between asthma, wheeze, and hay fever and IgG antibody concentrations to A actinomycetemcomitans and P gingivalis. For each association, the unadjusted OR was significantly less than 1.0, except for the association between A actinomycetemcomitans and asthma. After adjustment for the potential confounders, P gingivalis was inversely associated with each of the outcomes, whereas A actinomycetemcomitans was inversely associated with wheeze (Table II). To test for the potential effects of extreme IgG antibody concentrations, the adjusted analyses shown in Table II were repeated with the lower and upper 1% of values excluded. Each of the associations that were significant in Table II remained significant, and the ORs remained essentially the same (data not shown).
TABLE II.
Unadjusted and adjusted ORs for asthma, wheeze, and hay fever for a 1–log-unit increase in IgG antibody concentrations to A actinomycetemcomitans and P gingivalis
| OR (95% CI) for a 1–log-unit increase in IgG concentration |
||||||
|---|---|---|---|---|---|---|
| Asthma |
Wheeze |
Hay fever |
||||
| Pathogen | Unadjusted | Adjusted* | Unadjusted | Adjusted* | Unadjusted | Adjusted* |
| A actinomycetemcomitans | 0.51 (0.18-1.43) | 0.56 (0.19-1.72) | 0.35 (0.18-0.68) | 0.39 (0.17-0.86) | 0.37 (0.20-0.69) | 0.48 (0.23-1.03) |
| P gingivalis | 0.41 (0.21-0.79) | 0.41 (0.20-0.87) | 0.34 (0.18-0.66) | 0.43 (0.23-0.78) | 0.34 (0.17-0.68) | 0.45 (0.23-0.93) |
Adjusted by all variables shown in Table I.
The adjusted ORs reported in Table II did not statistically differ by age, sex, or race, with 2 exceptions. For P gingivalis and hay fever, the inverse association was much stronger among males than females: OR was 0.18 (95% CI, 0.04-0.73) in males and 0.82 (0.40-1.67) in females (interaction P value = .05). The inverse association for P gingivalis and hay fever was evident only among individuals younger than 50 years: OR was 0.29 (95% CI, 0.08-1.04) for ages 12 to 29 years, 0.21 (0.06-0.70) for ages 30 to 49 years, and 1.06 (0.52-1.67) for ages 50 to 90 years (interaction P value = .05).
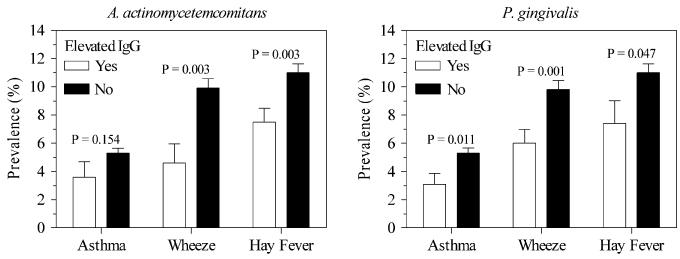
In the United States, 7.4% (SE, 0.72) and 7.2% (0.50) of the population had elevated antibody titers to A actinomycetemcomitans and P gingivalis, respectively, according to thresholds defined by Dye et al13 (see Methods). Fig 3 compares the prevalences of allergic disease for elevated versus nonelevated IgG antibody concentrations, and Table III shows the ORs for those associations. With IgG antibody concentrations dichotomized as elevated versus nonelevated, associations remained similar to those reported for the continuous IgG antibody concentrations.
FIG 3.
Prevalence (±SE) of asthma, wheeze, and hay fever for elevated versus nonelevated IgG antibody concentrations to A actinomycetemcomitans and P gingivalis.
TABLE III.
Unadjusted and adjusted ORs for asthma, wheeze, and hay fever for IgG antibody concentrations to A actinomycetemcomitans and P gingivalis categorized as elevated versus not elevated (see Methods)
| OR (95% CI) for elevated versus not elevated IgG |
||||||
|---|---|---|---|---|---|---|
| Asthma |
Wheeze |
Hay fever |
||||
| Pathogen | Unadjusted | Adjusted* | Unadjusted | Adjusted* | Unadjusted | Adjusted* |
| A actinomycetemcomitans | 0.68 (0.36-1.28) | 0.72 (0.38-1.36) | 0.43 (0.21-0.88) | 0.41 (0.18-0.96) | 0.66 (0.49-0.88) | 0.72 (0.54-0.95) |
| P gingivalis | 0.56 (0.33-0.96) | 0.57 (0.34-0.97) | 0.58 (0.41-0.82) | 0.70 (0.52-0.94) | 0.64 (0.38-1.08) | 0.73 (0.44-1.22) |
Adjusted by all variables shown in Table I.
Additional analyses
In a secondary analysis, the potential confounding effects of inhaled corticosteroid use was investigated. Inhaled corticosteroid use in the past month was reported by only 98 subjects (1.6% of total population, 16.4% of patients with asthma, 8.0% of patients with wheezing, and 5.2% of patients with hay fever). When adjusted for corticosteroid use, the ORs for the log10-transformed IgG concentrations remained similar to the unadjusted ORs reported in Table II. For P gingivalis, the corticosteroid-adjusted ORs were 0.47 (95% CI, 0.25-0.88) for asthma, 0.37 (0.19-0.71) for wheeze, and 0.35 (0.18-0.69) for hay fever. For A actinomycetemcomitans, those ORs were 0.55 (0.22-1.42), 0.37 (0.19-0.71), and 0.38 (0.20-0.70), respectively.
In another analysis, ORs for the associations between the log10-transformed IgG concentrations and a positive allergy skin test (yes vs no) among the 3702 subjects with data on both oral pathogens and skin test positivity were estimated. The ORs, which were unweighted but adjusted for the covariables listed in Table I, were 1.0 (95% CI, 0.7-1.4) for A actinomycetemcomitans and 1.1 (0.8-1.5) for P gingivalis.
DISCUSSION
Consistent with the hygiene hypothesis, this study found that higher concentrations of serum IgG antibodies to P gingivalis were significantly associated with lower prevalences of asthma, wheeze, and hay fever, whereas higher concentrations of IgG antibodies to A actinomycetemcomitans were significantly associated with a lower prevalence of wheeze. These findings are also consistent with a previous study of NHANES III data that found asthma and hay fever were less frequent in subjects seropositive for hepatitis A virus, Toxoplasma gondii, and herpes simplex virus 1.18
A actinomycetemcomitans has been implicated in an aggressive form of periodontal disease, whereas P gingivalis has been implicated in a chronic form, though both forms of periodontal disease are considered mixed bacterial diseases.19 Aggressive periodontitis, which has a US prevalence of less than 1%, is most common around puberty.20 Chronic periodontitis, which has a prevalence of roughly 20%, usually affects adults, but it can also occur in children.20 Although A actinomycetemcomitans and P gingivalis are considered pathogens, they are commonly found at very low levels in the mouths of healthy children and adults. Using DNA-based detection methods, 1 group of researchers found A actinomycetemcomitans and P gingivalis, respectively, in 48% and 36% of children age 0 to 18 years, and both species were detected in subjects as young as 20 days old.21 It is well established that these periodontal pathogens are transmitted from parent to child, from sibling to sibling, and between spouses.22 Transmission is most likely through contact with saliva and the sharing of objects such as cups, spoons, and toothbrushes.22
The microbial ecology of the oral cavity is very complex, with hundreds of species colonizing on mucosal and tooth surfaces. On teeth, bacteria reside in a biofilm known as dental plaque. In healthy mouths, the dental plaque consists primarily of Gram-positive facultative species.9,23 That composition is maintained through a complex interplay of host, bacterial, and environmental factors.9,23 Periodontal health has been described as “a state of balance when the bacterial population coexists with the host and no irreparable damage occurs to either the bacteria or the host tissues.”23 Of the host factors, the immune system plays an important role in controlling the composition of the dental plaque. Neutrophils play a primary role in the surveillance and control of the periodontal pathogens.24 In fact, neutrophil disorders, such as Papillon-Lefèvre syndrome, are associated with severe periodontal destruction at an early age.24
When the balance among host, bacteria, and environment favors the overgrowth of Gram-negative facultative and anaerobic species, such as A actinomycetemcomitans and P gingivalis, periodontal disease is initiated.9 With the initiation of disease, chronic inflammatory cells, macrophages, and lymphocytes appear in the periodontal tissues.24 Interactions between bacterial antigens and local B cells lead to the production of antibody within the periodontal tissues, which is essential for bacterial phagocytosis, and the interactions of bacterial antigens with peripheral dendritic cells lead to the production of systemic antibodies, such as the IgG antibodies measured in this study.24 Proinflammatory mediators such as IL-1β, TNF-α, and prostaglandin E2 are released, leading to the destruction of the soft tissues and bone surrounding the teeth, the hallmark of periodontitis.24 Periodontitis is a leading, if not the leading, cause of tooth loss among adults.25 Because bacterial plaque is dependent on the presence of teeth, tooth loss could be considered the body's way of limiting the infection. Treatment for periodontitis focuses on the removal and prevention of dental plaque. Surgical intervention is often required not only to reduce deep pockets around teeth that encourage the growth of dental plaque but also to restore the support and function of the teeth.
The proposed biological mechanism for the hygiene hypothesis that came out of animal studies in the late 1980s was that microbial infections induced a TH1 cell pattern of cytokine release, which inhibited the TH2 cell pattern involved in IgE-mediated allergy.5 Originally, it was thought that the balance between TH1 and TH2 cytokines was controlled by reciprocal inhibition; however, observations that the prevalences of both TH1-mediated autoimmune disease and TH2-mediated allergic disease were increasing and that autoimmune and allergic diseases often coexisted in the same individuals brought the TH1/TH2 paradigm into question.6,26 It is now thought that both TH1 cells and TH2 cells are under the control of regulatory T cells, which can inhibit both TH1 and TH2 cells through the release of mediators such as IL-10 and TGF-β.27 The observation that germ-free mice cannot develop oral tolerance to antigens has led to the speculation that microflora in the gut play an important role in promoting the development of regulatory T cells.7,28,29 It is unquestionable that the oral pathogens A actinomycetemcomitans and P gingivalis have local and systemic effects on the immune system; however, whether the immune system's interaction with these pathogens could prevent allergic disease by either shifting the balance toward a TH1 phenotype or encouraging the development of regulatory T cells is not known.
An important limitation to the study is the cross-sectional nature of NHANES III. As originally conceived by Strachan,4 the hypothesis is concerned with infections or microbial exposures that occur in early childhood or even prenatally. For this study, it cannot be determined when the oral pathogen challenge occurred or when the IgG antibody response developed. Because of that ambiguity, reverse causality—that allergic disease suppressed oral pathogen colonization or the IgG response—cannot be discounted. The facts that asthma is more prevalent in children than adults and that periodontal disease is more prevalent in adults than children provide support for reverse causality. However, colonization with these oral pathogens often occurs in early infancy, and an IgG antibody response to these pathogens is evident as early as 6 months of age.21,30,31 In addition, asthma and hay fever often develop during adulthood, as does allergic sensitization. Data from NHANES II and III indicate that the prevalence of allergic sensitization is greatest in the second and third decades of life.2,32 Perhaps some microbial challenges even in late childhood or young adulthood provide a beneficial immune stimulus, whereas the lack of some microbial challenges even in late childhood or early adulthood increases one's risk for allergic disease.
Related to the issue of reverse causality is the possibility that use of inhaled corticosteroids inhibited the IgG response. The secondary analysis did not indicate confounding by inhaled corticosteroid use; however, the prescription medication database, according to NHANES documentation, contains incomplete information on medication use.17
A second limitation of this study was the use of antibody levels as a marker of oral pathogen exposure. Although the pathogens can be readily sampled from the mouth and identified and quantified by any of a variety of laboratory techniques, oral sampling was not conducted in NHANES III. Several studies have shown significant, positive correlations between specific IgG antibody levels and levels of these pathogens in the mouth33-35; however, those studies were relatively small, and correlations were not consistently strong. Because antibody responses to these and other pathogens are influenced by a variety of host factors, serum antibody levels may not be an accurate measure of pathogen exposure. In future studies of associations between oral pathogens and allergic disease, direct measurements of the pathogens, in addition to measurements of serum antibodies, should be made.
A third limitation was the study's inability to assess adequately associations between the oral pathogens and allergic sensitization. If oral pathogen exposures truly influence allergic disease outcomes, then one would expect to see inverse relationships between measures of oral pathogen exposure and allergy skin test reactivity or serum IgE levels. Unfortunately, neither total nor allergenspecific IgE was measured in NHANES III, and allergy skin testing was not performed on the majority of subjects with oral pathogen data (see Methods). The unweighted analysis of subjects with both allergy skin test and oral pathogen data suggested null associations; however, the limited number of subjects with data and the lack of representativeness of the US population bring into question the validity of those results. The strong association between P gingivalis and hay fever, which some researchers have used as a proxy for IgE or allergy skin test data,36 also brings into question the validity of the oral pathogen and allergy skin test results.
The hygiene hypothesis contends that fewer opportunities for infection and microbial exposures are responsible, at least in part, for the rise in prevalences of allergic diseases. For decades, the dental profession and commercial companies that market oral hygiene products have waged war against the oral bacteria that cause tooth decay and periodontal disease, 2 oral conditions that have decreased in prevalence. Although it is tempting to speculate that reductions in oral bacteria have contributed to the rise in the prevalences of allergic diseases, no studies have examined whether levels or types of oral bacteria within the US population have changed over time. Although this study found a strong inverse association between IgG antibodies to oral bacteria and allergic diseases, it is impossible to know from this cross-sectional data whether oral bacteria inhibited the development of allergic disease or vice versa. More research is needed to clarify the potential roles of these and other bacteria in the etiology of allergic diseases.
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences.
Abbreviations used
- EU
ELISA unit
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds ratio
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
REFERENCES
- 1.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma—United States, 1980-1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 2.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Liu AH, Murphy JR. Hygiene hypothesis: fact or fiction? J Allergy Clin Immunol. 2003;111:471–8. doi: 10.1067/mai.2003.172. [DOI] [PubMed] [Google Scholar]
- 4.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis.”. Thorax. 2000;55(suppl 1):S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheikh A, Strachan DP. The hygiene theory: fact or fiction? Curr Opin Otolaryngol Head Neck Surg. 2004;12:232–6. doi: 10.1097/01.moo.0000122311.13359.30. [DOI] [PubMed] [Google Scholar]
- 7.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–8. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 9.Kuramitsu HK, Ellen RP. Oral bacterial ecology: the molecular basis. Horizon Scientific; Wymondham: 2000. [Google Scholar]
- 10.Pussinen PJ, Alfthan G, Tuomilehto J, Asikainen S, Jousilahti P. High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2004;11:408–11. doi: 10.1097/01.hjr.0000129745.38217.39. [DOI] [PubMed] [Google Scholar]
- 11.Mattila KJ, Pussinen PJ, Paju S. Dental infections and cardiovascular diseases: a review. J Periodontol. 2005;76:2085–8. doi: 10.1902/jop.2005.76.11-S.2085. [DOI] [PubMed] [Google Scholar]
- 12.Pussinen PJ, Jousilahti P, Alfthan G, Palosuo T, Asikainen S, Salomaa V. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:1250–4. doi: 10.1161/01.ATV.0000072969.71452.87. [DOI] [PubMed] [Google Scholar]
- 13.Dye BA, Choudhary K, Shea S, Papapanou PN. Serum antibodies to periodontal pathogens and markers of systemic inflammation. J Clin Periodontol. 2005;32:1189–99. doi: 10.1111/j.1600-051X.2005.00856.x. [DOI] [PubMed] [Google Scholar]
- 14.Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, et al. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation. 2005;112:19–24. doi: 10.1161/CIRCULATIONAHA.104.511998. [DOI] [PubMed] [Google Scholar]
- 15.Pussinen PJ, Nyyssonen K, Alfthan G, Salonen R, Laukkanen JA, Salonen JT. Serum antibody levels to Actinobacillus actinomycetemcomitans predict the risk for coronary heart disease. Arterioscler Thromb Vasc Biol. 2005;25:833–8. doi: 10.1161/01.ATV.0000157982.69663.59. [DOI] [PubMed] [Google Scholar]
- 16.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat. 1994;1:1–407. [PubMed] [Google Scholar]
- 17.Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. (Series 11).NHANES III prescription medicines data file documentation. 1998;(No 2A) Available at: http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm. Accessed April 17, 2006.
- 18.Matricardi PM, Rosmini F, Panetta V, Ferrigno L, Bonini S. Hay fever and asthma in relation to markers of infection in the United States. J Allergy Clin Immunol. 2002;110:381–7. doi: 10.1067/mai.2002.126658. [DOI] [PubMed] [Google Scholar]
- 19.Novak MJ. Classification of diseases and conditions affecting the periodontium. In: Newman MG, Takei HH, Carranza FA, editors. Carranza's clinical periodontology. WB Saunders Co; Philadelphia: 2002. pp. 64–73. [Google Scholar]
- 20.Beck JD, Arbes SJ., Jr . Epidemiology of gingival and periodontal diseases. In: Newman MG, Takei HH, Carranza FA, editors. Carranza's clinical periodontology. WB Saunders Co; Philadelphia: 2002. pp. 74–94. [Google Scholar]
- 21.Lamell CW, Griffen AL, McClellan DL, Leys EJ. Acquisition and colonization stability of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in children. J Clin Microbiol. 2000;38:1196–9. doi: 10.1128/jcm.38.3.1196-1199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darby I, Curtis M. Microbiology of periodontal disease in children and young adults. Periodontology. 2000 2001;26:33–53. doi: 10.1034/j.1600-0757.2001.2260103.x. [DOI] [PubMed] [Google Scholar]
- 23.Haake SK, Newman MG, Nisengard RJ, Sanz M. Periodontal microbiology. In: Newman MG, Takei HH, Carranza FA, editors. Carranza's clinical periodontology. WB Saunders Co; Philadelphia: 2002. pp. 96–112. [Google Scholar]
- 24.Haake SK, Nisengard RJ, Newman MG, Miyasaki KT. Microbial interactions with the host in periodontal diseases. In: Newman MG, Takei HH, Carranza FA, editors. Carranza's clinical periodontology. WB Saunders Co; Philadelphia: 2002. pp. 132–52. [Google Scholar]
- 25.Burt BA, Eklund SA, Ismail AI. Dentistry, dental practice, and the community. 5th ed. Saunders; Philadelphia: 1999. [Google Scholar]
- 26.Bjorksten B. Effects of intestinal microflora and the environment on the development of asthma and allergy. Springer Semin Immunopathol. 2004;25:257–70. doi: 10.1007/s00281-003-0142-2. [DOI] [PubMed] [Google Scholar]
- 27.Umetsu DT, Akbari O, Dekruyff RH. Regulatory T cells control the development of allergic disease and asthma. J Allergy Clin Immunol. 2003;112:480–7. quiz 8. [PubMed] [Google Scholar]
- 28.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–45. [PubMed] [Google Scholar]
- 29.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun. 2005;73:30–8. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClellan DL, Griffen AL, Leys EJ. Age and prevalence of Porphyromonas gingivalis in children. J Clin Microbiol. 1996;34:2017–9. doi: 10.1128/jcm.34.8.2017-2019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouton C, Hammond PG, Slots J, Genco RJ. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periodontal disease. Infect Immun. 1981;31:182–92. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gergen PJ, Turkeltaub PC, Kovar MG. The prevalence of allergic skin test reactivity to eight common aeroallergens in the U.S. population: results from the second National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 1987;80:669–79. doi: 10.1016/0091-6749(87)90286-7. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa S, Machida Y, Nakagawa T, Fujii H, Yamada S, Takazoe I, et al. Infection by Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans, and antibody responses at different ages in humans. J Periodontal Res. 1994;29:9–16. doi: 10.1111/j.1600-0765.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 34.Papapanou PN, Neiderud AM, Papadimitriou A, Sandros J, Dahlen G. “Checkerboard” assessments of periodontal microbiota and serum antibody responses: a case-control study. J Periodontol. 2000;71:885–97. doi: 10.1902/jop.2000.71.6.885. [DOI] [PubMed] [Google Scholar]
- 35.Kojima T, Yano K, Ishikawa I. Relationship between serum antibody levels and subgingival colonization of Porphyromonas gingivalis in patients with various types of periodontitis. J Periodontol. 1997;68:618–25. doi: 10.1902/jop.1997.68.7.618. [DOI] [PubMed] [Google Scholar]
- 36.Hoppin JA, Umbach DM, London SJ, Alavanja MC, Sandler DP. Diesel exhaust, solvents, and other occupational exposures as risk factors for wheeze among farmers. Am J Respir Crit Care Med. 2004;169:1308–13. doi: 10.1164/rccm.200309-1228OC. [DOI] [PubMed] [Google Scholar]