Abstract
Thin layer chromatography was used to analyze glycosaminoglycan oligosaccharides obtained through the use of polysaccharide lyases. This method allows for the rapid, semi-quantitative analysis of a wide variety of glycosaminoglycan oligosaccharides.
Introduction
Glycosaminoglycans (GAGs) are linear, acidic polysaccharides found on cell surfaces and in the surrounding extracellular matrix. GAGs participate in and regulate many cellular events in physiological and pathophysiological processes, such as cell proliferation and differentiation, cell-cell and cell-matrix interactions, and viral infection, through their interaction with different proteins [1–3]. GAGs are divided into four main categories, hyaluronic acid (HA), chondroitin sulfate/dermatan sulfate (CS/DS), heparosan/heparan sulfate/heparin (HN/HS/HP) and keratan sulfate, based on monosaccharide composition and the configuration and position of the glycosidic bonds between their monosaccharides. The specificity of the interactions between GAGs and proteins result from the structural diversity of GAG type, size, saccharide composition, charge density, and sequence [4, 5]. It is often necessary to determine the size and purity of GAG-derived oligosaccharides, analyze activity of enzymes acting on GAGs and to monitor the preparation of GAG-derived oligosaccharides. Polyacrylamide gel electrophoresis (PAGE) is a method routinely used for separation and analysis of GAGs and GAG-derived oligosaccharides and the resulting gels are usually visualized by staining with alcain blue [6–9]. Unfortunately, bands corresponding to small oligosaccharides with a low net charge show low sensitivity to alcain blue staining, i.e., HA and HN (smaller than hexasaccharides) and CS/DS and HS (smaller than tetrasaccharides). Fluorophore assisted carbohydrate electrophoresis (FACE) affords an alternative method to analyze such small oligosaccharides [10, 11]. However, both PAGE and FACE are time and labor consuming. In this communication, we describe a newly developed thin layer chromatography (TLC) method for the analysis of acidic GAG-derived oligosaccharides. In comparison with PAGE and FACE, this TLC method shows high sensitivity for small oligosaccharides having a low net charge and can be performed quickly and easily at a low cost. Multiple samples can be analyzed in parallel using TLC, suggesting the utility of this approach in high throughput applications.
Materials and Methods
Preparation of oligosaccharide mixtures
HN was isolated through fermentation of E. coli K5, and purified as previous described [12]. Purified HN (500 μg/50 μl) was incubated at 37°C for 10 h in 50 mM sodium phosphate buffer pH 7.0 in presence of varying amounts of heparin lyase III (1, 2, 5, 10, 15 munits, Sigma Co. St. Louis, MO). The reaction mixtures were heated in a boiling water bath for 10 min to thermally inactivate the enzyme, halting the reaction. HA, from rooster comb, was purchased from Sigma Co. CS-A (chondroitin-6-sulfate, from bovine trachea), dermatan disulfate (DDS, dermatan 4, 6-disulfate, from porcine intestinal mucosa) and HP (from porcine intestinal mucosa) were purchased from Celsus Co., Cincinnati, Ohio. Chondroitin lyase ABC, 10, 5, 20 munits, (Seikagaku Co., Tokyo, Japan) was used to digest HA, CS-A and DDS (500 μg/50 μl) in 50 mM sodium phosphate buffer pH 7.0 at 37°C for 10 h, respectively. Heparin lyase I (20 munits, Sigma) was used to digest HP in 50 mM sodium phosphate buffer pH 7.0 at 37°C for 10 h.
TLC conditions
Reaction product mixture (0.3 μl of each) were loaded on a pre-coated silica gel-60 TLC aluminum plates (Merck Germany) (3 × 5 cm) and developed with a solvent system consisting of n-butanol/formic acid/water, 4:8:1 (v/v). The developed plates were stained by dipping in diphenylamine-aniline-phosphoric acid reagent (1 ml of 37.5% HCl, 2 ml of aniline, 10 ml of 85% H3PO3, 100 ml of ethyl acetate and 2 g diphenylamine) for 3 s and heated at 150 °C for 10 sec [13]. TLC plates were scanned, digitized and analyzed by UN-scan-it gel scanning software (Silk Scientific, Orem, Utah).
Results and Discussion
HN was treated with varying amounts of heparin lyase III to obtain oligosaccharide product mixtures. The percentage completion of digestion was quantified by dividing the UV absorbance at 232 nm, of the products prepared using a given amount enzyme for 10 h, by the UV absorbance at 232 nm determined at reaction completion. TLC analysis was performed to follow the enzymatic depolymerization of HN, at 5%, 10%, 15%, 30%, 50% reaction completion (Figure 1A). The stained TLC plate was digitized to obtain a semi-quantitative analysis (Figure 1B). In the reaction mixture at 5% completion, chromatography showed much of product remained at the origin of TLC and only very faint spots corresponding to smaller oligosaccharides were observed, suggesting the product mixture primarily contained higher oligosaccharides (higher than hexadecasaccharide, >16 saccharide units). The reaction products obtained at 10% and 15% completion (Lane b and c in Figure 1A) clearly showed spots on the TLC plate corresponding to oligosaccharides having 4–14 saccharide units. At reaction completion 30% and 50%, oligosaccharides from disaccharide to tetradecasaccharide were observed as distinctive separated bands on the TLC plate (Lanes d and e in Figure 1A). Next, the TLC data were digitized and intensity was plotted as a function of distance from origin (Figure 1B). In all lanes the band remaining at the origin corresponding to polysaccharide and oligosaccharides (> 16 saccharide units) was the most intense. While the bands corresponding to the oligosaccharides having 2–10 saccharide units increased, the bands associated with oligosaccharides having 12 and 14 saccharide units decreased with percentage digestion completion. The change in total band intensity as a function of digestion completion (Figure 1C) confirms this trend and can be used to assess reaction kinetics. The total band intensity of disaccharide increased sharply only after the digestion completion passed 20% completion. Similarly, the intensity of tetrasaccharide increased sharply when the digestion passed 15%. The rate of increase for hexasaccharide and octasaccharide slowed after 30% digestion. The decasaccharide and dodecasaccharide decreased slightly and significantly after 30% digestion. The profiles of the product mixtures at different reaction completion on the TLC plate clearly demonstrate that heparin lyase III is an endolytic enzyme confirming the results of previous methods that relied on PAGE analysis and viscometry [14]. Time-course experiments using a fixed quantity of enzyme can also be conveniently monitored and analyzed by this method [15].
Figure 1.
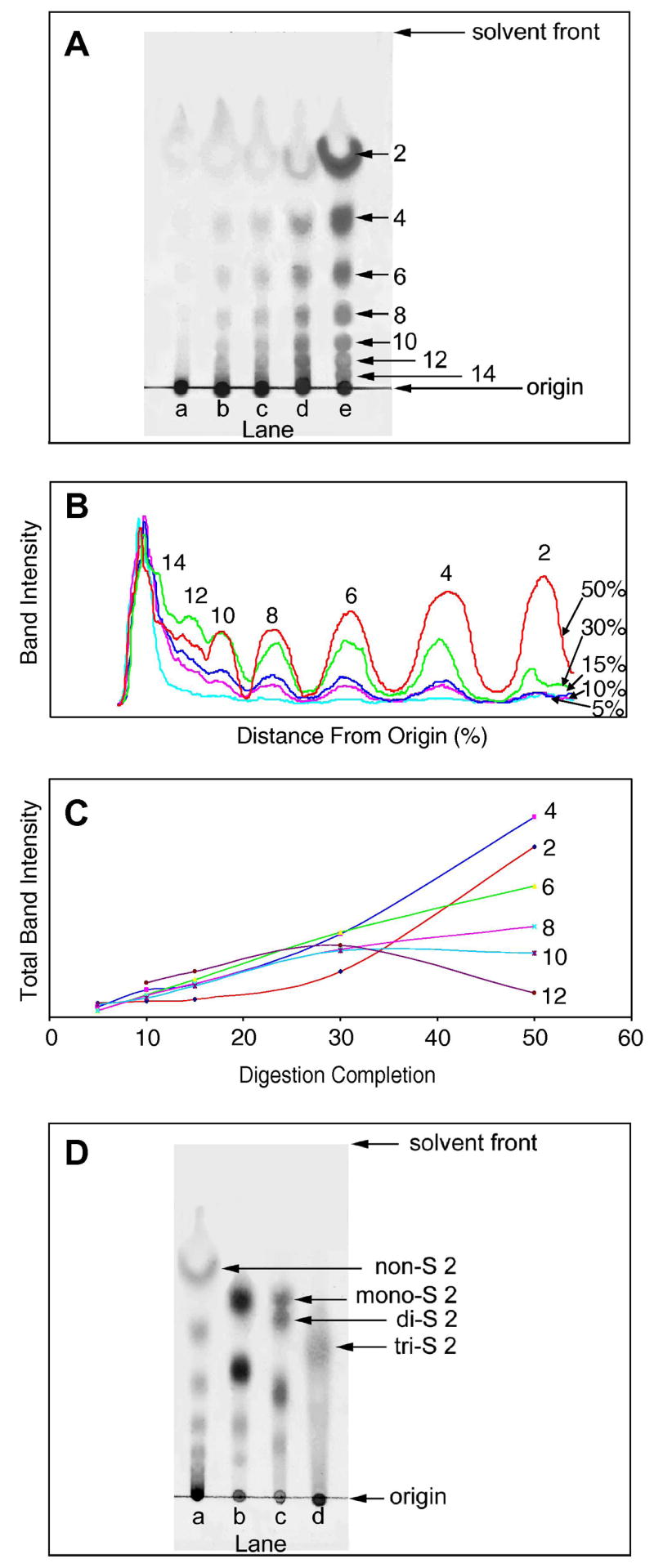
A. TLC of partially digested heparosan. The reaction products obtained at Lane a, 5% completion; Lane b, 10% completion; Lane c, 15% completion; Lane d, 30% completion; Lane e, 50% completion. B. Digitized TLC. C. A plot of total band intensity as a function of percentage digestion completion for each size oligosaccharide. D. TLC of other GAGs digested with polysaccharide lyases. Lane a, HA; Lane b, CS-A; Lane c, DDS; Lane d, HP. The major disaccharides found in each GAG and their number of sulfo groups (nonsulfated, monosulfated, disulfated and trisulfated) is indicated. Their identity was confirmed based on the use of disaccharide standards. The numbers 2–14 in panels A-D correspond to the number of saccharide units present in each oligosaccharide product.
Next, oligosaccharide mixtures, prepared from HA, CS-A, DDS and HP by polysaccharide lyase treatment, were analyzed by TLC plate (Figure 1D). The HA oligosaccharide mixture analyzed contained oligosaccharides between disaccharide and hexadecasaccharide (Lane a in Figure 1D). HA is a nonsulfated GAG consisting of the repeating units, →4) glucuronic acid (GlcA) (1 → 3) N-acetylglucosamine (GlcNAc) (1→. The absence of sulfation in HA oligosaccharides results in both the enhanced migration and resolution of these oligosaccharides. A second non-sulfated GAG, HN, analyzed by TLC under same conditions gave similarly high resolution (data not shown). Analysis of oligosaccharides prepared from CS-A (Lane b in Figure 1D) showed 4 spots corresponding to disaccharide, tetrasaccharide, hexasaccharide and octasaccharide. CS-A consists of repeating units →4) glucuronic acid (GlcA) (1→3) 6-O-sulfated N-acetyl galactosamine (GalNAc) (1→. The single sulfo group at position 6 of GalNAc in CS-A oligosaccharides retards their migration on TLC as a result of their higher polarity. Analysis of DDS (prepared by chemical sulfation of dermatan sulfate at the primary 6-hydroxyl group of GalNAc, resulting in GalNAc 4S6S) is shown in Figure 1D lane c. Bands corresponding to disaccharide, tetrasaccharide and hexasaccharide were observed in the TLC. The disaccharide band shows two closely spaced spots corresponding to mono-S and di-S disaccharides as expected based on incomplete chemical sulfation. Analysis of heparin, a very complex GAG having variable levels of sulfation, showed a broad smear extending from the origin to disaccharides, which consists primarily of a tri-S disaccharide.
In conclusion, TLC can be used to separate GAG oligosaccharides on the basis of size and disaccharides on the basis of sulfation level. Furthermore, TLC represents as a quick, easy and reliable method for the parallel analysis of GAG oligosaccharide samples and can be used to examine the active pattern of polysaccharide lyases.
Acknowledgments
This work was supported by the National Institute of Health (Grants HL62244 and GH38060).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu ZL, Zhang L, Beeler DL, Kuberan B, Rosenberg RD. A new strategy for defining critical functional groups on heparan sulfate. FASEB J. 2002;16:539–545. doi: 10.1096/fj.01-0807com. [DOI] [PubMed] [Google Scholar]
- 2.Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 4.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 5.Sasisekharan R, Raman R, Prabhakar V. Glycomics approach to structure-function relationships of glycosaminoglycans. Annu Rev Biomed Eng. 2006;8:181–231. doi: 10.1146/annurev.bioeng.8.061505.095745. [DOI] [PubMed] [Google Scholar]
- 6.Edens RE, al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Linhardt RJ. Gradient polyacrylamide gel electrophoresis for determination of molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci. 1992;81:823–827. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 7.Lee KB, Desai UR, Palcic MM, Hindsgaul O, Linhardt RJ. An electrophoresis-based assay for glycosyltransferase activity. Anal Biochem. 1992;205:108–114. doi: 10.1016/0003-2697(92)90586-v. [DOI] [PubMed] [Google Scholar]
- 8.Min H, Cowman MK. Combined alcian blue and silver staining of glycosaminoglycans in polyacrylamide gels: application to electrophoretic analysis of molecular weight distribution. Anal Biochem. 1986;155:275–285. doi: 10.1016/0003-2697(86)90437-9. [DOI] [PubMed] [Google Scholar]
- 9.Rice KG, Rottink MK, Linhardt RJ. Fractionation of heparin-derived oligosaccharides by gradient polyacrylamide-gel electrophoresis. Biochem J. 1987;244:515–522. doi: 10.1042/bj2440515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabro A, Hascall VC, Midura RJ. Adaptation of FACE methodology for microanalysis of total hyaluronan and chondroitin sulfate composition from cartilage. Glycobiology. 2000;10:283–293. doi: 10.1093/glycob/10.3.283. [DOI] [PubMed] [Google Scholar]
- 11.Karousou EG, Porta G, De Luca G, Passi A. Analysis of fluorophore-labelled hyaluronan and chondroitin sulfate disaccharides in biological samples. J Pharm Biomed Anal. 2004;34:791–795. doi: 10.1016/S0731-7085(03)00568-5. [DOI] [PubMed] [Google Scholar]
- 12.Vann WF, Schmidt MA, Jann B, Jann K. The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. Eur J Biochem. 1981;116:359–364. doi: 10.1111/j.1432-1033.1981.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Yu G, Zhao X, Liu H, Guan H, Lawson AM, Chai W. Sequence analysis of alginate-derived oligosaccharides by negative-ion electrospray tandem mass spectrometry. J Am Soc Mass Spectrom. 2006;17:621–630. doi: 10.1016/j.jasms.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Jandik KA, Gu K, Linhardt RJ. Action pattern of polysaccharide lyases on glycosaminoglycans. Glycobiology. 1994;4:289–296. doi: 10.1093/glycob/4.3.289. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Xie J, Liu J, Linhardt RJ. LC-MS/MS to distinguish Hyaluronic acid from N-Acetylheparosan. Anal Biochem. 2007 doi: 10.1016/j.jasms.2007.10.012. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]


