Abstract
Salvinorin A, a neoclerodane diterpenoid, isolated from the Mexican hallucinogenic plant, Salvia divinorum is a potent kappa-opioid receptor agonist. Its biosynthetic route was studied by NMR and HR-ESI-MS analysis of the products of the incorporation of [1-13C]-glucose, [Me-13C]-methionine, and [1-13C; 3,4-2H2]-1-deoxy-d-xylulose into its structure. The use of cuttings and direct stem injection were unsuccessful, however, incorporation of 13C into salvinorin A was achieved using in vitro sterile culture of microshoots. NMR analysis of salvinorin A (2.7 mg) isolated from 200 microshoots grown in the presence of [1-13C]-glucose established that this pharmacologically important diterpene is biosynthesized via the 1-deoxy-d-xylulose-5-phosphate pathway, instead of the classic mevalonic acid pathway. This was confirmed in plants grown in the presence of [1-13C; 3,4-2H2]-1-deoxy-d-xylulose. In addition, analysis of salvinorin A produced by plants grown in the presence of [Me-13C]-methionine indicates that the methylation of the C-4 carboxyl group is catalyzed by a type III S-adenosyl-l-methionine-dependent O-methyltransferase.
Key Words Index: salvinorin A, Salvia divinorum, deoxyxylulose phosphate pathway, 13C-labeling, biosynthesis, retrobiosynthetic NMR analysis
1. Introduction
Salvia divinorum, commonly referred to as Maria Pastora sage, has been known for its hallucinogenic properties for generations by Mazatec Indians of Oaxaca, Mexico (Valdés III et al., 1983). The active component of S. divinorum, salvinorin A (1) (Fig. 1), was discovered by Ortega and colleagues in 1982 (Ortega et al., 1982), and its psychoactive activity in mice tests were reported a few years later (Valdés III et al., 1987). Subsequent work established a threshold dose of 200 μg of 1 for humans (Siebert, 1994). Salvinorin A is a first non-nitrogenous, potent and selective kappa-opioid receptor agonist, and is being intensively studied as a lead compound for the treatment of mental disorders (Roth et al., 2002; Vortherms and Roth, 2006).
Fig. 1.
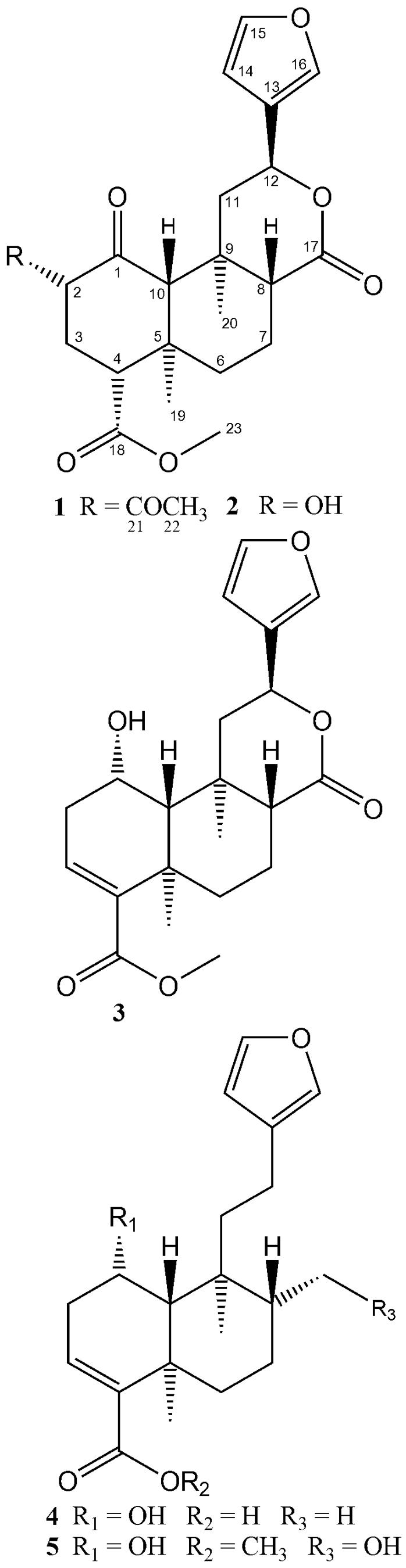
Structures of salvinorin A (1) with atoms numbered according to Ortega et al. (2), salvinorins B (2), F (3), and divinatorins A (4) and B (5) isolated from Salvia divinorum.
Terpenoids are among the most abundant plant secondary metabolites (Gershenzon and Croteau, 1991). All terpenoids result from the assembly of isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) building blocks (Eisenreich et al., 2004). It was long thought that these precursors originate exclusively from the mevalonic acid (MVA) pathway, which is ubiquitous in plants and animals (Porter and Spurgeon, 1981). However, this paradigm was challenged by Rohmer in labeling studies of bacterial hopanoids (Rohmer et al., 1993). Broers and Schwarz showed that the new pathway involves the monophosphate of 1-deoxy-D-xylulose (DOX), and that higher plants utilize both the MVA and DOX pathways (Broers, 1994; Schwarz, 1994). This biosynthetic route is now called the DOXP or MEP pathway, in reference to the early pentose intermediates, 1-deoxy-d-xylulose 5-phosphate and 2-C-methyl-D-erythritol 4-phosphate (Eisenreich et al., 2004; Eisenreich et al., 2001; Eisenreich et al., 1998; Rohmer, 1999).
The biosynthetic pathways of many different metabolites have been studied extensively using stable isotopes, primarily 13C and 2H (Simpson, 1998). The biosynthetic pathway of the hallucinogenic diterpenoid 1 has not yet been elucidated. Recent studies have shown that 1 is compartmentalized within the glandular trichomes located on abaxial side of the leaves of S. divinorum (Siebert, 2004). Since monoterpenes produced in glandular trichomes are normally derived from the DOXP pathway (Samanani and Facchini, 2006), we hypothesized that this psychoactive diterpenoid was also formed by this alternative route (Fig. 2). This hypothesis was tested by incorporation experiments with [1-13C]-glucose and [1-13C; 3,4-2H2]-1-deoxy-d-xylulose (DOX) in Salvia divinorum. The source of the methyl ester of 1 was also investigated in a feeding experiment with [Me-13C]-methionine.
Fig. 2.
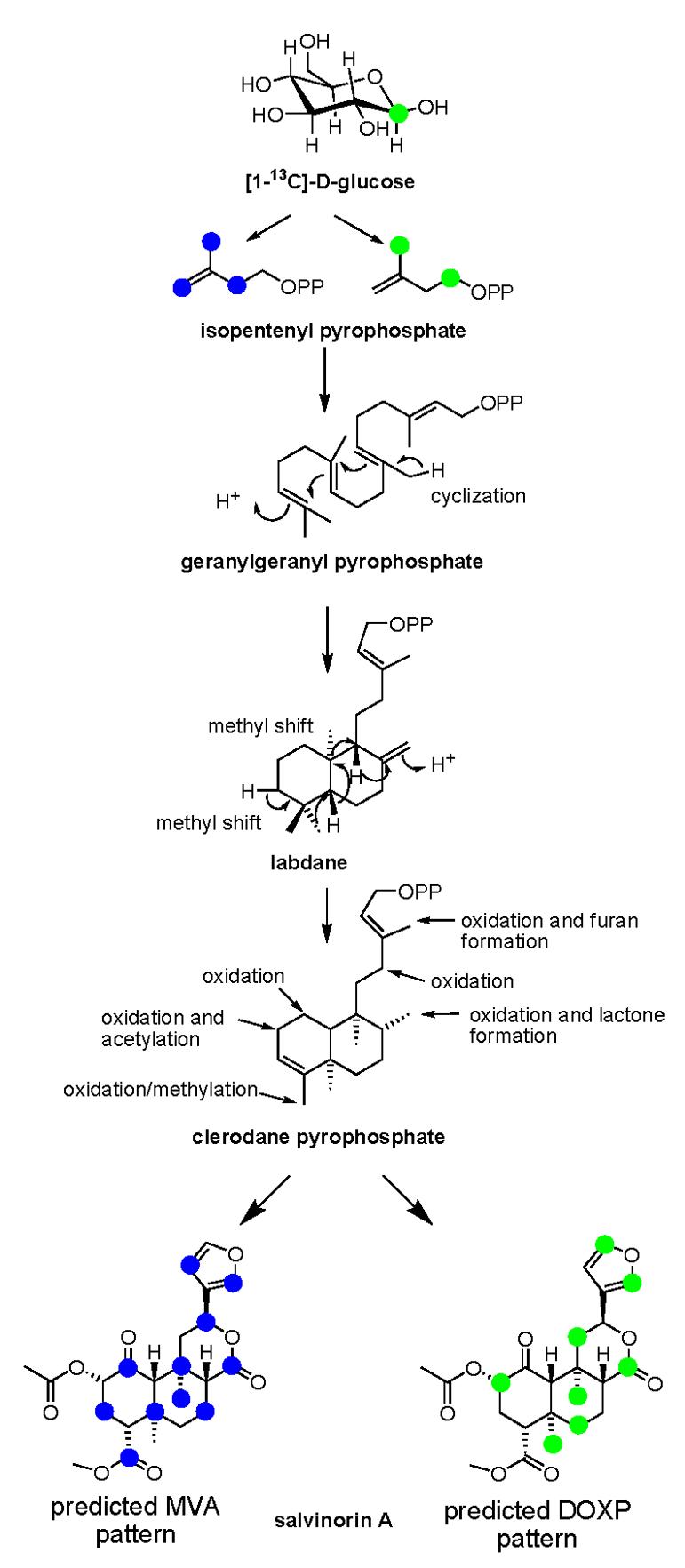
Simplified biosynthetic scheme showing the predicted incorporation pattern of [1-13C]-glucose isotopically labeled IPP in salvinorin A (1) derived from either the MVA route (blue labels) or DOXP pathway (green labels).
2. Results and Discussion
2.1. Method of incorporation
Salvinorin A (1) is being extensively studied as a lead compound for the treatment of mental disorders (Roth et al., 2002; Vortherms and Roth, 2006), however, its biosynthesis has not been investigated previously. Preliminary experiment with [2-13C]-glucose were designed to determine whether isotopically labeled substrates were taken up by S. divinorum cuttings and to estimate the duration of the feeding experiment required for sufficient incorporation. Our initial attempts to label 1 using plant cuttings or direct-stem injection techniques were not successful although these methods have often been used in other biosynthetic studies. For example, cuttings of Populus nigra L. were used to study biosynthesis of isoprene and phytol via DOXP pathway (Schwender et al., 1997), and 1-[5,5-2H2]-1-deoxy-d-xylulose was incorporated into germacrene D using cuttings of Solidago canadensis (Steliopoulos et al., 2002). In contrast, S. divinorum cuttings did not take the labeled substrate up very efficiently. The incorporation of 13C-glucose or 13C-DOX into 1 could not be detected by HR-MS or 13C-NMR even after up to 6 weeks of incubation (data not shown). Furthermore, fungal and algal contamination of the medium could not be eliminated completely, which may have reduced the availability of the substrates. Direct-stem injection has been a successful alternative method of introducing biosynthetic precursors into plants. Incorporation of [1-14C]-tryptophan into camptothecin was increased 25-100 fold by using direct-stem injection compared with feeding through roots (Sheriha and Rapoport, 1976). Another study also showed that more asparagine was introduced in plants by direct-stem injection than through root uptake (Oti-Boateng and Silsbury, 1993). However, this method of application has been reported to cause severe tissue damage and necrosis (Oti-Boateng and Silsbury, 1993; Sheriha and Rapoport, 1976). In our case, direct-stem injection method did not result in detectable incorporation of [2-13C]-glucose into 1. Growth inhibition and tissue darkening of terminal leaves reflected intolerance of S. divinorum to this type of treatment. Mass spectrometry analysis did not reveal noticeable isotope clusters that would suggest 13C enrichment in 1.
In order to overcome these problems, a method for in vitro tissue culture of S. divinorum was developed. This approach led to the incorporation of labeled substrates and the elucidation of the biogenic pathway of 1. Microshoots transferred to new tissue culture tubes required 7-10 days of adaptation and usually grew at an average rate of 1 cm per week, reaching 2-3 cm height after 21 days of incubation in labeled medium. The contamination rate among initial nodal explants taken from mother plants grown in the greenhouse was 46%, but clonal subcultures of healthy aseptic samples remained free from microbial infection indefinitely.
Incorporation of [13C]-glucose in 1 was achieved in microshoots grown in 1% labeled/1% unlabeled glucose. The 1:1 ratio of labeled to unlabeled glucose in the medium was used to enhance uptake of the isotopically labeled substrate (Wungsintaweekul and De-Eknamkul, 2005). Glucose was used in the tissue culture medium instead of the more commonly used sucrose (Arikat et al., 2004; Avato et al., 2005) to maximize the biosynthetic incorporation.
2.2. Spectroscopic analysis of salvinorins labeled with [2-13C]-glucose
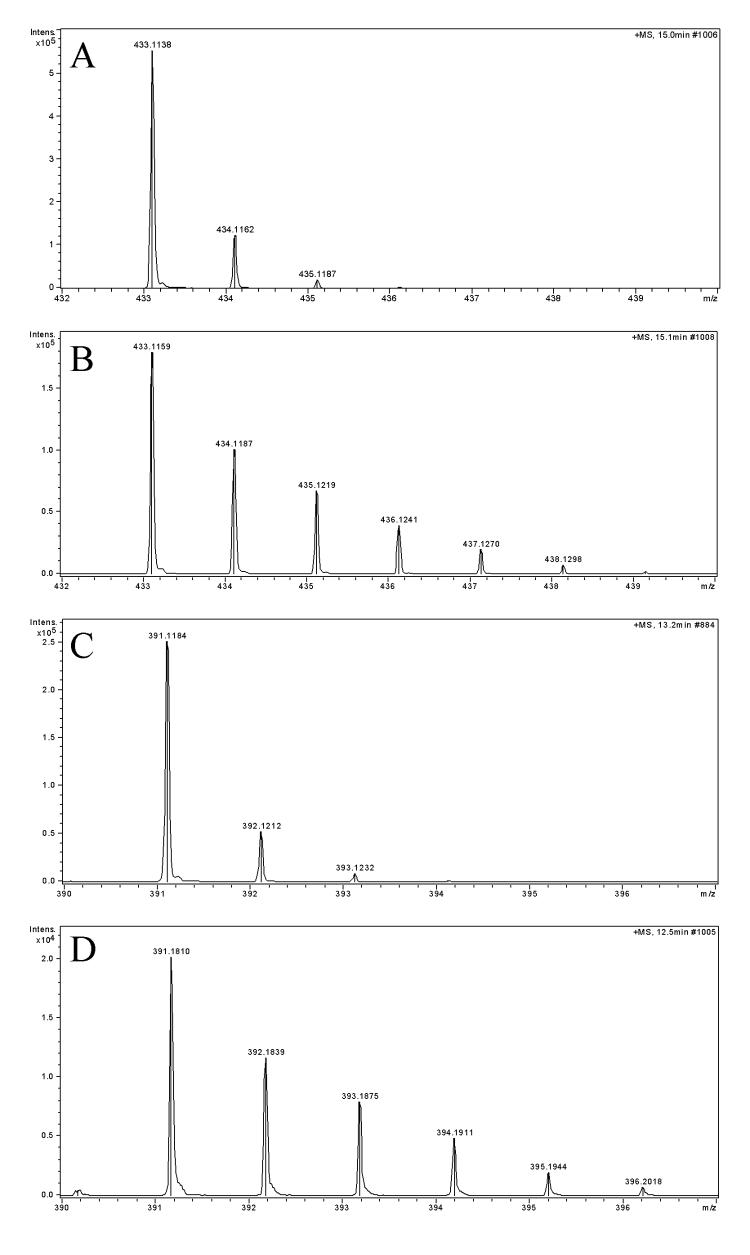
LC-MS analysis of leaf extracts obtained after 3 days, and then later after 11 days of incubation, showed 13C originated from labeled glucose was successfully incorporated into the core structure of salvinorin A (1) and salvinorin B (2) (Fig. 3 A and B). Salvinorin A showed [M+1]+ peak at m/z 433.2 and three other peaks for [M+2]+, [M+3]+, [M+4]+ and [M+5]+. The signal of the parent ion of 2 occurred at m/z 391.2 and had a clear isotope cluster when compared to standards. Peaks from 13C enrichment also appeared for [M+2]+, [M+3]+, [M+4]+ and [M+5]+. Calculated percent of enrichment from [M+2]+ to [M+4]+, was in the range between 2.1% to 7.8% for 1, and 2.6% to 13.1% for 2, in comparison to standard (see Fig. 3 A and C).
Fig. 3.
Mass spectra showing incorporation of 13C from the experiment with [2-13C]-glucose. A) standard of salvinorin A (1), B) m/z 433.1159 [M+H]+ refers to salvinorin A parent peak. Subsequent peaks form an isotope cluster reflecting the incorporation of 13C into the molecule of 1. C) standard of salvinorin B (2), D) m/z 391.1810 [M+H]+ corresponds to 2. Isotope cluster is spread to [M+5]+.
2.3. Spectroscopic analysis of salvinorins labeled with [1-13C]-glucose
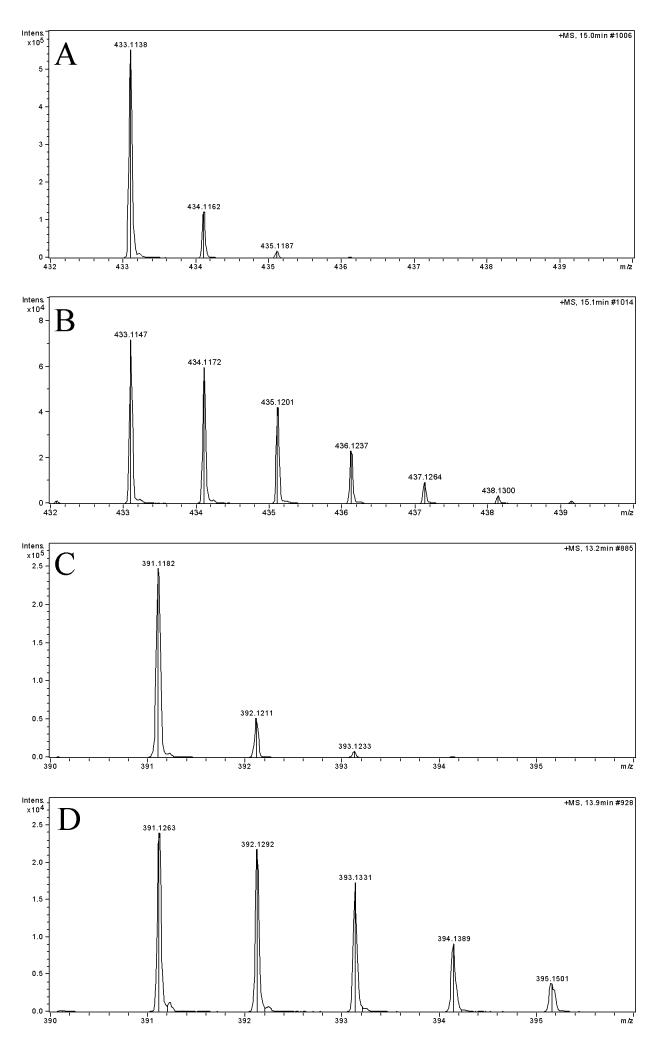
The large scale experiment consisted of 200 microshoots grown aseptically for 4 weeks in the presence of 2% glucose (1% isotopically labeled). Salvinorin A (1) was isolated and purified by HPLC, resulting in a total of 2.5 mg of labeled 1 and 0.5 mg of salvinorin B (2). salvinorin F (3) (Munro and Rizzacasa, 2003) along with divinatorins A (4) and B (5) (Bigham et al., 2003) were also isolated in small quantities (50-100 μg). LC-MS analysis confirmed incorporation of [1-13C]-glucose into 1 and 2 (Fig. 4 B and D). Analysis of the relative signal intensity of 1 revealed that [M+2]+ was 3.6% enriched, whereas 2 reached 4.4% of incorporation, compared to standards (Fig. 4A and C).
Fig. 4.
Mass spectra of: A) standard of salvinorin A (1), B) 1 isolated from experiment with [1-13C]-glucose, C) standard of salvinorin B (2), and D) 2 isolated from experiment with [1-13C]-glucose. Isotope clusters are spread up to [M+5]+ peak in case of isotopically labeled 1 and to [M+5]+ in 2.
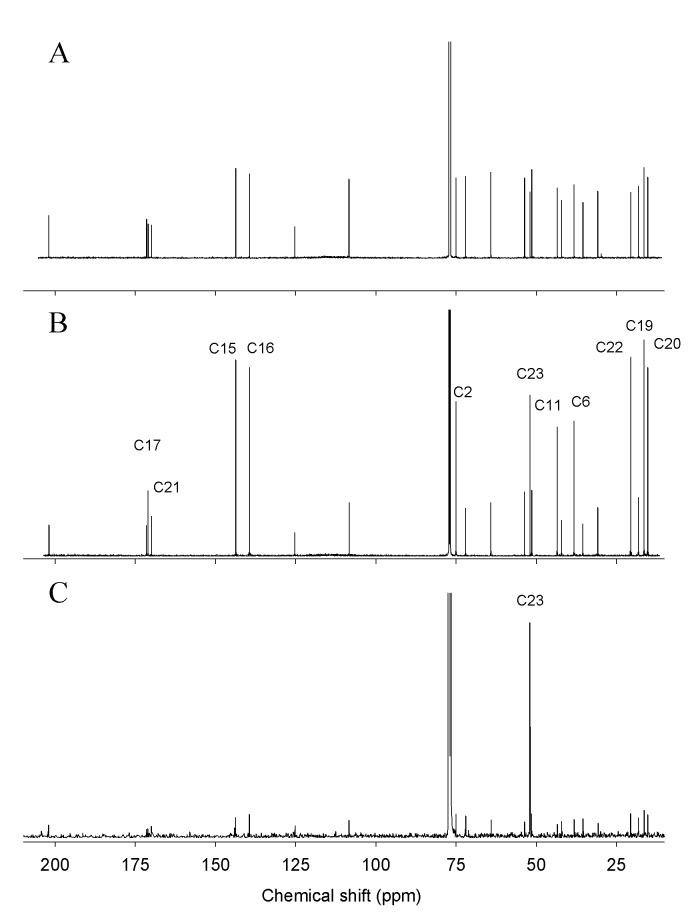
Carbon NMR spectra of 1-5 (Fig. 5B and supplemental data), revealed an incorporation pattern of 13C consistent with that predicted for the DOXP pathway (Fig. 2). The 13C enrichment measured by 151 MHz 13C-NMR (Table 1) shows that methyl carbons C-19 (16.4 ppm), C-20 (15.2 ppm), C-22 (20.6 ppm), C-23 (51.9 ppm) are enriched by 3.0%, 3.1%, 3.6% and 2.9 %, respectively; methylene carbons C-6 (38.2 ppm), and C-11 (43.5 ppm) were also each enriched by 2.8%; methine carbons C-2 (75.0 ppm), C-15 (143.7 ppm), C-16 (139.4 ppm), were enriched at 2.7 %, 3.0% and 3.3%, respectively; and carbonyl carbons C-17 (171.1 ppm) and C-21 (169.9 ppm) showed 13C enrichments of 3.6% and 1.2%, respectively. The 13C enrichments of unlabeled carbons ranged from 0.0 to 0.7 for unlabeled carbons, and 1.2 to 3.6 for labeled ones. Average 13C enrichment for unenriched carbons was 0.4, and for enriched carbons was 2.9. These numbers are consistent with the percent of incorporation calculated from HR-MS data. Enrichment of carbons C-2, C-6, C-11 and C-15 of 1 was the result of the incorporation of carbon C-1 of IPP/DMAPP, whereas enrichment of carbons C-16, C-17, C-19 and C-20 is due to the incorporation of carbon C-5 of IPP/DMAPP. As expected, methyl groups C-19 and C-20 migrated during the biosynthesis from C-4 to C-5, and C-10 to C-9, respectively (Fig. 2). The observed pattern is in complete agreement with the expected incorporation of glucose via the DOXP pathway. In addition, carbonyl C-21 and methyl C-22 from the acetate functional group also were isotopically labeled with [1-13C]-glucose in 1. This is the due to the nonspecificity of glucose as a biosynthetic substrate, since it is a major metabolic intermediate in primary metabolism. Apparently, glycolysis generated isotopically labeled acetyl-CoA which served as a substrate for the acetylation of the hydroxyl group at C-2 by an acetyltransferase (Laflamme et al., 2001). Labeling of the methyl ester in 1 at position C-23 was also observed, and most likely results from the enzymatic action of a type III S-adenosyl-l-methionine - dependent O-methyltransferase (Noel et al., 2003; Roje, 2006). As with acetyl-CoA, the methyl group of SAM was apparently derived from [1-13C]-glucose.
Fig. 5.
A) Reference carbon NMR spectrum of salvinorin A (1); B) Spectrum of 1 labeled with [1-13C]-glucose; C) Spectrum of 1 labeled with [Me-13C]-methionine. Spectra were recorded in CDCl3 with a Bruker NMR with BBO 5 mm carbon probe at 151 MHz (standard, and labeled with [1-13C]-glucose 1), and Bruker NMR with 3 mm carbon direct probe at 100 MHz (1 labeled with [Me-13C]-methionine). Carbons with enhanced peak heights relative to the reference spectrum are labeled accordingly.
Table 1.
Incorporation of [1-13C]-glucose and [1-13C, 3,4-2H2]-DOX into salvinorin A (1). Numbering schemes corresponds to that shown in Figure 1.
| Carbon No. | Chemical shift (ppm) | Integration ratioa | |
|---|---|---|---|
| [1-13C]Glucose | [1-13C, 3,4-2H2]-DOX | ||
| 1 | 202.0 | 0.7 | 0.0 |
| 2 | 75.0 | 2.9 | 0.1 |
| 3 | 30.8 | 0.3 | 0.1 |
| 4 | 53.6 | 0.6 | 0.1 |
| 5 | 42.1 | 0.3 | 0.0 |
| 6 | 38.2 | 3.0 | 0.1 |
| 7 | 18.2 | 0.6 | 0.1 |
| 8 | 51.4 | 0.4 | 0.1 |
| 9 | 35.5 | 0.0b | 0.0b |
| 10 | 64.2 | 0.5 | 0.1 |
| 11 | 43.5 | 3.0 | 0.1 |
| 12 | 72.0 | 0.3 | 0.1 |
| 13 | 125.3 | 0.4 | 0.1 |
| 14 | 108.4 | 0.2 | 0.1 |
| 15 | 143.7 | 3.2 | 0.1 |
| 16 | 139.4 | 3.6 | 0.5 |
| 17 | 171.1 | 3.9 | 0.6 |
| 18 | 171.5 | 0.7 | 0.1 |
| 19 | 16.4 | 3.2 | 0.4 |
| 20 | 15.2 | 3.4 | 0.4 |
| 21 | 169.9 | 1.3 | 0.1 |
| 22 | 20.6 | 3.9 | 0.1 |
| 23 | 51.9 | 3.1 | 0.3 |
Numbers in bold indicates carbons with 13C incorporation
Denotes the carbon used as reference to calculate integration ratio.
Integration ratio was calculated according to formula = ((Enriched integral - unlabeled integral)/unlabeled integral)*1.07
Proton NMR analysis showed clear satellites around proton peaks at H-19 (1.11 ppm, s), H-23 (3.72 ppm, s), H-2 (5.14 ppm, dd) and H-16 (7.4 ppm, dd) that are associated with the protons directly attached to the 13C-enriched carbons (supplemental data). Coupling between 13C and 1H appears as symmetrical satellite peaks that can be detected around the 1H-NMR signal (Eisenreich and Bacher, 2000). The satellites of the proton signals of methyl groups (H-19 and H-23, 1.11 ppm s and 3.72 ppm s) are easily identified because their signals are sharp and tall (supplemental data). Methylene and methine satellites are more difficult to observe because their signals are typically weaker and are often overlapped with other signals. Their signals are typically split because of coupling to other protons, which further reduces their relative height. Due to those factors, the satellites of proton signals of H-2 (5.14 ppm dd) and H-16 (7.4 ppm dd) are more difficult to observe than those of the methyl groups.
The signals showing 13C enrichment in the 13C spectra were clearly distinct from the non-enriched positions. Every position in the terpenoid core of salvinorin A (1) (as well as its analogues 2-4), which was expected to be enriched if derived from the DOXP pathway, had a higher level of 13C incorporation than the other carbons (Fig. 5 and Table 1). Mass spectrometry data also supported this observation, with the presence of an isotope cluster up to [M+6]+. The relative intensity of the [M+2]+ signal of 1, which at natural 13C abundance is about 25% of the height of [M+1]+, increased to 47.1%, indicating that a majority of the isotopomers have at least 1 enriched carbon in the salvinorin core structure (Fig. 4).
It has been shown experimentally that 1 is stored in glands, but there is no genomic or biochemical confirmation that biogenesis of this compound is localized in this structures. Our data indicates that 1 is derived from the DOXP biosynthetic pathway, providing additional evidence that the site of biosynthesis is compartmentalized in glandular trichomes. Indeed, many secondary metabolites that are produced in plant secretory glands, such as the cannabinoids and other terpenoids, are produced via the DOXP pathway (Gang et al., 2001; Mahlberg and Kim, 2004).
2.4. [1-13C,3,4-2H2]-1-deoxy-d-xylulose incorporation
To confirm the results obtained with the relatively nonspecific precursor glucose, an experiment was carried out to label 98 S. divinorum microshoots with [1-13C,3,4-2H2]-1-deoxy-d-xylulose. A multiply labeled substrate containing deuterium was used to provide additional biosynthetic information. Because of phytotoxicity problems, the concentration of DOX could not exceed 0.1%. Analysis of the salvinorin A (1) isolated from these plants using 13C-NMR (Table 1) showed low, but significant, 13C enrichments of 0.3% to 0.6% in four peaks: C-16, C-17, C-19, and C-20, which is consistent with the DOXP pathway. The methyl carbon of the carbomethoxy group (C-23) was also enriched to the extent of 0.3%. The unenriched carbons showed enrichments of 0.0-0.1%.
Despite the low incorporation, the experiment with labeled DOX clearly showed the expected pattern of incorporation and was consistent with the results of [1-13C]-glucose incorporation. However, the low enrichment obtained in this experiment meant that the anticipated information from the deuterium labeling was lost, since the presence of deuterium could not be observed by 2H-NMR, nor by the influence of 2H on the 13C-chemical shifts. Because the microshoots could not tolerate concentrations of the DOX greater than 0.1%, the incorporation rate was limited. Low incorporation of DOX has been reported to be the result of poor phosphorylation rate of the exogenous DOX (Adam et al., 1999), or to possibly be due to the partial degradation of DOX into acetate, which reduces the pool of DOX available for terpenoid biosynthesis (Thiel and Adam, 2002). It has also been suggested that, unlike glucose, DOX may face limitations associated with active transport to plastids and with their membrane permeability (Fluegge and Gao, 2005). Finally, there is a significant possibility that kinetic isotopic effects associated with deuterium labeling at positions that undergo enzymatic oxidation may have led to the low incorporation rate.
2.5. [Me-13C]-methionine incorporation
An experiment with 0.2% labeled methionine caused phytotoxic damage to the microshoots which ultimately resulted in the death of the plants. However, 0.1 mg of salvinorin A (1) was extracted and purified from these samples and evaluated using 100 MHz 13C-NMR spectroscopy. Because of the very small amount of the compound extracted, none of the carbon signals were detectable except for a very strong resonance at 51.9 ppm, indicating the incorporation of the methyl group of methionine into C-23 of 1. Reducing the concentration to 0.1% of 13C-labeled methionine allowed the plants to grow for 21 days. This experiment yielded 0.5 mg of pure 1, which was combined with the 1 from the first methionine incorporation experiment, and analyzed by NMR. The calculated percent of incorporation based on the 100 MHz 13C-NMR spectrum was 2.9% for C-23. Very good incorporation of methionine was observed, since the incubation of microshoots with 0.2 and 0.1% of methionine resulted in a similar level of incorporation of 13C into 1 (around 3%), as was observed with a 10 fold higher concentration of labeled glucose.
The retrobiosynthetic NMR analysis of 1 showed that the methoxy ester at C-23 probably originates from SAM. Incubation of microshoots in the presence of [Me-13C]-methionine led to the specific labeling of C-23. As mentioned above, such reactions are catalyzed by type III SAM-dependent O-methyltransferases (Noel et al., 2003). Methyltransferases play important roles in the biosynthesis of primary and secondary metabolites in plants (Roje, 2006). While some O-methyltransferases have high substrate specificity, some others are quite promiscuous (Dayan et al., 2003; Pichersky and Gang, 2000).
3. Concluding remarks
Retrobiosynthetic NMR analysis of the biogenic origin of salvinorin A (1) yielded an incorporation pattern consistent with the DOXP-dependent pathway. Labeling with [1-13C]-glucose and [1-13C; 3,4-2H2]-1-deoxy-d-xylulose were in agreement with each other. Additionally, enrichment of the C-23 methoxy group in samples grown in the presence of [Me-13C]-methionine strongly suggested the participation of a SAM-dependent type III O-methyltransferase. The microshoots tissue culture technique developed to obtain this data provides a valuable tool to continue our on-going biochemical characterization of the individual steps involved in formation salvinorin in isolated glands.
4. Experimental Procedures
4.1. General Experimental Procedures
All chemicals were purchased from Fisher Scientific unless specified otherwise. [2-13C]-Glucose (99% 13C enrichment) was purchased from Sigma-Aldrich (St. Louis, MO), [1-13C]-glucose (99% 13C enrichment) and [Me-13C]-methionine (99% 13C enrichment) were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). 1-Deoxy-d-xylulose (DOX), [1-13C]-1-deoxy-d-xylulose ([1-13C]-DOX) and [1-13C, 3,4-2H2]-1-deoxy-d-xylulose ([13C,2H2]-DOX) were synthesized as previously described (Giner, 1998). The crude plant extracts were purified using Waters XTerra C18 7.8×100 mm (5 μm particle size) column connected to an HPLC (Delta Prep 4000, Waters Corporation, Milford, MA) equipped with a dual wavelength detector (Model 2487, Waters Corporation, Milford, MA). Purification of the metabolites was achieved using an MeCN:H2O (40:60) isocratic mobile phase and a UV detector (λ = 210 nm). Purified samples were evaluated by LC-MS (Bruker Daltonik microTOF, Leipzig Germany). Analysis was performed using a 150 × 4.6 mm C8 column (Luna Phenomenex, Torrance, CA) with 3 μm particles size and a linear gradient starting at 20% of MeCN and reaching 100% over 20 min. The LC was equipped with PDA detector (Agilent 1100, Palo Alto, CA). HR-ESI-MS analysis was done in positive ionization mode.
NMR analysis was performed on either a 600 MHz Bruker Avance instrument with a 5 mm BBO broadband probe or a 400 MHz Bruker UltraShield with a 3 mm direct carbon probe. NMR samples were dissolved in CDCl3, except for salvinorin B (2), which was dissolved in d6-acetone. All NMR experiments (13C and 1H) were recorded using standard Bruker software settings. Chemical shifts were standardized to solvent signals. NMR peak assignments of 1 and 2 were according to Giner et al. (Giner et al., 2007).
4.2. Plants growth
Rooted cuttings of S. divinorum (Hofmann & Wasson strain) were purchased from Theatrum Botanicum (Laytonville, CA) in February 2006. Plants were transferred to a commercial peat-lite medium in one-gallon pots and grown in the greenhouse (Oxford, MS) under natural light. The plants were watered and fertilized as needed.
4.3. Direct-stem injection
A two-week old plant (6 cm high, 3 mm stem width) was immobilized on a ring stand. A needle (0.31 mm nominal OD) with a 10°-12° beveled needle point attached to a 100 μl syringe (Hamilton, NE) was inserted approximately 1.5 mm in the stem just below the terminal node. Twenty five μl of 1% [2-13C]-glucose solution was injected with a flow rate of about 3 - 4 μl per hour. New leaves developing above the point of injection were harvested 7 days later and extracted. The extract was analyzed by LC-MS for incorporation of [2-13C]-glucose into salvinorin A (1).
4.4. Plant cuttings
Plant cuttings were grown in non-sterile conditions for about 10 days in 15 ml Hoagland’s medium. The plants were then transferred to solutions containing either 1% total glucose (0.5% actual [2-13C]-glucose) or 0.1 % [1-13C]-1-deoxy-d-xylulose. Preliminary experiments determined that DOX was toxic to S. divinorum cuttings at concentrations above 0.1%. Plants were supplemented daily with 2 ml of labeled substrate in Hoagland’s medium. Cuttings were incubated with labeled glucose for 2 weeks, and those exposed to [1-13C]-DOX maintained in the solution for 6 weeks. The culture media were filtered through sterile 0.2 mm filters every other day to limit the microbial contamination. Leaf extracts were prepared and submitted for spectroscopic analyses as described below.
4.5. Tissue culture
A method to culture microshoots of S. divinorum was adapted from previously published protocols (Arikat et al., 2004). Briefly, nodal stem sections (4-6 mm) were excised from greenhouse grown plants, washed with 70% ethanol for 40 sec followed by a 60 sec sterile water rinse. Explants were then surface-sterilized in a 10% solution of commercial bleach (0.6% sodium hypochlorite) with 0.1% Tween-20 for 10 min followed by 5 sterile DDI water washing. The sterile nodal sections were stripped of their larger leaves and placed into 16×100 mm sterile glass culture tubes containing Murashige and Skoog medium supplemented with 2% glucose, Gamborg’s Vitamin solution, 0.2% Phytagel, and 5 μM 6-benzylaminopurine (MSG, pH 5.8) (0.2%). The microshoots were then placed into a growth chamber with a 14-h photoperiod at 400 μmol m-2s-1 and maintained at 22-23°C.
4.6. Small scale incorporation with [2-13C]-glucose
For the small scale incorporation experiment, MSG medium was prepared as described above except that 1% glucose plus 1% [2-13C]-glucose (2% total glucose concentration) were used. Nine microshoots were transferred aseptically to each of four Magenta GA7 culture vessels containing 45 ml of medium and grown as described above. Plant tissue was harvested after 11 days. Salvinorin A (1) was extracted with chloroform, purified by HPLC, and analyzed for incorporation by HR-ESI-MS as described below.
4.7. Large scale experiment with [1-13C]-glucose
A large scale experiment was designed to produce sufficient amount of labeled 1 for 13C-NMR analysis. MSG medium was prepared as above except that labeled [1-13C]-glucose was used instead of [2-13C]-glucose. Two milliliter aliquots of medium were pipetted into 200 glass tubes (16×100 mm). Terminal and axillary microshoots were excised from sterile mother plants grown on MSG medium and transferred into the glass tubes. The cultures were maintained in a growth chamber at 22-23°C with a 14-h photoperiod at 400 μmol m-2s-1 for approximately 4 weeks to obtain sufficient biomass. 1, along with other compounds, was extracted, isolated and purified as described below.
4.8. Experiment with [1-13C,3,4-2H2]-1-deoxy-d-xylulose
Labeling with the specific precursor of the DOXP pathway was done as described above with 98 microshoots grown in 25×95 mm flat-bottomed culture tubes (Phytotechnology Labs, Shawnee Mission, KS), with MSG medium (2.75 ml/tube) containing 0.1 % [1-13C,3,4-2H2]-DOX. Plants were grown for 21 days in growth chamber in the same conditions as previously described. Preliminary experiments using unlabeled DOX determined that higher concentrations of substrates were toxic to the microshoots. After the experiment was terminated, 1 was extracted, and purified as described below.
4.9. Experiment with [Me-13C]-methionine
[Me-13C]-methionine incorporation was performed at two concentrations. Initially, 68 microshoots were transferred into MSG medium (3 ml/tube) containing 0.2% of [Me-13C]-methionine. This concentration of the substrate, however, inhibited the growth of the microshoots and eventually led to necrosis of the foliage. Therefore, the experiment was terminated after 19 days, and material was exhaustively extracted, followed by purification of compounds and their spectroscopic analysis as described below. The experiment was subsequently repeated using 56 microshoots grown with 0.1% of [Me-13C]-methionine for 21 days, after which plants were extracted.
4.10. Extraction and purification (HPLC) of salvinorin A
Salvinorin A (1) and its derivatives were extracted from the microshoots grown in vitro by sonicating the tissue 3 times in 50 ml of chloroform for 5 min each (Siebert, 2004). The solvent was evaporated in vacuo. The residue was redissolved in acetonitrile (MeCN) and filtered through 0.2 μm HPLC filter (Millex®-GN, Bedford, MA) and separated by HPLC.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Jeremy Stewart for scientific inspiration on research of Salvia divinorum, Dr. Ruslan Bikbulatov for valuable discussion during the preparation of this manuscript and Dr. Abbas Shilabin for the mass spectrometric analysis. We also thank David J. Kiemle (Analytical and Technical Services, SUNY-ESF, Syracuse, NY) for assistance with the spectrometry involving the 600 MHz Bruker NMR instrument. This work was supported by NIH grant P20 RR 021929-01 (Center of Research Excellence in Natural Products Neuroscience), and a USDA cooperative research agreement 58-6408-2-0009. The research was conducted in a facility constructed with support from research facilities improvement program grant C06 RR-14503-01 from National Center for Research Resources, National Institutes of Health, Bethesda, MD, USA.
Abbreviations
- DOXP
1-deoxy-d-xylulose phosphate
- MEP
2-C-Methyl-d-erythritol phosphate
- DOX
1-Deoxy-d-xylulose
- [1-13C]-DOX
[1-13C]-1-deoxy-d-xylulose
- [13C,2H2]-DOX
[1-13C, 3,4-2H2]-1-deoxy-d-xylulose
- HR-ESI-MS
High Resolution Electron Spray Impact Mass Spectrometry
- SAM
S-adenosyl-l-methionine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam KP, Thiel R, Zapp J. Incorporation of 1-[1-13C]deoxy-D-xylulose in chamomile sesquiterpenes. Arch. Biochem. Biophys. 1999;369:127–132. doi: 10.1006/abbi.1999.1346. [DOI] [PubMed] [Google Scholar]
- Arikat NA, Jawad FM, Karam NS, Shibli RA. Micropropagation and accumulation of essential oils in wild sage (Salvia fruticosa Mill.) Sci. Hort. 2004;100:193–202. [Google Scholar]
- Avato P, Fortunato IM, Ruta C, D’Elia R. Glandular hairs and essential oils in micropropagated plants of Salvia officinalis L. Plant Sci. 2005;169:29–36. [Google Scholar]
- Bigham AK, Munro TA, Rizzacasa MA, Robins-Browne RM. Divinatorins A-C, New neoclerodane diterpenoids from the controlled sage Salvia divinorum. J. Nat. Prod. 2003;66:1242–1244. doi: 10.1021/np030313i. [DOI] [PubMed] [Google Scholar]
- Broers STJ. Regarding the early steps of the biosynthesis of isoprenoids in Escherichia coli. 1994. Dissertation, ETH-Zurich Dissertation #10978. [Google Scholar]
- Dayan FE, Kagan IA, Rimando AM. Elucidation of the biosynthetic pathway of the allelochemical sorgoleone using retrobiosynthetic NMR analysis. J. Biol. Chem. 2003;278:28607–28611. doi: 10.1074/jbc.M304185200. [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Bacher A. Elucidation of biosynthetic pathways by retrodictive/predictive comparison of isotopomer patterns determined by NMR spectroscopy. In: Setlow JK, editor. Genetic Engineering. Vol. 22. Kluwer Academic/Plenum Publishers; 2000. pp. 121–153. [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Bacher A, Arigoni D, Rohdich F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell. Mol. Life Sci. 2004;61:1401–1426. doi: 10.1007/s00018-004-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Rohdich F, Bacher A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 2001;6:78–84. doi: 10.1016/s1360-1385(00)01812-4. [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem. Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- Fluegge U-I, Gao W. Transport of isoprenoid intermediates across chloroplast envelope membranes. Plant Biol. 2005;7:91–97. doi: 10.1055/s-2004-830446. [DOI] [PubMed] [Google Scholar]
- Gang DR, Wang J, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol. 2001;125:539–555. doi: 10.1104/pp.125.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, Croteau R. Terpenoids. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: Their Interactions with Secondary Plant Metabolites. Academic Press; New York: 1991. pp. 165–209. [Google Scholar]
- Giner J-L. New and efficient synthetic routes to 1-deoxy-D-xylulose. Tetrahedron Lett. 1998;39:2479–2482. [Google Scholar]
- Giner J-L, Kiemle D, Kutrzeba L, Zjawiony JK. Unambiguous NMR spectral assignments of salvinorin A. Magnet. Reson. Chem. 2007;45:351–354. doi: 10.1002/mrc.1972. [DOI] [PubMed] [Google Scholar]
- Laflamme P, St-Pierre B, De Luca V. Molecular and biochemical analysis of a Madagascar periwinkle root-specific minovincinine-19-hydroxy-O-acetyltransferase. Plant Physiol. 2001;125:189–198. doi: 10.1104/pp.125.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlberg PG, Kim ES. Accumulation of cannabinoids in glandular trichomes of Cannabis (Cannabaceae) J. Ind. Hemp. 2004;9:15–36. [Google Scholar]
- Munro TA, Rizzacasa MA. Salvinorins D-F, new neoclerodane diterpenoids from Salvia divinorum, and an improved method for the isolation of salvinorin A. J. Nat. Prod. 2003;66:703–705. doi: 10.1021/np0205699. [DOI] [PubMed] [Google Scholar]
- Noel JP, Dixon RA, Pichersky E, Zubieta C, Ferrer J-L. Structural, functional, and evolutionary basis for methylation of plant small molecules. Rec. Adv. Phytochem. 2003;37:37–58. [Google Scholar]
- Ortega A, Blount JF, Manchand PS. Salvinorin, a new trans-neoclerodane diterpene from Salvia divinorurn (Labiatae) J. Chem. Soc. Perk. Trans. 1. 1982;10:2505–2508. [Google Scholar]
- Oti-Boateng C, Silsbury JH. The effects of exogenous amino acid on acetylene reduction activity of Vicia faba L. cv. Fiord Ann. Bot. 1993;71:71–74. [Google Scholar]
- Pichersky E, Gang DR. Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends Plant Sci. 2000;5:439–445. doi: 10.1016/s1360-1385(00)01741-6. [DOI] [PubMed] [Google Scholar]
- Porter JW, Spurgeon SL. Biosynthesis of Isoprenoid Compounds. Wiley; New York: 1981. [Google Scholar]
- Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roje S. S-Adenosyl-L-methionine: Beyond the universal methyl group donor. Phytochemistry. 2006;67:1686–1698. doi: 10.1016/j.phytochem.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: A potent naturally occurring nonnitrogenous Kappa opioid selective agonist. Proc. Nat. Acad. Sci. USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanani N, Facchini PJ. Compartmentalization of plant secondary metabolism. In: Romeo JT, editor. Rec. Adv. Phytochem. Vol. 40. Elsevier New York: 2006. pp. 53–83. [Google Scholar]
- Schwarz MK. Terpene biosynthesis in Gingko biloba: a surprising story. 1994. ETH-Zurich Dissertation #10951. [Google Scholar]
- Schwender J, Zeidler J, Groner R, Muller C, Focke M, Braun S, lichtenthaler FW, Lichtenthaler HK. Incorporation of 1-deoxy-D-xylulose into isoprene and phytol by higher plants and algae. FEBS Lett. 1997;414:129–134. doi: 10.1016/s0014-5793(97)01002-8. [DOI] [PubMed] [Google Scholar]
- Sheriha GM, Rapoport H. Biosynthesis of Camptotheca acuminate alkaloids. Phytochemistry. 1976;15:505–508. [Google Scholar]
- Siebert D. Localization of salvinorin A and related compounds in glandular trichomes of the psychoactive sage, Salvia divinorum. Ann. Bot. 2004;93:763–771. doi: 10.1093/aob/mch089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert DJ. Salvia divinorum and salvinorin A: new pharmacologic findings. J. Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Simpson TJ. Application of isotopic methods to secondary metabolic pathways. Top. Cur. Chem. 1998;195:1–48. [Google Scholar]
- Steliopoulos P, Wüst M, Adam KP, Mosandl A. Biosynthesis of the sesquiterpene germacrene D in Solidago canadensis: 13C and 2H labeling studies. Phytochemistry. 2002;60:13–20. doi: 10.1016/s0031-9422(02)00068-7. [DOI] [PubMed] [Google Scholar]
- Thiel R, Adam KP. Incorporation of [1-13C]1-deoxy-D-xylulose into isoprenoids of the liverwort Conocephalum conicum. Phytochemistry. 2002;59:269–274. doi: 10.1016/s0031-9422(01)00453-8. [DOI] [PubMed] [Google Scholar]
- Valdés LJ, III, Díaz JL, Paul AG. Ethnopharmacology of Ska María Pastora (Salvia divinorum, Epling and Játiva-M.) J. Ethnopharmacol. 1983;7:287–312. doi: 10.1016/0378-8741(83)90004-1. [DOI] [PubMed] [Google Scholar]
- Valdés LJ, III, Hatfeild GM, Koreeda M, Paul AG. Studies of Salvia divinorum (Lamiaceae), an hallucinogenic mint from the Sierra Mazateca in Oaxaca, Central Mexico. Econ. Bot. 1987;41:283–291. [Google Scholar]
- Vortherms TA, Roth BL. Salvinorin A: From natural product to human therapeutics. Mol. Intervent. 2006;6:259–267. doi: 10.1124/mi.6.5.7. [DOI] [PubMed] [Google Scholar]
- Wungsintaweekul J, De-Eknamkul W. Biosynthesis of plaunotol in Croton stellatopilosus proceeds via the deoxyxylulose phosphate pathway. Tetrahedron Lett. 2005;46:2125–2128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.