Abstract
Purpose
To determine the incidence of thiazide-associated hypercalcemia and clarify its clinical features and natural history.
Methods
In a population-based descriptive study, Olmsted County, Minnesota, residents with thiazide-associated hypercalcemia were identified through the Rochester Epidemiology Project and the Mayo Clinic Laboratory Information System. Changes in incidence rates were evaluated by Poisson regression.
Results
Seventy-two Olmsted County residents (68 women and 4 men; mean age, 64 years) with thiazide-associated hypercalcemia first recognized in 1992 to 2001 were identified. The overall annual age- and sex-adjusted (to 2000 U.S. whites) incidence was 7.7 (95% CI, 5.9 to 9.5) per 100,000. There was an increase in incidence after 1996, peaking at 16.3 (95% CI, 8.3 to 24.3) per 100,000 in 1998. The highest rate was 55.3 per 100,000 in 70 to 79 year-old women. Hypercalcemia was identified a mean of 6 ± 7 years after thiazide initiation, and the average highest serum calcium was 10.7 ± 0.3 mg/dL with serum parathyroid hormone (obtained in 53 patients) of 4.8 ± 2.7 pmol/L. Of 33 patients who stopped the thiazide, 21 (64%) had persistent hypercalcemia. Patients subsequently diagnosed with primary hyperparathyroidism had the highest average serum calcium and parathyroid hormone levels of 11.0 ± 0.3 mg/dL and 6.3 ± 2.4 pmol/L, respectively.
Conclusion
Persistence of hypercalcemia in patients discontinuing thiazides, and similarities in the clinical spectrum, suggest that underlying primary hyperparathyroidism is common in patients who develop hypercalcemia while on thiazide diuretics.
Keywords: Epidemiology, Hypercalcemia, Incidence, Thiazide diuretic, Trends, Hyperparathyroidism
INTRODUCTION
Hypercalcemia associated with thiazide diuretic use is a well recognized clinical entity.1 Mean 24-hour plasma calcium concentrations are increased with thiazide use, but mean 24-hour plasma parathyroid hormone levels remain unchanged in subjects with normal baseline parathyroid hormone levels and no evidence of hypercalciuria.2 Thiazides have several metabolic effects that may contribute to increased calcium levels. A reduction in urine calcium excretion is the most likely explanation,3-6 but a metabolic alkalosis associated with diuretic use could also cause an elevation in total serum calcium through a pH-dependent increase in protein-bound calcium. Although plasma 1,25(OH)2D levels are unchanged,7 increased intestinal calcium absorption in response to thiazide treatment has been noted and could also contribute to an increase in serum calcium.8,9 A final factor that may lead to the development of hypercalcemia is hemoconcentration associated with diuresis.10
Despite the well-known effect of thiazides increasing serum calcium levels, the incidence of thiazide-associated hypercalcemia has never been reported. Furthermore, prior prevalence studies were done before the introduction of automated serum calcium measurements. The only available data are from the early 1970′s in Sweden where, in a health screen, 1034 of 15,903 persons between 20 and 63 years of age (66% women) were on thiazides; among the thiazide-treated subjects, 20 (1.9%) were found to have hypercalcemia.11 The prevalence of hypercalcemia in this group was greater than that in the entire population (0.6%). Stenstrom and Heedman estimated the overall prevalence of hypercalcemia to be 0.1%-0.2%, with a prevalence of 0.4% in thiazide-treated subjects.12
The primary aim of this study was to determine the incidence of thiazide-associated hypercalcemia in the era of routine serum calcium measurement. Additionally, we sought to clarify the clinical characteristics and natural history of thiazide-associated hypercalcemia in the general population. Given the demonstrated benefits of thiazide diuretics13 and their status as preferred first-step agents in the treatment of hypertension,14 we also wanted to provide timely clinical guidance in the evaluation and management of this disorder.
METHODS
Population-based research is feasible in Olmsted County, Minnesota, because medical care is essentially self-contained within this community of approximately 124,000 residents (90% Caucasian) and the area has relatively few providers. Most endocrinologic care is provided by the Mayo Clinic, which has maintained a common medical record with its two hospitals (St. Marys and Rochester Methodist) for over 100 years. The diagnoses and surgical procedures recorded in these records are indexed, as are the medical records of other providers who serve the local population.15 Utilizing this unique record system (the Rochester Epidemiology Project), all Olmsted County residents diagnosed with hypercalcemia from 1992 through 2001 were identified. We also identified all patients with primary hyperparathyroidism as described elsewhere.16 The following diagnostic rubrics were searched through 2003 (to allow patients in the process of being examined to be included on the diagnostic index): hyperparathyroidism (ICD-9 code, 252.0), parathyroid adenoma (ICD-9 code, 227.1), osteitis fibrosa cystica (ICD 9-code, 588.8), malignant hypercalcemia/ectopic hormone secretion (ICD-9 code, 259.3), and hypercalcemia not otherwise specified (ICD-9 code, 275.4). In addition, all Olmsted County residents with serum calcium levels exceeding 10.1 mg/dL at least twice, between 1992 and 2001, were identified directly from Mayo’s Laboratory Information System.
Patients were accepted as having thiazide-associated hypercalcemia if they met the following criteria: sustained hypercalcemia (serum calcium > 10.1 mg/dL) documented on two or more measurements with concomitant thiazide diuretic use for which no other cause (e.g., pathologically or biochemically proven primary hyperparathyroidism before initiation of the thiazide diuretic, malignancy, family history of familial benign hypercalcemia, creatinine level > 2 mg/dL, or lithium therapy) was identified. The methods used at the Mayo Clinic to measure serum calcium levels changed over time; however, the normal range (8.9 to 10.1 mg/dL) remained the same because the instrumentation was calibrated not against the manufacturer’s standard but against atomic absorption spectrophotometry (according to certified references from the National Bureau of Standards). The date of diagnosis of thiazide-induced hypercalcemia was the date of the first elevated calcium level while on a thiazide diuretic. Patients with thiazide-associated hypercalcemia who subsequently had biochemically proven primary hyperparathyroidism must have met the inclusion criteria outlined in our previous studies16,17 three months or longer after thiazide discontinuation.1 Serum intact parathyroid hormone was measured by a two-site immunochemiluminometric assay (normal range: 1.0-5.2 pmol/L). To qualify for the study, patients must have been residents of Olmsted County on the date the first elevation in serum calcium was discovered. In addition, to assure comparability with the earlier study of primary hyperparathyroidism,16,17 we also established residency in Olmsted County for at least one year before the diagnosis of thiazide-associated hypercalcemia.
For each case identified, the complete (inpatient and outpatient) medical record in the community was reviewed by one of the investigators (RAW). Mayo Clinic records contain the details of every inpatient hospitalization at its two hospitals, every outpatient office or clinic visit, all emergency room and nursing home care, as well as all laboratory, radiographic and pathology reports, including autopsies. This information was supplemented by that available from the other providers of care to local residents, most notably the Olmsted Medical Center.15
Incidence rates were calculated as of the date of the initial elevated serum calcium level after initiation of the thiazide. The denominator age- and sex-specific person-years for the entire population of Olmsted County were estimated from decennial census data with interpolation between census years.18 Standard errors and 95% confidence intervals were calculated for the rates assuming that they follow a Poisson distribution. Incidence rates were directly age- and/or age- and sex-adjusted to the population structure of white persons in the United States in 2000. Poisson regression was used to compare male and female incidence rates adjusted for age. The cumulative probability of primary hyperparathyroidism diagnosis after thiazide discontinuation was calculated using the Kaplan-Meier method.19
RESULTS
We identified 72 Olmsted County residents with thiazide-associated hypercalcemia (68 women and 4 men) during the 10-year study period, 1992-2001. The overall annual age- and sex-adjusted incidence was 7.7 (95% CI, 5.9-9.5) per 100,000 (Table 1). The incidence of thiazide-associated hypercalcemia increased after 1996, with a peak incidence of 16.3 (95% CI, 8.3-24.3) per 100,000 in 1998 (Table 2). The age-adjusted incidence was higher in women (13.5 per 100,000 person-years; 95% CI, 10.3-16.8) than in men (0.9 per 100,000 person-years; 95% CI, 0.0-1.8; P<.0001). The highest incidence was 55.3 per 100,000 person-years in 70-79 year-old women.
Table 1.
Incidence of thiazide-associated hypercalcemia among Olmsted County, Minnesota, residents, 1992-2001, by gender and age-group
| Women | Men | Both sexes | ||||
|---|---|---|---|---|---|---|
| Age-group (yrs) | n | Rate* (95% CI) | n | Rate* (95% CI) | n | Rate* (95% CI) |
| < 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30-39 | 1 | 1.0 | 0 | 0 | 1 | 0.5 |
| 40-49 | 5 | 5.5 | 1 | 1.2 | 6 | 3.4 |
| 50-59 | 21 | 35.4 | 1 | 1.8 | 22 | 19.0 |
| 60-69 | 18 | 45.5 | 1 | 2.8 | 19 | 25.1 |
| 70-79 | 17 | 55.3 | 1 | 4.4 | 18 | 33.8 |
| 80-89 | 5 | 25.0 | 0 | 0 | 5 | 17.0 |
| ≥ 90 | 1 | 18.1 | 0 | 0 | 1 | 14.6 |
| Total | 13.5 (10.3-16.8) † | 0.9 (0.0-1.8)† | 7.7 (5.9-9.5) ‡ | |||
Incidence per 100,000 person-years.
Incidence per 100,000 person-years directly age-adjusted to the U.S. white population in 2000.
Incidence per 100,000 person-years directly age- and sex-adjusted to the U.S. white population in 2000.
Table 2.
Trends in the incidence of thiazide-associated hypercalcemia among Olmsted County, Minnesota, residents, 1992-2001, by year
| Year | n | Rate* (95% CI) |
|---|---|---|
| 1992 | 4 | 4.9 (0.1-9.8) |
| 1993 | 3 | 3.6 (0-7.8) |
| 1994 | 6 | 7.1 (1.4-12.8) |
| 1995 | 4 | 4.6 (0.1-9.0) |
| 1996 | 3 | 3.0 (0.0-6.5) |
| 1997 | 8 | 8.6 (2.6-14.6) |
| 1998 | 16 | 16.3 (8.3-24.3) |
| 1999 | 12 | 12.3 (5.3-19.2) |
| 2000 | 6 | 5.8 (1.1-10.5) |
| 2001 | 10 | 9.5 (3.5-15.4) |
Incidence per 100,000 person-years directly age- and sex-adjusted to the U.S. white population in 2000.
The mean age at diagnosis was 64 ± 11 years, and women comprised the majority of cases (94%) (Table 3). Laboratory results indicated mild hypercalcemia, with a mean highest serum calcium on thiazides of 10.7 ± 0.3 mg/dL (range, 10.2 to 11.5 mg/dL). Prior to initiation of thiazides, the mean serum calcium was 9.7 ± 0.4 mg/dL as measured in 57 subjects. Of those without a serum calcium level prior to thiazide initiation, 10 patients (67%) had a normal serum calcium measurement prior to the detection of hypercalcemia. The elevated serum calcium level was identified on average 6.0 ± 7.2 years after the initiation of thiazide treatment (range, 14 days to 27 years). Serum parathyroid hormone was measured in 53 patients after the identification of hypercalcemia and averaged 4.8 ± 2.7 pmol/L (range, 0.4 to 13 pmol/L). The serum parathyroid hormone was ≤ 2.1 pmol/L in 7 subjects (13.2 %), 2.2-5.2 pmol/L in 29 subjects (54.7%), and > 5.2 pmol/L in 17 subjects (32.1%). None of the patients with a suppressed parathyroid hormone level had an identifiable secondary cause of their hypercalcemia and the onset of hypercalcemia in all of these patients was more than 1 year after thiazide initiation. Patients without a serum parathyroid hormone measurement had less severe hypercalcemia (10.46 ± 0.19), but were otherwise similar to the overall cohort. The most common reason for thiazide initiation was hypertension (94%); edema (3%) and hypercalciuria/nephrolithiasis (3%) were the other rationales for its use.
Table 3.
Clinical and laboratory spectrum of thiazide-associated hypercalcemia among Olmsted County, Minnesota, residents, 1992-2001, overall and subset later found to have primary hyperparathyroidism
| Characteristic | All patients mean ± SD, or n (%) | Primary Hyperparathyroidism subset mean ± SD, or n (%) |
|---|---|---|
| Female gender | 68 (94.4) | 19 (95.0) |
| Age at onset of hypercalcemia, yrs | 63.9 ± 11.3 | 66.2 ± 11.2 |
| Serum calcium prior to thiazide use, mg/dL | 9.7 ± 0.4 | 9.7 ± 0.5 |
| Maximum serum calcium on thiazides, mg/dL | 10.7 ± 0.3 | 11.0 ± 0.4 |
| Serum parathyroid hormone, pmol/L | 4.8 ± 2.7 | 6.3 ± 4.4 |
| Years from thiazide start to hypercalcemia | 6.0 ± 7.2 | 7.3 ± 8.5 |
| Reason for thiazide use | ||
| Hypertension | 68 (94.4) | 20 (100) |
| Edema | 2 (2.8) | 0 (0) |
| Hypercalciuria/nephrolithiasis | 2 (2.8) | 0 (0) |
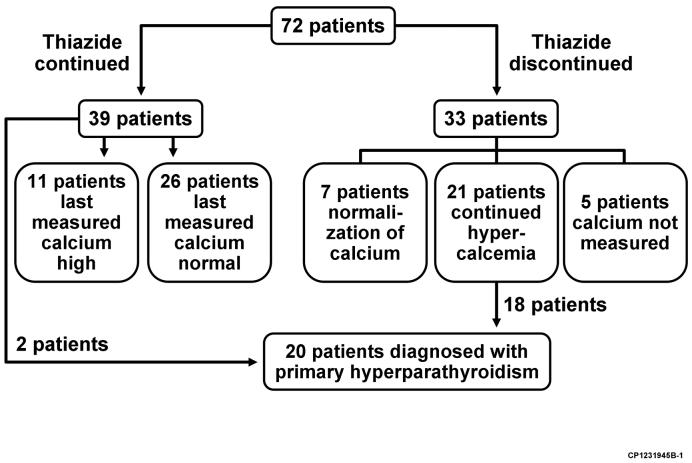
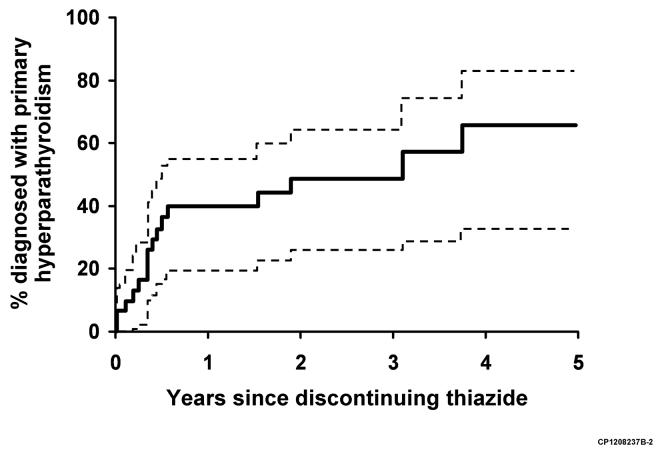
The thiazide was abruptly discontinued in 33 patients (in 12.5% [95% CI, 4.5-19.8%] within 1 year, 25.1% [95% CI, 14.3-34.4%] within 3 years, and 34.6% (95% CI, 22.2-45.1%) within 5 years following initiation of treatment), and was then reinitiated in 2 patients (Figure 1). Serum calcium stayed persistently normal in 7 patients discontinuing thiazides, with a mean last serum calcium measurement 5 ± 4.0 years after the thiazide was stopped. Twenty one patients (64%) continued to have hypercalcemia despite stopping the thiazide, 18 of whom were formally diagnosed with primary hyperparathyroidism (39.7 % [95% CI, 19.2-55.2%] within 1 year, 48.3% [95% CI, 25.8-68.4%] within 3 years, and 65.5% [95% CI, 32.5-83.7%] within 5 years since discontinuation) (Figure 2). In patients with continued hypercalcemia after stopping the thiazide, 5 patients had initial normalization of their serum calcium level, followed by recurrent hypercalcemia an average of 3.1 ± 1.2 years later. Five patients who stopped thiazides did not have their serum calcium level measured after discontinuation.
Figure 1.
Figure 2.
Thirty seven subjects (51%) continued the thiazide and did not have parathyroid surgery (2 subjects had parathyroid surgery without stopping the thiazide). Serum calcium levels were measured in all patients after meeting the inclusion criteria for thiazide-associated hypercalcemia. Their last measured mean serum calcium level was 9.9 ± 0.4 mg/dL (range, 8.8 to 11.1 mg/dL) an average of 5.7 years ± 2.1 years (range, 2.6 to 11.1 years) after detection of hypercalcemia, with continued hypercalcemia on the last measurement in 11 subjects (30%). In the 26 patients with a last measured serum calcium level in the normal range, 5 patients (19%) had a single measurement, 7 patients (27%) had persistently normal calcium levels, and 14 patients (54%) had intermittent hypercalcemia. When compared to those in whom the thiazide was discontinued, the mean serum calcium and parathyroid hormone levels were lower in patients remaining on thiazides (Table 4).
Table 4.
Clinical and Laboratory Spectrum of Thiazide-Associated Hypercalcemia Among Olmsted County, Minnesota, Residents, 1992-2001, Comparing Subsets Continuing and Discontinuing Thiazide.
| Characteristic | Thiazide discontinuation mean ± SD, or n (%) | Thiazide continuation without parathyroid surgery mean ± SD, or n (%) |
|---|---|---|
| Female gender | 30 (90.9) | 36 (97.3) |
| Age at onset of hypercalcemia, yrs | 67.48 ± 10.24 | 60.76 ± 11.03 |
| Serum calcium prior to thiazide use, mg/dL | 9.64 ± 0.37 | 9.71 ± 0.37 |
| Maximum serum calcium on thiazides, mg/dL | 10.80 ± 0.38 | 10.56 ± 0.20 |
| Serum parathyroid hormone, pmol/L | 5.38 ± 2.82 | 3.66 ± 1.83 |
| Range, pmol/L | 1.8-13 | 0.4-7.6 |
| Not measured | 5 | 14 |
| Parathyroid hormone ≤2.1 pmol/L | 2 (7.1) | 5 (21.7) |
| Parathyroid hormone 2.2-5.2 pmol/L | 16 (57.1) | 13 (56.5) |
| Parathyroid hormone > 5.2 pmol/L | 10 (35.7) | 5 (21.7) |
| Years from thiazide start to hypercalcemia | 7.92 ± 8.40 | 4.67 ± 6.01 |
| Reason for thiazide use | ||
| Hypertension | 31 | 35 |
| Edema | 2 | 0 |
| Hypercalciuria/nephrolithiasis | 0 | 2 |
Primary hyperparathyroidism was diagnosed in 20 patients with thiazide-associated hypercalcemia. Of the patients with primary hyperparathyroidism, 10 (50%) had pathologic confirmation, 7 (35%) had an inappropriate parathyroid hormone in the setting of hypercalcemia, and 3 (15%) had persistent hypercalcemia for a year or more after stopping the thiazide. In patients diagnosed with primary hyperparathyroidism, 18 (90%) were asymptomatic. The two patients with symptomatic disease had kidney stones. The mean age at onset of hypercalcemia in the patients diagnosed with primary hyperparathyroidism was 66 years, and 19 (95%) were women (Table 3). The mean maximum serum calcium in this subset of patients was 11.0 ± 0.3 mg/dL, with a mean PTH level of 6.3 ± 2.4 pmol/L (range, 3.4 to 12.0 pmol/L) at the time closest to the diagnosis of primary hyperparathyroidism. The mean time to identification of hypercalcemia after initiation of the thiazide in patients diagnosed with primary hyperparathyroidism was 7.3 years.
DISCUSSION
In this study, the first to estimate the incidence of thiazide-associated hypercalcemia, we found an overall age- and sex-adjusted incidence of 7.7 per 100,000 person-years in our community. In general, the incidence increased after 1996, with a peak of 16.3 per 100,000 in 1998. One potential explanation for this trend could be the increased use of thiazides since the ALLHAT trial.20 However, due to significant changes in our prescription ordering system during the period of interest, we were unable to confidently quantify thiazide prescription trends at our institution.
Factors other than thiazides may be important in the development of hypercalcemia. Patients with a clear etiology for hypercalcemia were excluded from our results. Thus, the primary diagnostic considerations in this setting would be primary hyperparathyroidism or the thiazide itself. To distinguish between these two conditions, it is often necessary to discontinue the thiazide for up to three months1 since hypercalcemia has been reported to resolve once thiazides are discontinued. However, Christensson and colleagues reported that hypercalcemia resolved in only 5 of 20 subjects 1-3 months after thiazide discontinuation and 14 of the 15 subjects with persistent hypercalcemia had pathologically confirmed primary hyperparathyroidism.11 This is consistent with our finding that 64% of community patients identified with thiazide-associated hypercalcemia remained hypercalcemic after treatment was discontinued, suggesting another underlying cause, e.g., primary hyperparathyroidism.
Indeed, the clinical characteristics of thiazide-associated hypercalcemia are similar to those seen for primary hyperparathyroidism in this population.16,17 The average age was 64 years with 94% females in the thiazide cohort compared to an average age of 56 years and 69% women in the primary hyperparathyroidism cohort from the same period.17 The observed gender discrepancy suggests that postmenopausal women are predisposed to developing hypercalcemia while on thiazides, but an additional factor may be that more women than men are on thiazides for the treatment of hypertension.21 The mean maximum serum calcium (10.7 mg/dL) in our subjects was identical to that seen in primary hyperparathyroidism.17 Furthermore, in subjects who remained on thiazides, the last serum calcium measurement was 9.9 mg/dL, suggesting a non-progressive process as is often seen in mild primary hyperparathyroidism.22 Consistent with previous literature, complications were limited to 2 patients, both of whom were later diagnosed with primary hyperparathyroidism.16 Although patients diagnosed with primary hyperparathyroidism appeared to be similar to the overall group of patients with thiazide-associated hypercalcemia, on average, they had higher maximum serum calcium (11.0 vs 10.7 mg/dL) and parathyroid hormone (6.3 vs 4.8 pmol/L) levels. It is also likely that several of the subjects remaining on thiazides with unsuppressed parathyroid hormone levels or continued hypercalcemia had underlying primary hyperparathyroidism.
The specific relationship between thiazide use and primary hyperparathyroidism is uncertain. Work from Pickleman and colleagues suggested that thiazide use induced enlargement of the parathyroid glands.23 Alternatively, the association may simply represent a chance association between two conditions (hypertension and primary hyperparathyroidism) which are more prevalent with increasing age. In addition, the renal tubular Na-Cl co-transporter (NCTT) has been suggested as a link between hypertension and calcium homeostasis.24 Specifically, higher urinary calcium excretion and higher parathyroid hormone levels have been reported in patients with essential hypertension.24 Finally, thiazides might unmask mild or normocalcemic primary hyperparathyroidism.25 Conversely, thiazide use theoretically could reduce parathyroid gland stimulation through renal3-6 and intestinal8,9 mechanisms and delay the development of primary hyperparathyroidism.26
Our study has several limitations due to its design. We did not have prospective measurements of serum calcium in all patients on thiazides, and serum calcium was not measured at specific time points prior to or after thiazide initiation. Also, we did not have routine measurements of serum albumin or a formal assessment of dietary calcium and vitamin D intake, all of which may influence serum calcium measurements. In addition, the population of Olmsted County is primarily Caucasian, limiting the application of study results to more ethnically diverse populations. Nonetheless, the results would be representative of typical patients encountered in clinical practice. Furthermore, our unique record system allowed comprehensive follow-up of all community medical care. A comparable prospective study to identify thiazide-associated hypercalcemia would require an estimated 3600 study subjects followed for at least 6 years.
The appropriate clinical management of patients with thiazide-associated hypercalcemia is poorly delineated. After confirming sustained hypercalcemia, a serum parathyroid hormone determination is useful in narrowing the diagnostic considerations. Although thiazides will often be discontinued to determine if they are the cause of the hypercalcemia, the hypercalcemia has been described as mild, non-progressive, and often consists of a temporary elevation shortly after the onset of therapy.12,27 Our results are consistent with mild, asymptomatic and non-progressive disease. In patients without an easily identifiable cause of hypercalcemia on thiazides and an unsuppressed parathyroid hormone level, a reasonable strategy may be to follow guidelines for asymptomatic primary hyperparathyroidism.28 Although exacerbation of hypercalcemia is a concern if thiazides are continued, it appears that thiazides do not worsen hypercalcemia in patients who develop mild primary hyperparathyroidism.29 However, there are case reports of severe hypercalcemia in patients with primary hyperparathyroidism who are started on a thiazide diuretic.30 This study did not address the issue of starting thiazides in patients with known primary hyperparathyroidism.
In summary, the overall age- and sex adjusted annual incidence of thiazide-associated hypercalcemia in Olmsted County in 1992-2001 was estimated at 7.7 per 100,000 with an increasing trend since 1996 for unclear reasons. The typical patients were women with mild, uncomplicated, and non-progressive hypercalcemia that was discovered approximately 6 years after thiazide initiation. Excluding readily identifiable causes of hypercalcemia, we estimate that approximately two-thirds of the patients with thiazide-associated hypercalcemia have underlying primary hyperparathyroidism. Since there is increasing utilization of thiazides as first-line antihypertensive agents, combined with an aging population at increased risk for parathyroid disease, a further increase in the incidence of thiazide-associated hypercalcemia might be expected. It will be important to monitor long-term trends in this condition, as well as to consider prospective studies to further characterize its long-term natural history.
ACKNOWLEDGMENT
The authors thank Mrs. Mary Roberts for assistance in preparing the manuscript.
This project was supported in part by an ENHANCE award from the Department of Internal Medicine, Mayo Clinic Rochester, and by a grant (RO1 AR 30582) from the National Institutes of Health, U.S. Public Health Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Significance
- The annual incidence of thiazide-associated hypercalcemia was 7.7 per 100,000, with an increasing trend after 1996.
- Hypercalcemia was detected 6 years after thiazide initiation. The majority of the patients with hypercalcemia were women.
- Clinical features of thiazide-associated hypercalcemia were similar to those seen in primary hyperparathyroidism.
- Persistence of hypercalcemia in 64% of patients discontinuing thiazides suggests that underlying primary hyperparathyroidism is common in patients who develop hypercalcemia while on thiazide diuretics.
References
- 1.Silverberg S, Bilezikian JP. Clinical course of primary hyperparathyroidism. In: Bilezikian JP, Marcus R, Levine MA, editors. The Parathyroids, Basic and Clinical Concepts. 2nd ed. Academic Press; San Diego: 2001. pp. 387–398. [Google Scholar]
- 2.Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Loop diuretics alter the diurnal rhythm of endogenous parathyroid hormone secretion. A randomized-controlled study on the effects of loop- and thiazide-diuretics on the diurnal rhythms of calcitropic hormones and biochemical bone markers in postmenopausal women. Eur J Clin Invest. 2001;31:764–772. doi: 10.1046/j.1365-2362.2001.00883.x. [DOI] [PubMed] [Google Scholar]
- 3.Coe FL, Canterbury J, Reiss E. Hyperparathyroidism in idiopathic hypercalciuria: primary or secondary? Trans Assoc Am Physicians. 1971;84:152–161. [PubMed] [Google Scholar]
- 4.Brickman AS, Massry SG, Colburn JW. Changes in serum and urinary calcium during treatment with hydrochlorothiazide: study on mechanisms. J Clin Invest. 1972;51:945–954. doi: 10.1172/JCI106889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middler S, Pak CY, Murad F, Bartter FC. Thiazide diuretics and calcium metabolism. Metabolism. 1973;22:139–146. doi: 10.1016/0026-0495(73)90264-3. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen FS, Brunner S. The long-term effect of bendroflumethiazide on renal calcium and magnesium excretion and stone formation in patients with recurring renal stones. Scand J Urol Nephrol. 1974;8:128–131. doi: 10.3109/00365597409132117. [DOI] [PubMed] [Google Scholar]
- 7.Riis B, Christiansen C. Actions of thiazide on vitamin D metabolism: a controlled therapeutic trial in normal women early in the postmenopause. Metabolism. 1985;34:421–424. doi: 10.1016/0026-0495(85)90206-9. [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen FS, Transbol I. The effect of bendroflumethiazide on the intestinal absorption of calcium in normocalcaemic renal stone formers and in hyperparathyroidism. Acta Med Scand. 1974;195:33–36. [PubMed] [Google Scholar]
- 9.Bazzini C, Vezzoli V, Sironi C, et al. Thiazide-sensitive NaCl-cotransporter in the intestine: possible role of hydrochlorothiazide in the intestinal Ca2+ uptake. J Biol Chem. 2005;280:19902–19910. doi: 10.1074/jbc.M411961200. [DOI] [PubMed] [Google Scholar]
- 10.Heath H., III Postural and venous stasis-induced changes in total calcium. Mayo Clin Proc. 2005;80:1101. doi: 10.4065/80.8.1101. [DOI] [PubMed] [Google Scholar]
- 11.Christensson T, Hellstrom K, Wengle B. Hypercalcemia and primary hyperparathyroidism. Prevalence in patients receiving thiazides as detected in a health screen. Arch Intern Med. 1977;137:1138–1142. doi: 10.1001/archinte.137.9.1138. [DOI] [PubMed] [Google Scholar]
- 12.Stenstrom G, Heedman P-A. Clinical findings in patients with hypercalcemia. A final investigation based on biochemical screening. Acta Med Scand. 1974;195:473–477. doi: 10.1111/j.0954-6820.1974.tb08174.x. [DOI] [PubMed] [Google Scholar]
- 13.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid- Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 16.Wermers RA, Khosla S, Atkinson EJ, Hodgson SF, O’Fallon WM, Melton LJ., III The rise and fall of primary hyperparathyroidism: a population-based study in Rochester, Minnesota, 1965-1992. Ann Intern Med. 1997;126:433–440. doi: 10.7326/0003-4819-126-6-199703150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wermers RA, Khosla S, Atkinson EJ, et al. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease. J Bone Miner Res. 2006;21:171–177. doi: 10.1359/JBMR.050910. [DOI] [PubMed] [Google Scholar]
- 18.Bergstralh EJ, Offord KP, Chu CP, Beard CM, O’Fallon WM, Melton LJ., IIICalculating Incidence, Prevalence and Mortality Rates for Olmsted County, Minnesota: An Update 1992. Technical Report Series, No. 49 Mayo Clinic; Rochester, MN [Google Scholar]
- 19.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. J Royal Stat Soc, Series B (Methodological) 1976;38:290–295. [Google Scholar]
- 20.Austin PC, Mamdani MM, Tu K, Zwarenstein M. Changes in prescribing patterns following publication of the ALLHAT Trial. JAMA. 2004;291:44–45. doi: 10.1001/jama.291.1.44-b. [DOI] [PubMed] [Google Scholar]
- 21.Weiss R, Buckley K, Clifford T. Changing patterns of initial drug therapy for the treatment of hypertension in a Medicaid population, 1997-2000. Clin Ther. 2002;24:1451–1462. doi: 10.1016/s0149-2918(02)80048-0. [DOI] [PubMed] [Google Scholar]
- 22.Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–1255. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 23.Pickleman JR, Straus FH, Forland M, Paloyan E. Thiazide induced parathyroid stimulation. Metabolism. 1969;18:867–873. doi: 10.1016/0026-0495(69)90062-6. [DOI] [PubMed] [Google Scholar]
- 24.McCarron DA, Pingree PA, Rubin RJ, Gaucher SM, Molitich M, Krutzik S. Enhanced parathyroid function in essential hypertension: a homeostatic response to a urinary calcium leak. Hypertension. 1980;2:162–168. doi: 10.1161/01.hyp.2.2.162. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88:5348–5352. doi: 10.1210/jc.2003-031014. [DOI] [PubMed] [Google Scholar]
- 26.Arnold A, Brown MF, Urena P, Gaz RD, Sarfati E, Drueke TB. Monoclonality of parathyroid tumors in chronic renal failure and in primary parathyroid hyperplasia. J Clin Invest. 1995;95:2047–2053. doi: 10.1172/JCI117890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duarte CG, Winnacker JL, Becker KL, Pace A. Thiazide-induced hypercalcemia. N Engl J Med. 1971;284:828–830. doi: 10.1056/NEJM197104152841506. [DOI] [PubMed] [Google Scholar]
- 28.Bilezikian JP, Potts JT, Jr, Fuleihan GH, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century J Bone Miner Res 200217Suppl 2N2–N11. 30 [PubMed] [Google Scholar]
- 29.Farquhar CW, Spathis GS, Barron JL, Levin GE. Failure of thiazide diuretics to increase plasma calcium in mild primary hyperparathyroidism. Postgrad Med J. 1990;66:714–716. doi: 10.1136/pgmj.66.779.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strong P, Jewell S, Rinker J, Hoch D, Crapo L. Thiazide therapy and severe hypercalcemia in a patient with hyperparathyroidism. West J Med. 1991;154:338–340. [PMC free article] [PubMed] [Google Scholar]