Abstract
Condensed abstract
To evaluate the relation between statin use and breast cancer risk, we conducted a cohort study within an integrated healthcare delivery system. The study does not support an association between statin use and breast cancer risk.
Background
Mechanistic studies suggest that HMG-CoA inhibitors (statins) reduce the risk of breast cancer. Observational studies offer mixed results.
Methods
To evaluate the relation between statin use and breast cancer risk, we conducted a cohort study among women aged 45–89 years within an integrated healthcare delivery system. Information on statin use and covariates were obtained from automated databases. We identified breast cancer cases through the Surveillance, Epidemiology, and End Results registry. We used Cox proportional hazards models to estimate the hazard ratios (HR) and 95% confidence intervals (CI) for invasive breast cancer among statin users compared to non-users.
Results
Among 92,788 women studied from 1990–2004, median follow-up time was 6.4 years and 2,707 breast cancer cases were identified. During the study period, 7.4% of women used statins for at least one year and the median duration of use was 3.1 years. We found no difference in breast cancer risk among statin users (HR =1.07; 95% CI, 0.88–1.29) compared to non-users. Risk of breast cancer did not differ by duration of use (1–2.9 years, 3–4.9 years or 5+ years) or hydrophobic statin use. We found a suggestive increased risk of breast cancer among statin users of 5+ years (HR=1.27; 95% CI, 0.89–1.81 for any statins and 1.47; 95% CI, 0.89–2.44 for hydrophobic statins) and of estrogen receptor negative tumors with increasing duration of statin use (1–2.9 years: HR=1.33; 95% CI, 0.64–2.77; 3–4.9 years: HR=1.68; 95% CI, 0.72–3.92; 5+ years: HR=1.81; 95% CI, 0.75–4.36).
Conclusion
This study does not support an association between statin use and breast cancer risk.
Keywords: Statins, breast cancer, antilipemics, HMG-CoA Reductase Inhibitors
Introduction
HMG-CoA inhibitors (statins) are a therapeutic class of drugs that reduce plasma cholesterol levels and are used to manage and prevent coronary heart disease.1 Statin use has increased dramatically in the past decade and is likely to continue rising. Statins are currently the most widely prescribed lipid-lowering agents on the United States pharmaceutical market; five of the six statins on the market were among the top 200 prescribed drugs for 2004.2
While an early review of studies in rodents,3 and at least two clinical trials of pravastatin raised concerns that statins may have carcinogenic properties,4,5 a growing body of mechanistic studies suggest statins may in fact have chemopreventive potential against cancer.6,7 Statins appear to induce apoptosis and reduce cell invasiveness in various cells lines,7 including mammary carcinoma.8–12
Epidemiologic studies that report on statin use and breast cancer risk are varied and report no effect on risk, and both increases and decreases in risk.13–21 To assess the association between statin use and breast cancer risk, we conducted a cohort study within a large integrated healthcare delivery system that contains computerized information on medication use, incident cancers, and risk factors for breast cancer.
Methods
Study Population
We conducted a dynamic, retrospective cohort study among women enrolled in Group Health Cooperative (GHC), a non-profit integrated delivery system that provides comprehensive health care on a prepaid basis to approximately 550,000 individuals throughout western Washington State. GHC’s Institutional Review Board approved the study.
Women were included in the study if they met all the following criteria: 1) between the age of 45 to 89 years during the study period of January 1, 1990 to July 31, 2004, 2) continuously enrolled in GHC’s integrated group practice for at least two years during the study period, 3) residing in one of 13 counties covered by the Surveillance, Epidemiology, and End Results (SEER) cancer registry, 4) no prior history of any type of breast cancer as identified in the SEER registry and the self-administered Breast Cancer Screening Program risk survey, and 5) no prior preventive mastectomy or breast conserving surgery.
Data collection
We used automated health plan data to ascertain information on medication use, and potential confounders. Information on health plan enrollment, health care utilization including medication use, diagnoses, and procedures are recorded and maintained in GHC’s automated databases that can be linked by a unique consumer number assigned to each enrollee.22 GHC automated pharmacy data are considered a complete source of medication use and it is estimated that GHC enrollees obtain about 97% of their medications at GHC pharmacies.23,24 Computer linkage between active GHC enrollees and the western Washington SEER registry provides complete ascertainment of cancer cases.25 Similarly, Washington State Death records for active GHC enrollees are regularly obtained.26
GHC has a Breast Cancer Screening Program (BCSP) that women are invited to join when they turn 40 years old or when they enroll in GHC after 40 years of age.27 Women participating in the BCSP complete breast cancer risk factor questionnaires at program enrollment, and this information is updated at the time of each mammogram. Approximately 86% of women complete the questionnaires, and the data are available in automated databases.25 We collected risk factor data from the survey closest in time to entry into the study (median time interval between survey and study entry was 1.3 years). However, data are missing on a proportion of subjects partly because risk factor questions were added to the survey over the course of the study period (e.g., questions on race was added in 1993 and on education was added in 1996).
Statin use
We used automated pharmacy data to identify all statins (atorvastatin, cerivastatin, lovastatin, fluvastatin, pravastatin, simvastatin, and rosuvastatin) dispensed at GHC-owned pharmacies during the study period. For each statin dispensing, we estimated the date when the pills should have run out (run-out date) based on quantity dispensed and instructions for use. A new run-out date was set with each successive dispensing. A sixty-day lag period between the run-out date of one dispensing and fill date of the successive dispensing was used to define continuous use. Periods of continuous use were summed for total duration of use.
We defined statin users as women with at least two dispensings for a statin within any 6-month period and who used statins for at least one year. Non-users included women with no or one dispensing for a statin or less than one year of statin use. We considered women a current user if statins were used within the previous 12 months and a past user if statins were used more than 12 months prior. Among statin users, we calculated the cumulative lengths of statin use. Cumulative duration of statin use was categorized as 1–2.9 years, 3–4.9 years, and 5+ years. Statin use was further classified as hydrophobic only users (lovastatin, simvastatin, fluvastatin, atorvastatin and cerivastatin) or hydrophilic only users (pravastatin and rosuvastatin). We were unable to evaluate dose because there were few high dose statin users.
Breast cancer
We identified all incident, invasive breast cancer cases (N = 2,707) from the SEER registry. Only invasive breast cancer cases were included.
Covariates
The majority of information on risk factors for breast cancer was self-reported and determined from the BCSP survey completed at a time closest to the beginning of study period: weight, height, race, education, parity, menopausal status, and family history of breast cancer.
We used GHC outpatient, inpatient, laboratory, pharmacy, and administrative data to identify other possible confounders. We defined diabetics as women with at least one of the following: 1) 2+ fills for a medication used to treat diabetes (e.g., sulfonylureas, insulin), 2) a fasting glucose >125 mg/dL confirmed by a second out-of range test within one year, 3) a random glucose >200 mg/dL also confirmed by a second test within one year, or 4) a hospital discharge of diabetes at any time during GHC enrollment.28 Hormone therapy (HT) use was defined as 2+ dispensings for systemic estrogen (oral or transdermal) either alone or in combination with progestin. Other lipid lowering drug use was defined as 2+ dispensings for bile acid sequestrants, niacin, or fibrates.
Statistical Analysis
Follow-up of the study cohort extended from January 1, 1991 or one year after enrollment in GHC if later than January 1, 1990 until the earliest of the following: diagnosis of an incident invasive breast cancer, 90 years of age, death, disenrollment from GHC, preventive mastectomy, or the end of the study period (July 31, 2004). We used Cox proportional hazards models to examine the association between statin use and risk of incident invasive breast cancer.29 In separate subgroup analyses, we evaluated estrogen receptor (ER) positive and ER negative (ER-) tumors.
Statin use was modeled as a time-varying exposure and women were allowed to transition from being non-users to users or from non-users to current users to past users over time. For analyses of statin type, women were censored once they began using a different type of statin (hydrophobic or hydrophilic). Final models were adjusted for age at the beginning of the study period, HT use (estrogen only user, estrogen and progestin user, or non-user), diabetes (yes or no), and other lipid lowering drug use (yes or no) which were time-varying covariates, and body mass index which was based on weight and height information obtained from the BCSP survey. We used a cubic smoothing spline with five and three knots to model age and body mass index, respectively. Cubic smoothing splines provide a flexible approach for modeling the non-linear relationship between cancer risk and these important covariates to more completely adjust for confounding.30 We tested for the difference in the effects of statin use on breast cancer risk between HT users and non-users by including an interaction of statin use with HT use. The proportional hazards assumption was evaluated in all models by testing for the interaction of statin use with follow-up time. The assumption was met in all models (data not shown). Analyses were performed using the SAS statistical package, version 9.1 (Cary, NC).
In a subgroup of women who were enrolled in GHC for at least 5 years during the study period (January 1, 1990 – July 31, 2004), we evaluated the relation between duration of statin use (1–2.9 years, 3–4.9 years, and 5+ years) and breast cancer risk. Follow-up began on January 1, 1995 or 5 years after enrollment in GHC if enrollment occurred after January 1, 1990. Women who died or developed invasive breast cancer during the first 5 years of the study period were excluded.
In sensitivity analyses, we only considered the true non-users of statins in the comparison group by excluding women with either one dispensing for a statin or use of less than one year. We also adjusted for regular mammography screening in the models, which was defined as having at least one screening mammogram every three years among the subgroup of women enrolled in GHC for at least 5 years during the study period.
Results
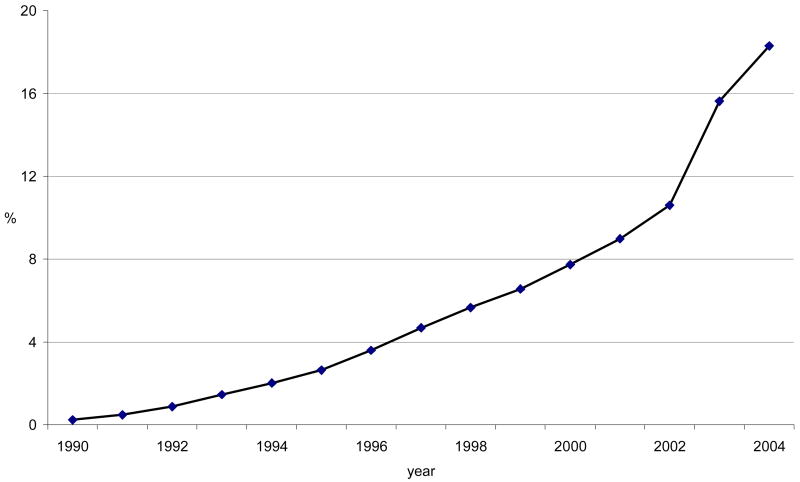
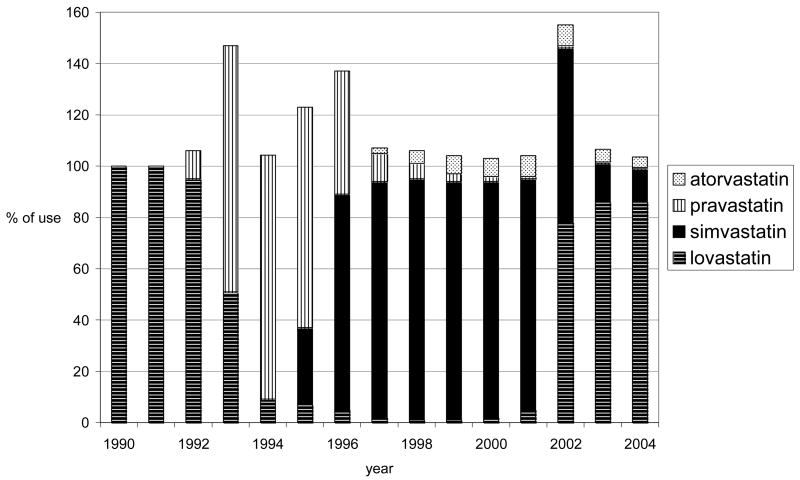
Among 92,788 women, the median duration of follow-up was 6.4 years (range, 2 days – 13.6 years). 7.4% of women used statins for at least a year during the study period. The prevalence of statin use gradually climbed from 0.2% in 1990 to 18.3% in first half of 2004 (Figure 1) and the median cumulative duration of statin use during the study period was 3.1 years (range, 1–14.5 years). Lovastatin and simvastatin were the most commonly used statins overall, but specific statin use varied throughout the study period due to changes in the GHC drug formulary (Figure 2).
Figure 1.
Prevalence of statin use by year
Figure 2.
Specific statins used among all users by year
Characteristics of the cohort by statin use and breast cancer status are described in Tables 1 and 2. Statin users were older, less educated, had a higher BMI, and more likely to be parous and peri/postmenopausal at the beginning of the study than non-users (Table 1). There were a higher proportion of Caucasians, diabetics, HT users, and other lipid lowering medication users among statin users than non-users. Among women who were enrolled in GHC for at least 5 years during the study period, a higher proportion of statin users (78.4%) than non-users (70.1%) had at least one screening mammogram in the 3 years prior to end of follow-up (p<0.0001). Statin users were less likely to have missing BCSP survey data on risk factors such as education, race, and family history of breast cancer than non-users.
Table 1.
Demographic characteristics of the study population by statin use
| Characteristics | Statin User (N=6,836) | Non-User (N=85,952) | p value* | ||
|---|---|---|---|---|---|
| Age in years, mean (SD) | 61.4 | (8.7) | 59.7 | (11.0) | <0.0001 |
| Body mass index in kg/m2, mean (SD) | 27.9 | (6.1) | 25.9 | (5.7) | <0.0001 |
| n | (%) | n | (%) | ||
| Race | <0.0001 | ||||
| White | 5,325 | (89.3) | 48,614 | (87.6) | |
| African American | 192 | (3.2) | 1,583 | (2.9) | |
| Asian/Pacific Islander | 316 | (5.3) | 3,928 | (7.1) | |
| Other | 128 | (2.1) | 1,366 | (2.5) | |
| No information | 875 | 30,461 | |||
| Education | <0.0001 | ||||
| Below high school | 740 | (12.9) | 4,660 | (9.3) | |
| High school/GED | 1,920 | (33.5) | 14,023 | (27.9) | |
| Some college | 1,932 | (33.8) | 17,481 | (34.8) | |
| College graduate | 563 | (9.8) | 6,796 | (13.5) | |
| Graduate school | 568 | (9.9) | 7,263 | (14.5) | |
| No information | 1,113 | 35,729 | |||
| Family history of breast cancer (1st or 2nd degree relatives) | 0.79 | ||||
| No | 4,790 | (74.3) | 54,004 | (74.4) | |
| Yes | 1,660 | (25.7) | 18,566 | (25.6) | |
| No information | 386 | 13,382 | |||
| Parous | <0.0001 | ||||
| No | 609 | (9.4) | 9,217 | (12.6) | |
| Yes | 5,882 | (90.6) | 63,981 | (87.4) | |
| No information | 345 | 12,754 | |||
| Diabetes | <0.0001 | ||||
| No | 4,397 | (64.3) | 78,372 | (91.2) | |
| Yes | 2,439 | (35.7) | 7,580 | (8.8) | |
| Hormone therapy use | <0.0001 | ||||
| Never | 3,137 | (45.9) | 49,170 | (57.2) | |
| Estrogen only | 1,709 | (25.0) | 16,126 | (18.8) | |
| Estrogen plus progestin | 1,990 | (29.1) | 20,656 | (24.0) | |
| Menopausal status | <0.0001 | ||||
| Pre-menopausal | 29 | (0.4) | 3,655 | (4.4) | |
| Peri or Post-menopausal | 6,793 | (99.6) | 78,532 | (95.6) | |
| No information | 14 | 3,765 | |||
| Other lipid lowering agents use | <0.0001 | ||||
| Never | 5,070 | (74.2) | 83,682 | (97.4) | |
| Ever | 1,766 | (25.8) | 2,270 | (2.6) | |
Chi square test for categorical variables or t-test for continuous variables
Table 2.
Demographic characteristics of the study population by breast cancer status
| Characteristics | Breast cancer cases (N=2,707) | Disease free (N=90,081) | p value* | ||
|---|---|---|---|---|---|
| Age in years, mean (SD) | 61.7 | (9.9) | 59.7 | (10.9) | <0.0001 |
| Body mass index in kg/m2, mean (SD) | 25.9 | (5.3) | 26.0 | (5.8) | 0.19 |
| n | (%) | n | (%) | ||
| Race | <0.0001 | ||||
| White | 2,206 | (93.1) | 51,733 | (87.6) | |
| African American | 56 | (2.4) | 1,719 | (2.9) | |
| Asian/Pacific Islander | 74 | (3.1) | 4,170 | (7.1) | |
| Other | 34 | (1.4) | 1,460 | (2.5) | |
| No information | 337 | 30,999 | |||
| Education | 0.10 | ||||
| Below high school | 223 | (9.6) | 5,177 | (9.7) | |
| High school/GED | 702 | (30.1) | 15,241 | (28.4) | |
| Some college | 770 | (33.0) | 18,643 | (34.8) | |
| College graduate | 284 | (12.2) | 7,075 | (13.2) | |
| Graduate school | 351 | (15.1) | 7,480 | (14.0) | |
| No information | 377 | 36,465 | |||
| Family history of breast cancer (1st or 2nd degree relatives) | <0.0001 | ||||
| No | 1,773 | (68.7) | 57,021 | (74.6) | |
| Yes | 808 | (31.3) | 19,418 | (25.4) | |
| No information | 126 | 13,642 | |||
| Parous | 0.39 | ||||
| No | 306 | (11.8) | 9,520 | (12.3) | |
| Yes | 2,291 | (88.2) | 67,572 | (87.7) | |
| No information | 110 | 12,989 | |||
| Diabetes | 0.23 | ||||
| No | 2,434 | (89.9) | 80,335 | (89.2) | |
| Yes | 273 | (10.1) | 9,746 | (10.8) | |
| Hormone therapy use | <0.0001 | ||||
| Never | 1,335 | (49.3) | 50,972 | (56.6) | |
| Estrogen only | 522 | (19.3) | 17,313 | (19.2) | |
| Estrogen plus progestin | 850 | (31.4) | 21,796 | (24.2) | |
| Menopausal status | <0.0001 | ||||
| Pre-menopausal | 23 | (0.9) | 3,661 | (4.2) | |
| Peri or Post-menopausal | 2,670 | (99.1) | 82,655 | (95.8) | |
| No information | 14 | 3,765 | |||
| Other lipid lowering agent use | <0.01 | ||||
| Never | 2,561 | (94.6) | 86,191 | (95.7) | |
| Ever | 146 | (5.4) | 3,890 | (4.3) | |
Chi square test for categorical variables or t-test for continuous variables
Breast cancer cases were older and more likely to be peri/postmenopausal and Caucasian than disease-free women (Table 2). We observed a higher frequency of HT use, other lipid lowering medication use, and a positive family history of breast cancer among breast cancer cases than disease-free women. Cases were less likely to have missing survey data than disease-free women. The majority of breast cancer cases were diagnosed at American Joint Committee on Cancer (AJCC) stage 1 (61.7%) or stage 2 (28.2%); most tumors were ER+ (84.7%); and histology was primarily ductal (80.3%). There were no differences in these breast cancer characteristics by ever use of statins compared to non-users (data not shown).
Overall, we found no association between statin use and breast cancer risk (Table 3). Risk of breast cancer did not differ by ever use (hazard ratio (HR) =1.07; 95% confidence interval (CI), 0.88–1.29) or current use (HR =1.08; 95% CI, 0.89–1.31) of statins compared with non-users. Compared to non-users, type of statin use was not associated with risk of breast cancer (HR=1.01; 95% CI, 0.80–1.26 for hydrophobic only and HR=1.01; 95% CI, 0.48–2.13 for hydrophilic only). Among women enrolled at GHC for at least 5 years during the study period, examination of the risk of incident breast cancer by duration of statin use and duration of hydrophobic statin use revealed no significant trend. There was, however, a suggestive increased risk of breast cancer among the highest category of 5+ years of statin use (HR=1.27; 95% CI, 0.89–1.81 for any statins and 1.47; 95% CI, 0.89–2.44 for hydrophobic statins).
Table 3.
Association between statin use and breast cancer risk
| Statin use | Breast cancer cases | Disease free | Age-adjusted HR (95% CI) | Multivariable-adjusted HR (95% CI)* |
|---|---|---|---|---|
| All women |
n=2,707
% |
n=90,081
% |
||
| Never | 95.2 | 92.6 | referent | referent |
| Ever | 4.8 | 7.4 | 1.15 (0.96–1.38) | 1.07 (0.88–1.29) |
| Current | 4.5 | 6.7 | 1.17 (0.97–1.41) | 1.08 (0.89–1.31) |
| Hydrophobic only** | 3.1 | 6.3 | 1.09 (0.88–1.36) | 1.01 (0.80–1.26) |
| Hydrophilic only*** | 0.3 | 0.1 | 1.05 (0.50–2.21) | 1.01 (0.48–2.13) |
| Duration of use among women with 5+ years of data† |
n=1,682
% |
n= 57,666
% |
||
| Never | 93.4 | 89.9 | referent | referent |
| All statins | ||||
| 1–2.9 yrs | 2.7 | 4.4 | 0.96 (0.71–1.29) | 0.96 (0.71–1.31) |
| 3–4.9 yrs | 1.8 | 2.4 | 1.09 (0.76–1.55) | 1.04 (0.72–1.51) |
| 5+ yrs | 2.1 | 3.3 | 1.28 (0.91–1.79) | 1.27 (0.89–1.81) |
| Hydrophobic statins only** | ||||
| 1–2.9 yrs | 2.7 | 4.5 | 0.90 (0.64–1.26) | 0.90 (0.64–1.26) |
| 3–4.9 yrs | 1.5 | 2.2 | 1.19 (0.79–1.79) | 1.12 (0.74–1.71) |
| 5+ yrs | 1.0 | 2.2 | 1.51 (0.92–2.49) | 1.47 (0.89–2.44) |
HR=hazard ratio and CI=confidence interval
Multivariable-adjusted models include age, hormone therapy use (HT), diabetes, other lipid lowering drug use, and BMI
Includes users of lovastatin, simvastatin, fluvastatin, atorvastatin, and cerivastatin
Includes users of pravastatin and rosuvastatin
p=0.3 from linear trend test for the effect of the duration of statin use and duration of hydrophobic statin use on breast cancer risk
Compared with non-users, there was a suggestive increased risk of ER- breast cancer tumors associated with statin use (HR=1.28; 95% CI, 0.78–2.08 for ever use; HR=1.16; 95% CI, 0.69–1.95 for current use) although the hazard ratios were not statistically significant (Table 4). The suggested increased risk of ER- tumors was also observed for increasing duration of statin use (1–2.9 years: HR=1.33; 95% CI, 0.64–2.77; 3–4.9 years: HR=1.68; 95% CI, 0.72–3.92; 5+ years: HR=1.81; 95% CI, 0.75–4.36) compared to non-users, but the findings were limited by a small number of exposed cases.
Table 4.
Association between statin use and estrogen receptor positive (ER+) and negative (ER−) tumors
| Statin use | ER+ cases
|
ER− cases
|
||
|---|---|---|---|---|
| Multivariable-adjusted HR (95% CI)* | Multivariable-adjusted HR (95% CI)* | |||
| All women |
n=2,067
% |
n=373
% |
||
| Never | 95.1 | Referent | 94.4 | referent |
| Ever | 4.9 | 1.06 (0.85–1.32) | 5.6 | 1.28 (0.78–2.08) |
| Current | 4.7 | 1.10 (0.88–1.37) | 4.8 | 1.16 (0.69–1.95) |
| Duration of use among women with 5+ years of data** |
n=1,315
% |
n=218
% |
||
| Never | 93.6 | referent | 90.7 | referent |
| 1–2.9 yrs | 2.6 | 0.93 (0.66–1.32) | 3.7 | 1.33 (0.64–2.77) |
| 3–4.9 yrs | 1.7 | 0.98 (0.63–1.51) | 2.8 | 1.68 (0.72–3.92) |
| 5+ yrs | 2.1 | 1.24 (0.83–1.86) | 2.8 | 1.81 (0.75–4.36) |
HR=hazard ratio and CI=confidence interval
Multivariable-adjusted models include age, HT use, diabetes, other lipid lowering drug use, and BMI.
p=0.5 from linear trend test for the effect of the duration of statin use on risk of ER+ tumor
p=0.08 from linear trend test for the effect of the duration of statin use on risk of ER− tumor
In sensitivity analyses where only the true non-users of statin were included in the comparison group, the results remained the same. Additional adjustment for regular mammography screening did not alter our findings. The interaction between statin use and HT use was tested in all models, including ER+ and ER- tumors, but only significant in the model of ever statin use and overall breast cancer incidence. When we evaluated statin use and breast cancer risk separately by HT use, we found a suggestive difference in risk among non-users of HT (HR=1.29; 95% CI, 0.99–1.68) and estrogen plus progestin users (HR=0.83; 95% CI, 0.59–1.17) (Table 5).
Table 5.
Association of statin use and breast cancer risk by hormone use
| Statin users | Breast cancer cases | Disease free | Multivariable-adjusted HR (95% CI)* | P for interaction |
|---|---|---|---|---|
|
n=2,707
% |
n=90,081
% |
|||
| Non-users of HT | 49.3 | 56.6 | 1.29 (0.99–1.68) | |
| Estrogen only users | 19.3 | 19.2 | 1.02 (0.70–1.49) | 0.31 |
| Estrogen + progestin users | 31.4 | 24.2 | 0.83 (0.59–1.17) | 0.04 |
HR=hazard ratio and CI=confidence interval
Multivariable-adjusted models include age, HT use, diabetes, other lipid lowering drug use, and BMI
Discussion
This population-based cohort study does not suggest an association between statin use and breast cancer risk. Overall, we found no significant difference in breast cancer risk by ever use, current use, duration of use, or use of hydrophobic statins compared to non-users. We did find a suggestive increased risk of breast cancer among statin users of 5+ years, and a suggestive increased risk of ER- tumors with increasing duration of statin use compared to non-users. However, confidence intervals included 1.0 and the findings were limited by a small number of exposed cases. We are unaware of any biologic mechanisms to support an increased risk of ER- tumors with statin use; no difference was seen in the one other study that evaluated type of breast cancer tumor in relation to statin use.21
This was the first large cohort study conducted in an integrated practice setting where women receive almost all their care within the system, and information on breast cancer risk factors is available. Other strengths of the study include the stability of the population over time, extensive and unbiased data on medication use and covariates, and reliable data on cancer incidence. Our large population-based cohort demonstrates the dramatic increase in statin use among women over the past decade.
While we found no association between statin use and breast cancer risk, it remains plausible that statins reduce breast cancer risk. The mechanistic data is relatively strong and suggests statins inhibit cancer cell growth and lead to apoptotic cell death through their inhibition of the mevalonate pathway, although other mechanisms have also been suggested.6 Many products of the mevalonate pathway are necessary for critical cellular functions such as membrane integrity, cell signaling, protein synthesis, and cell cycle progression.6,7 Disruptions of these processes in neoplastic cells by statins may result in control of tumor initiation, growth, and metastasis.7 Our study and others15,19,21 suggest that experimental models may not apply to humans, but there may be reasons for not observing an association in our study such as residual confounding, use of HT by the majority of statin users (54%), and a relatively short average duration of statin use.
Our results are similar to at least two other recently published large cohort studies,19,21 and one small case-control study15 that report no overall association between statin use and breast cancer risk. However, one of these large cohort studies by Cauley and colleagues found statin use was associated with an 18% lower risk of breast cancer when statin use was limited to hydrophobic statin users among women enrolled in the Women’s Health Initiative.21 Other observational studies have reported sometimes large reductions in the risk of breast cancer (30–72%) associated with use of statins.16,18,20 Cauley et al. also report no association between statin use and breast cancer risk among users of estrogen plus progestin or among never/past HT user, but a non-statistically significant reduced risk (22%) among users of estrogen alone. Our study and the study by Cauley and colleagues were not powered to evaluate an interaction between HT use and statins on breast cancer risk and, therefore, findings may be due to chance.
There are several limitations in our study. First, our study subjects are from a single healthcare system in western Washington State and may not be representative of other populations. Second, we cannot rule out exposure misclassification. Subjects who fill prescriptions but do not subsequently take the medication may be misclassified as users. Additionally, pharmacy utilization is only captured for enrollees who fill prescriptions at GHC-pharmacies and for enrollees with a drug benefit through GHC who fill prescriptions at contracting pharmacies. Therefore, subjects who fill prescriptions at non-GHC pharmacies may be erroneously classified as non-users. However, misclassification of medication use is relatively unlikely since previous GHC studies have found that GHC enrollees obtain 97% of their medications at GHC pharmacies,23,24 and we required 2+ dispensings and a year of use to be considered a statin user. We did not have information on medication use before enrollment in GHC. Any bias resulting from classifying a user as a non-user would bias our findings towards the null. Third, BCSP survey data were disproportionately missing for non-statin users compared to statin users and for disease-free women compared to breast cancer cases. While these risk factors were not included in the multivariable-adjusted models, the differential missing data indicate a difference in screening practice by statin use and disease status. Lastly, residual confounding is always possible in observational studies. We lacked information on potential confounders such as diet and level of physical activity, and women prescribed and adherent to statins may differ from non-users by factors not measured in this study.
In conclusion, our study and others indicate that statins are safe in relation to breast cancer risk but any chemopreventive effect in humans remains to be established. Researchers have hypothesized hydrophobic statins to have antiproliferative effects on breast cancer cells31,32 and hydrophilic statins to promote cancer.33 We were unable to evaluate hydrophilic statin use due to the small number of hydrophilic only statin users in our cohort. Further evaluations of longer durations of statin use by type of statins (hydrophobic and hydrophilic) in relation to breast cancer risk are warranted. The effects of statins on cancer are not completely understood. The NCI recently funded two phase-II trials of: 1) statin use and colorectal cancer risk among patients with an increased risk of colorectal cancer, and 2) the effect of statin use on precancerous changes in atypical nevi, which are a precursor for melanoma.34 These trials are likely to be useful in disentangling the complex relation between statins and cancer. Clinical trials of the effects of statins on breast cancer risk and biologic endpoints such as breast density have been suggested but are not supported by our study findings.31,32,35,36
Acknowledgments
We thank Deborah Seger for her valuable contributions.
Funding/support: This study was supported by NCI grant number CA108357 and CA63731
Reference List
- 1.Sewester CS, Dombek CE, Olin BR, Kastrup EK, Hebel SK. Drug Facts and Comparisons. St. Louis, MO; 2004. [Google Scholar]
- 2.RX List. The Top 200 Prescriptions for 2004 by U.S. Sales. [Accessed May 10, 2005]; Available at: http://www.rxlist.com/top200_sales_2004.
- 3.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996;275:55–60. [PubMed] [Google Scholar]
- 4.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 6.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–19. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 7.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–9. [PubMed] [Google Scholar]
- 8.Denoyelle C, Vasse M, Korner M, et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22:1139–48. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 9.Alonso DF, Farina HG, Skilton G, Gabri MR, De Lorenzo MS, Gomez DE. Reduction of mouse mammary tumor formation and metastasis by lovastatin, an inhibitor of the mevalonate pathway of cholesterol synthesis. Breast Cancer Res Treat. 1998;50:83–93. doi: 10.1023/a:1006058409974. [DOI] [PubMed] [Google Scholar]
- 10.Inano H, Suzuki K, Onoda M, Wakabayashi K. Anti-carcinogenic activity of simvastatin during the promotion phase of radiation-induced mammary tumorigenesis of rats. Carcinogenesis. 1997;18:1723–7. doi: 10.1093/carcin/18.9.1723. [DOI] [PubMed] [Google Scholar]
- 11.Seeger H, Wallwiener D, Mueck AO. Statins can inhibit proliferation of human breast cancer cells in vitro. Exp Clin Endocrinol Diabetes. 2003;111:47–8. doi: 10.1055/s-2003-37501. [DOI] [PubMed] [Google Scholar]
- 12.Denoyelle C, Albanese P, Uzan G, et al. Molecular mechanism of the anti-cancer activity of cerivastatin, an inhibitor of HMG-CoA reductase, on aggressive human breast cancer cells. Cell Signal. 2003;15:327–38. doi: 10.1016/s0898-6568(02)00124-9. [DOI] [PubMed] [Google Scholar]
- 13.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363–8. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 14.Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Shapiro S. Statin use and the risk of breast and prostate cancer. Epidemiology. 2002;13:262–7. doi: 10.1097/00001648-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kaye JA, Meier CR, Walker AM, Jick H. Statin use, hyperlipidaemia, and the risk of breast cancer. Br J Cancer. 2002;86:1436–9. doi: 10.1038/sj.bjc.6600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudreau DM, Gardner JS, Malone KE, Heckbert SR, Blough DK, Daling JR. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women: a case-control study. Cancer. 2004;100:2308–16. doi: 10.1002/cncr.20271. [DOI] [PubMed] [Google Scholar]
- 17.Beck P, Wysowski DK, Downey W, Butler-Jones D. Statin use and the risk of breast cancer. J Clin Epidemiol. 2003;56:280–5. doi: 10.1016/s0895-4356(02)00614-5. [DOI] [PubMed] [Google Scholar]
- 18.Cauley JA, Zmuda JM, Lui LY, et al. Lipid-lowering drug use and breast cancer in older women: a prospective study. J Womens Health (Larchmt) 2003;12:749–56. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- 19.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch Intern Med. 2005;165:2264–71. doi: 10.1001/archinte.165.19.2264. [DOI] [PubMed] [Google Scholar]
- 20.Kochhar R, Khurana V, Bejjanki H, Caldito G, Fort C. Statins reduce breast cancer risk: a case control study in US female veterans, 2005 ASCO Annual Meeting Proceedings. Journal of Clinical Oncology. 2005;23(16S):Abstract 514. [Google Scholar]
- 21.Cauley JA, McTiernan A, Rodabough RJ, et al. Statin use and breast cancer: prospective results from the Women's Health Initiative. J Natl Cancer Inst. 2006;98:700–7. doi: 10.1093/jnci/djj188. [DOI] [PubMed] [Google Scholar]
- 22.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom B, editor. Pharmacoepidemiology. Chichester: John Wiley and Sons; 2005. pp. 223–239. [Google Scholar]
- 23.Boudreau DM, Doescher MP, Jackson JE, Fishman PA. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38:1317–8. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- 24.Buist DS, LaCroix AZ, Brenneman SK, Abbott T. A population-based osteoporosis screening program: who does not participate, and what are the consequences? J Am Geriatr Soc. 2004;52:1130–7. doi: 10.1111/j.1532-5415.2004.52311.x. [DOI] [PubMed] [Google Scholar]
- 25.Taplin SH, Ichikawa L, Buist DS, Seger D, White E. Evaluating organized breast cancer screening implementation: the prevention of late-stage disease? Cancer Epidemiol Biomarkers Prev. 2004;13:225–34. doi: 10.1158/1055-9965.epi-03-0206. [DOI] [PubMed] [Google Scholar]
- 26.Washington State Department of Health, Center for Health Statistics 2003. Washington State Vital Statistics, 2002. [Accessed September 17, 2004];2003 Available at: http://www.doh.wa.gov/ehsphl/chs/chs-data/Public/AnnSum_2002.
- 27.Taplin SH, Thompson RS, Schnitzer F, Anderman C, Immanuel V. Revisions in the risk-based breast cancer screening program at Group Health Cooperative. Cancer. 1990;66:812–818. doi: 10.1002/1097-0142(19900815)66:4<812::aid-cncr2820660436>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Newton KM, Wagner EH, Ramsey SD, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol. 1999;52:199–207. doi: 10.1016/s0895-4356(98)00161-9. [DOI] [PubMed] [Google Scholar]
- 29.Hosmer DW, Jr, Lemeshow S. Applied survival analysis: regression modeling of time to event data. New York: John Wiley & Sons; 1999. [Google Scholar]
- 30.Hastie T, Tibshirani R, Friedman J, editors. The elements of statistical learning; data mining, inference, and prediction. New York: Springer; 2001. Chapter 5. [Google Scholar]
- 31.Prowell TM, Stearns V, Trock B. Lipophilic statins merit additional study for breast cancer chemoprevention. J Clin Oncol. 2006;24:2128–9. doi: 10.1200/JCO.2005.05.1649. author reply 2129. [DOI] [PubMed] [Google Scholar]
- 32.Sprague JR, Wood ME. Statins and breast cancer prevention: time for randomized controlled trials. J Clin Oncol. 2006;24:2129–30. doi: 10.1200/JCO.2005.05.5392. author reply 2130–1. [DOI] [PubMed] [Google Scholar]
- 33.Duncan RE, El-Sohemy A, Archer MC. Statins and cancer development. Cancer Epidemiol Biomarkers Prev. 2005;14:1897–8. doi: 10.1158/1055-9965.EPI-05-0027. [DOI] [PubMed] [Google Scholar]
- 34.Zielinski SL. Following positive epidemiologic studies, statins to enter clinical trials for cancer prevention. J Natl Cancer Inst. 2005;97:1172–3. doi: 10.1093/jnci/dji267. [DOI] [PubMed] [Google Scholar]
- 35.Boudreau DM, Rutter CM, Buist DS. The influence of statin use on breast density. Cancer Epidemiol Biomarkers Prev. 2006;15:1026–9. doi: 10.1158/1055-9965.EPI-05-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar AS, Campbell M, Benz CC, Esserman LJ. A call for clinical trials: lipophilic statins may prove effective in treatment and prevention of particular breast cancer subtypes. J Clin Oncol. 2006;24:2127. doi: 10.1200/JCO.2005.04.9882. author reply 2127–8. [DOI] [PubMed] [Google Scholar]