Abstract
Ovarian hormones play a role in the use of drugs of abuse in women. In female rats estradiol has been shown to enhance acquisition of cocaine self-administration and behavioral sensitization induced by repeated cocaine treatment. Experiments were conducted to determine the effects of estradiol and/or progesterone on cocaine self-administration and behavioral sensitization to cocaine (10 mg/kg; in animals with unilateral 6-hydroxydopamine lesions). Five groups of ovariectomized females were tested: 1) oil vehicle; 2) estradiol (E); 3) progesterone (P); 4) estradiol and progesterone given concurrently (EPC); and 5) estradiol and progesterone given sequentially (EPS 3 days of estradiol, 1 day progesterone, 1 day oil). All animals were tested during the dark phase of the light:dark cycle at ZT1600 and ZT2000-2100.
Behavioral Sensitization Results
There was substantial conditioned turning throughout the habituation periods, and all animals exhibited behavioral sensitization with repeated cocaine treatment. Multivariate analysis indicated a significant effect of hormone treatment, time of day and day of testing. When individual groups were compared, however, only at ZT1600 did the E-treated and the EPS-treated animals show a trend (p<0.06) for greater behavioral sensitization to cocaine relative to the oil-treated animals.
Self-Administration Results
All groups showed rapid acquisition of cocaine self-administration at 0.3 mg/kg/inf, so we did not see an effect of ovarian hormones on acquisition, or a difference between groups tested at ZT1600 vs. ZT2100 (p<0.005). There was, however, enhanced total intake of cocaine at 0.75 mg/kg/inf in the E and the EPS groups. Concurrent administration of progesterone with estradiol counteracted the effect of estradiol on cocaine intake at 0.75 mg/kg/inf, while progesterone alone did not enhance cocaine self-administration.
Keywords: Estradiol, progesterone, drug abuse, circadian rhythms, rats
Introduction
Abuse of cocaine by women has increased markedly in the last decade so that women now account for approximately 40% of regular cocaine users [34]. Women begin using cocaine at younger ages and enter treatment programs after shorter periods of drug use than men [54]. At the time they enter treatment, however, women are taking greater amounts of cocaine and have more severe psychological and physiological problems associated with drug use than do men seeking treatment [35, 47, 51].
Ovarian hormones are thought to play a significant role in the use of drugs of abuse in women. This is seen in the reported subjective effects of drugs, where women report a greater subjective response to cocaine [31, 83] and amphetamine (AMPH) [43, 44] in the late follicular phase of the menstrual cycle (when estradiol is elevated), compared to the luteal phase (when both estradiol and progesterone are elevated). The subjective effects of AMPH are also positively correlated with salivary estradiol levels and negatively correlated with progesterone levels [44]. Together, these results suggest that estradiol potentiates the subjective responses to psychomotor stimulants relative to the combined effects of estradiol with progesterone in women.
Sex differences and effects of ovarian hormones similar to those seen in humans are also seen in laboratory rats. Female rats acquire cocaine self-administration more rapidly than males [17, 53] and at lower doses of cocaine [40]. Furthermore, females self-administer more cocaine than males do, once both have acquired drug cocaine self-administration [40]. Cocaine self-administration in female rodents also varies across the estrous cycle [52]. When tested under a progressive ratio schedule of responding for cocaine, female rats demonstrate greater motivation to take cocaine by exhibiting higher breaking points (they will work harder for cocaine by making more operant responses) during behavioral estrus than will females in other phases of the cycle, and females work harder than male rats [71]. In contrast, sucrose self-administration does not vary across the estrous cycle [37]. Although estrous/menstrual cycle effects demonstrate the involvement of ovarian hormones, they do not identify which specific ovarian hormones are involved.
Estradiol administration to ovariectomized (OVX) females affects many psychostimulant drug-induced behaviors, including self-administration of cocaine [7, 36, 55, 57, 62, 65, 68, 81, 86]. In research from this laboratory [40] we found that exogenous estradiol treatment in OVX female rats was sufficient to facilitate acquisition of cocaine self-administration [40]. Estradiol-facilitated cocaine self-administration has also been shown in other studies [55, 71]. Estradiol also enhances behavioral sensitization to cocaine in OVX rats [39]. In contrast, in male rats estradiol does not enhance cocaine self-administration [42] or cocaine-induced behavioral sensitization [10].
As mentioned above, the subjective effects of psychomotor stimulant drugs are negatively correlated with salivary progesterone levels in women [44]. Furthermore, exogenous progesterone has been shown to attenuate the subjective effects of smoked cocaine in women [30]. In rodents, progesterone attenuates estradiol-enhancement of locomotor activity and sensitization of cocaine-induced stereotyped behavior, compared to OVX females treated with estradiol [65, 68]. Furthermore, progesterone counteracts the estradiol-induced enhancement of cocaine self-administration in OVX females [42]. When animals are tested for self-administration of cocaine and other drugs of abuse, animals are usually tested during the dark phase of the light:dark cycle since this is when they are naturally awake and active. When animals are tested for amphetamine or cocaine-induced behavioral sensitization we have typically tested animals during the light phase of the light dark cycle (e.g., [39]). The current studies were conducted to determine the effects of estradiol and/or progesterone on cocaine self-administration and behavioral sensitization to cocaine. So that the results of the studies could be readily compared all animals were tested during the dark phase of the light:dark cycle. Unexpectedly, we found an effect of the time of testing on behavioral sensitization to cocaine, but not cocaine self-administration.
Materials and Methods
Animals
Female Sprague Dawley rats (Harlan, Indianapolis, IN), weighting 250-275 grams at the start of the experiment, were housed 2-3 per cage for rotational behavior tests, and 1 per cage for the self-administration tests, under a 14:10 hour light:dark cycle (by definition lights go on at ZT000, the dark period began at 8:00AM or ZT1400). Most neuroendocrine experiments have typically utilized a 14:10 light:dark cycle for hormone analysis [14, 38], and for that reason the colonies are maintained on this light schedule. Animals were housed in a room maintained at a constant temperature of 20-21°C, with phytoestrogen-free rodent chow (2014 Tekad Global, 14% protein rodent maintenance diet, Harlan rat chow; Harlan Teklad, Madison, WI) and water available ad libitum. All procedures were performed according to a protocol approved by the University of Michigan Committee for Use and Care of Animals. The number of subjects for each experimental group is given in the figures.
Experiment 1. Sensitization of Cocaine-Induced Rotational Behavior
Sensitization to cocaine was determined in animals with a unilateral 6-hydroxydopamine (6-OHDA) lesion of the nigrostriatal dopamine (DA) pathway as rotational behavior induced by cocaine with this model is linearly related to the dose of cocaine and to the effects of sensitization, while locomotor behavior or stereotyped behaviors exhibit qualitative as well as quantitative changes with dose and sensitization [21]. This method has been used routinely to demonstrate sex differences and effects of ovarian hormones on behavioral sensitization to psychomotor stimulants [12, 15, 39, 73].
Unilateral 6-OHDA Lesion
Approximately 1 week after arrival, animals received unilateral 6-OHDA lesions of the nigrostriatal DA pathway according to the following protocol. Animals were anesthetized with ketamine (45 mg/kg, i.p.) and medetomidine (0.3 mg/kg, i.p.), and then received pargyline (35 mg/kg, i.p.) to inhibit monoamine oxidase metabolism of 6OHDA and desipamine (15 mg/kg, i.p.) to protect noradrenergic cells. Lesion coordinates measured from bregma, skull flat, were as follows (left/right random): posterior 5.0 mm; lateral 2.0 mm; and ventral 7.7 mm. Thirty minutes after receiving pargyline and desipramine, a solution containing 8.26 μg 6-OHDA hydrobromide (2 μg/ul in 0.1mg/ml L-ascorbic acid in 0.9% sterile saline) was infused into the unilateral substantia nigra through a 29 gauge cannula at a rate of 0.5μl/min for 8 minutes and 16 seconds (for a total infusion of ∼4.13μl). The infusion cannula was left in place for additional 2 minutes, to allow the infusate to disperse, before being slowly raised.
Ovariectomy (OVX)
About 2 weeks after unilateral 6-OHDA lesion, all rats underwent bilateral OVX [39]. OVX was conducted using a dorsal approach. The skin was opened with an incision ∼1cm long along the midline just below the ribs, and a small incision ∼0.5cm is made through the muscle ∼1.5-2cm lateral to the midline. The ovary was externalized with blunt forceps, and the tissue between the ovary and uterus was clamped with a hemostat. The ovary was removed, and the hemostat remained in place until there was no bleeding when it was released. The uterus with associated tissue was then returned to the abdomen. The procedure was repeated on the other side, and the wound was closed with 11 mm wound clips. After 7 days of recovery, all animals underwent vaginal lavage testing daily for 10 consecutive days to confirm cessation of cycling.
Rotational Behavior Testing
Four weeks after 6-OHDA lesion, all the rats underwent behavioral testing during the dark period (ZT1400 – ZT2400). Animals were assigned to the following 5 groups: 1) OIL (0.1 ml peanut oil vehicle); 2) E (1 μg, 2 μg or 5 μg 17β-estradiol in 0.1 ml peanut oil; there were no differences among the groups and so data from these animals were combined). In an independent study, serum estradiol concentrations were determined from trunk blood 1 hr after hormone injection according to a previously published method [29]; pg/ml ± SEM were - 1 μg: 245±52, 2 μg: 408±59, 5 μg: 1146±108 (n=4-5/group); 3) P (125 μg or 500 μg progesterone, progesterone doses based on [12, 42]; there was no effect of dose of P, so data from these groups were combined); 4) 5 μg E with 125 μg or 500 μg P given concurrently (EPC) and 5) 5 μg E with P sequential (5 μg E for 3 days, 125 μg or 500 μg P on day 4, oil on day 5; EPS). As illustrated in Figure 1, animals were placed into the rotometers to habituate for 30 minutes (Habituation), then they received hormone or oil treatment according to their group assignment (Hormone/Oil), thirty minutes later (the time when we see behavioral and neurochemical effects of estradiol [e.g., 10, 12]) half of the rats in each group received cocaine (10 mg/ kg) and the other half received saline as a control (Cocaine/Saline). Rotational behavior induced by cocaine/ saline was measured for 60 minutes on 5 consecutive days followed by 2 days off per week for 3 weeks for a total of 15 test sessions, using an automated system previously described in detail [39, 58]. In each condition, half of the rats were tested in the first half of the dark period at ZT1600 hours, and the other half of the rats were tested at ZT2000. Times were selected to allow two waves of animals to be tested each day, not anticipating an effect of the time of testing. Rotational behavior was recorded in the habituation, hormone and cocaine/saline periods.
Figure 1.
Schematic of behavioral testing protocol for sensitization experiments. This protocol was followed daily for 5 days each week, for 3 weeks.
Striatal Dopamine (DA) Determination
Three weeks after the last sensitization day, all rats were decapitated. Their striata on each side were removed rapidly. The two striatal samples were each weighed and then homogenized in 400μl ISS (internal standard solution containing 200μg/L dihydroxybenzylamine as internal standard with 1.6 mmol/L ethylenediaminetetraacetic acid, 4 mmol/L Na2S2O5 in 0.05 N HClO4). These samples were then centrifuged at 3,500 g for 15 min at 4°C, and then filtered through 0.2 μm filters and transferred to autosampler vials for DA analysis using HPLC with electrochemical detection (Coulochem II; ESA, Waltham, MA), as described previously [9]. Percentage DA depletions were calculated by subtracting the concentration of DA per milligram of wet tissue weight on the lesion side from the value on the intact side. This result is divided by the value on the intact side, providing a percentage depletion of DA. Rats whose percentage depletion was <96% were eliminated from the study [18, 75].
Statistical Analyses
The total number of net rotations (Net Full Turns) in each testing period were calculated as the sum of the number of rotations contralateral to the intact striatum during each period minus the number of rotations in the other direction for each rat. All data were analyzed by multivariate analysis with factors using SPSS.
Experiment 2. Cocaine Self-Administration
Ovariectomy
Approximately 1 week after arrival, all rats underwent bilateral OVX as described above.
Catheter Implantation
Approximately 2–3 weeks after OVX, rats were prepared with indwelling intravenous jugular catheters connected to a backport. Jugular catheter construction and implantation were based on procedures described previously [20, 40, 88]. Briefly, catheters were constructed by gluing Silastic tubing (0.51mm ID, 0.94mm OD; Dow Corning, Midland, MI) to an external guide cannula. The cannulae were then glued to polypropylene mesh with cranioplastic cement. Rats were anesthetized with a combination of ketamine (45 mg/kg, i.p.) and medetomidine (0.3 mg/kg, i.p.). The free end of the Silastic tubing was inserted into the right jugular vein and secured with 4.0 silk sutures around the venous tissue. The catheters exited dorsally on the animal's back. Dummy stylets were inserted into the catheters when rats were not connected to infusion pumps. Catheters were flushed daily with 0.1 ml heparinized saline (30 U/ml, in 0.9% sterile saline buffered at pH 7.4) and a 0.1 ml gentamicin solution (0.8 mg/ml) to prevent occlusions and to circumvent microbial buildup in the catheter. For each self-administration session, catheters were flushed with 0.1 ml of heparin before sessions began and with 0.1 ml gentamicin after each session.
Self-Administration
5 days after recovery from catheter surgery, rats were placed into standard operant chambers (Med Associates, Inc., Georgia, VT) and were allowed to nose poke to obtain an i.v. infusion of cocaine on an FR1 schedule of reinforcement during a 60 min session each day. Animals were not pre-trained to nose poke.
Animals were connected to an infusion syringe and tethered via a steel cable to a swivel, which was mounted on a counterbalanced arm. This allowed the animal to move freely in the test cage. Each response in the active hole produced a compound stimulus consisting of a white stimulus light, a tone (85 dB), and an intravenous injection of 50 _l of cocaine-HCl in saline delivered over 2.8 s. The compound stimulus occurred simultaneously with the cocaine injection and was followed by a 5 sec time-out period, during which time further responses had no programmed consequences but nose pokes were recorded. Nose pokes in the inactive hole were also recorded, but had no programmed consequences.
Animals were assigned to the following 5 groups: 1) OIL (oil vehicle); 2) 5 μg estradiol benzoate (EB); 3) 500 μg progesterone (P); 4) 5 μg EB with 500 μg P concurrent (EBPC) and 5) 5 μg EB with 500 μg P sequential (EB for 3 days, P on day 4, oil on day 5; EBPS). Half of the rats in each group were tested during the dark phase of the cycle at ZT1600 hours and the other half were tested at ZT2100 hours. Thirty minutes prior to each self-administration session, rats received an injection of hormone or vehicle in 0.1 ml peanut oil (s.c.). For the 1st 5 days of testing, responses at the ‘active’ hole resulted in the administration of 0.3 mg/kg infusion of cocaine HCl. The animals were then given 2 days off, during which time hormones were not administered. During the 2nd 5 days of testing, the dose was raised to 0.4 mg/kg per infusion for 5 days followed by 2 days off. During the 3rd week, there were 5 days of self-administration testing with 0.5 mg/kg cocaine per infusion were available. After another 2 days off, the dose was raised to 0.75 mg/kg per infusion for the last 5 days of testing. To prevent an overdose of cocaine, during each testing session the maximum number of infusions an animal could receive was 50. Thus, after 50 infusions, the computer program would automatically shut down the infusion system, but would still record the active and inactive nose pokes. Only the data from the rats that survived all 4 weeks of testing are reported here.
Statistical Analyses
The total number of infusions, as well as active and inactive nose pokes was recorded for each testing. All data were analyzed by multivariate analysis with factors using SPSS.
Results
Experiment 1: Sensitization of Cocaine-Induced Rotational Behavior
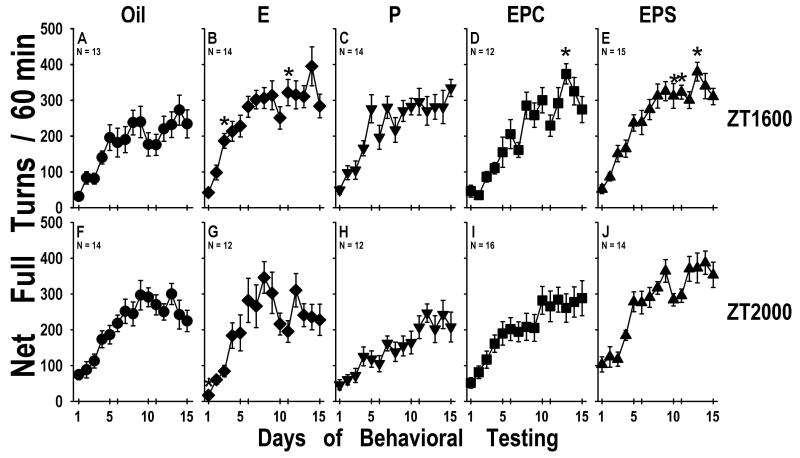
As illustrated in Figure 4, when all groups were analyzed by multivariate anlaysis there was a significant effect of treatment group, time of day and day of testing (details of statistics are below). Nevertheless, on individual comparisons there was only a trend (p<0.06) for the E-treated animals and the EPS-treated animals to show greater sensitization to cocaine than the oil-treated animals at ZT1600, and there were no individual group differences in the effect of hormones on behavioral sensitization at ZT2000. There was a substantial amount of conditioned turning during habituation (Figures 2, 5) and again after s.c. or i.p. injections of hormone/oil (Figures 3, 6) or saline (Figure 7) in animals tested during the dark phase of the light:dark cycle. Animals tested at ZT1600, in general, exhibited greater conditioned turning than those tested at ZT2000. The EPS group exhibited greater conditioned turning than all other groups.
Figure 4.
Rotational behavioral induced by repeated treatment with 10 mg/kg cocaine. Mean±SEM number of net rotations in cocaine treatment period for 60min during each of the 15 test sessions. A-E ZT1600 groups; F-J ZT2000 groups. Hormone treatment groups: A&F - Oil; E&G - E; C&H - P; D&I -EPC; E& J – EPS (N's are given for each group on the figure).
* indicates significantly different from Oil group (p<0.05).
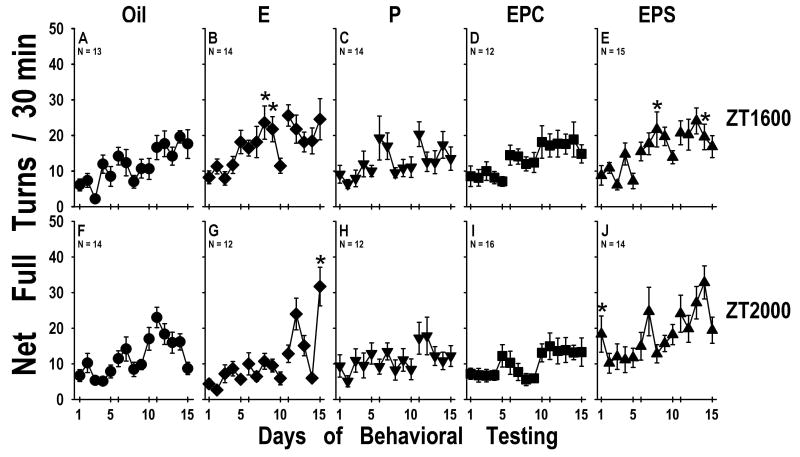
Figure 2.
Rotational behavioral during habituation period for animals that received cocaine during the test interval (mean±SEM number of net rotations) for first 30 min during each of the 15 test sessions. Animals were placed into chambers without treatment. A-E ZT1600 groups; F-J ZT2000 groups. Hormone treatment was given after this test interval: A&F - Oil; E&G -E; C&H - P; D&I - EPC; E& J – EPS (Group abbreviations defined in the text; N's are given for each group on the figure).
* indicates significantly different from Oil group (p<0.03).
Figure 5.
Rotational behavioral during habituation period for animals that received saline during the test interval (mean±SEM number of net rotations) for first 30 min during each of the 15 test sessions. Animals were placed into chambers without treatment. A-E ZT1600 groups; F-J ZT2000 groups. Hormone treatment was given after this test interval: A&F - Oil; E&G -E; C&H - P; D&I - EPC; E& J – EPS (Group abbreviations defined in the text; N's are given for each group on the figure).
* indicates significantly different from Oil group (p<0.05).
Figure 3.
Rotational behavioral following hormone or vehicle treatment for animals that received cocaine during the test interval (mean±SEM number of net rotations) for 30 min during each of the 15 test sessions. Animals received an injection s.c. prior to this test period. A-E ZT1600 groups; F-J ZT2000 groups. Hormone treatment: A&F - Oil; E&G E; C&H - P; D&I - EPC; E& J – EPS (N's are given for each group on the figure).
* indicates significantly different from Oil group (p<0.05).
Figure 6.
Rotational behavioral following hormone or vehicle treatment for animals that received saline during the test interval (mean±SEM number of net rotations) for 30 min during each of the 15 test sessions. Animals received an injection s.c. prior to this test period. A-E ZT1600 groups; F-J ZT2000 groups. Hormone treatment: A&F - Oil; E&G E; C&H - P; D&I - EPC; E& J – EPS (N's are given for each group on the figure).
*indicates significantly different from Oil group (p<0.05).
Figure 7.
Rotational behavioral induced by repeated treatment with 1ml/kg 0.9% saline. Mean±SEM number of net rotations for 60 min during each of the 15 test sessions. A-E ZT1600 groups; F-J ZT2000 groups. Hormone treatment: A&F - Oil; E&G - E; C&H - P; D&I - EPC; E& J – EPS (N's are given for each group on the figure).
*indicates significantly different from Oil group (p<0.05).
Cocaine-Treated Rats (Figures 2-4)
Habituation Period (Figure 2)
During the habituation period, at ZT1600 and ZT2000, animals exhibited conditioned turning behavior (F[1,14]=16.88, p<0.0001). Previous hormone treatment significantly affected the amount of turning seen during the habituation periods in the cocaine-treated animals (Hormone F[1,4]=5.50, p<0.0001) and there was a Day X Hormone interaction (F[4,56]=2.074, p<0.0001). When individual groups were analyzed, only the P treated animals (Fig. 2C and 2H), at both test times, and the EPC at ZT2000 (Fig. 2I) did not show conditioned turning. At ZT1600, the EPS (Fig. 2E) rats showed significantly greater conditioned turning behavior in the Habituation Period, relative to the Oil-treated group (Fig. 2A; p<0.03). The ZT2000 EPC rats (Fig. 2I) exhibited significantly less conditioned turning than did EPS rats at this testing time (Fig. 2J; p<0.009).
Hormone Treatment Period (Figure 3)
Animals at both testing times continued to exhibit conditioned turning behavior during the period after hormone treatment (F[1,14]=18.66, p<0.0001). There was a main effect of hormone treatment (F[1,4]=3.35, p<0.012) and a day X hormone interaction (F[4,56]=2.57, p<0.0001). When individual groups were compared, P treated animals at ZT2000 (Fig. 3H), and the EPC (ZT2000; Fig. 3I), again, did not show conditioned turning. The ZT2000 EPC rats (Fig. 3I) exhibited significantly less conditioned turning than did EPS rats at this testing time (Fig. 3J; p<0.015).
Cocaine Treatment Period (Figure 4)
Animals in all groups, at both testing times, exhibited sensitization of cocaine-induced turning behavior. There was a main effect of day of testing (F[1,14]=62.66, p<0.0001), a main effect of hormone treatment F[1,4]=5.44, p<0.0001), a hormone X time of day effect (F[1,4]=2.90, p<0.025) and day X hormone interaction (F[4,56]=1.92, p<0.001). On analysis of individual group effects, P treated animals exhibited more cocaine-induced turning behavior at ZT1600 than at ZT2000 (p<0.01; Fig. 4C vs 4H).
Contrary to what has been seen when animals were tested during the light phase of the day [39], comparisons between Oil and hormone treatment 30 min earlier did not find dramatic effects on cocaine-induced turning behavior. At ZT1600 E (Fig. 4B; p=0.054) and EPS (Fig. 4E; p=0.06) tended to exhibit more cocaine-induced turning throughout the test period than oil treated animals (Fig. 4A), but the effect was not as robust as in our previous report. The ZT2000 P animals (Fig. 4H) exhibited significantly less turning than did the EPS animals (Fig. 4J; P<0.0001). There were no other differences among the cocaine-treated groups.
Saline-Treated Rats (Figures 5-7)
Habituation Period (Figure 5)
Animals in all saline-treated groups, at both testing times, exhibited conditioned turning behavior during the habituation period (F[1,14]=11.57, p<0.0001). Previous hormone treatment and time of day significantly affected the amount of turning seen during the habituation periods in the saline-treated animals (Hormone X Time F[1,4]=4.59, p<0.002; Day X Hormone F[4,56]=2.02, p<0.0001). The ZT1600 EPC animals (Fig. 5D) exhibited significantly more turning than did the P rats at that time of testing (Fig. 5C; p<0.03), and the ZT2000 EPS rats (Fig. 5J) exhibited more turning than all other groups throughout the experiment (p<0.015).
Hormone Treatment Period (Figure 6)
Animals in all saline-treated groups, at both testing times, exhibited conditioned turning behavior during the period after hormone treatment (F[1,14]=16.58, p<0.0001). There was a significant hormone X time interaction (F[1,4]=6.51, p<000.1) and a significant day X hormone interaction F[4,56]=1.39, p<0.035). When individual groups of animals were compared over time, P rats at ZT1600 (Fig. 6C) as well as the Oil (Fig. 6F) and E (Fig. 6G) rats at ZT2000 did not exhibit conditioned turning across the 15 test days. The ZT1600 EPC animals (Fig. 6D) exhibited significantly more turning than did the P rats at this time of day (p<0.025), and the ZT2000 EPS rats (Fig. 6J) exhibited more turning than all other groups throughout the experiment (p<0.009).
Saline Treatment Period (Figure 7)
Animals in all groups, at both testing times, exhibited conditioned turning behavior during the period following saline treatment (F[1,14]=24.44, p<0.0001). Interestingly, hormone treatment 30 min earlier significantly affected the amount of turning seen in the saline-treated animals (Hormone X Time F[1,4]=6.20, p<0.0001; Day X Hormone F[4,56]=1.09, p=0.310). When individual groups were analyzed separately, the ZT2000 E (Fig. 7G) treated rats did not exhibit conditioned turning. The ZT2000 EPS group (Fig. 7J) exhibited more turning than the other groups throughout the experiment (p<0.03).
Cocaine vs. Saline Treatment
Habituation Period
The amount of conditioned turning seen during the habituation period was affected by whether animals received saline vs. cocaine during the test periods (Fig. 2 vs. Fig. 5), as animals that had received cocaine exhibited greater conditioned turning on average (Main effect of saline vs. cocaine F[1,1]=15.11, p<0.0001). Time of testing (F[1,1]=5.89, p<0.015) and hormone treatment (F[1,4]=9.07, p<0.0001) were also factors, as animals tended to turn less at ZT2000, and as discussed above, the EPS group exhibited greater conditioned turning than some of the other groups.
Hormone Treatment Period
The amount of conditioned turning seen during the habituation period was similarly affected by whether animals received saline or cocaine in the test period (F[1,1]=25.09, p<0.0001). Time of testing (F[1,1]=4.08, p<0.210) was not a factor, but there was a main effect of hormone treatment (F[1,4]=6.16, p<0.0001), and a hormone X time interaction (F[1,4]=1.60, p<0.003).
Saline/Cocaine Treatment Period
Animals exhibited greater turning following cocaine treatment than they did after saline treatment, as would be expected (F[1,1]=962.41, p<0.0001). There were also effects of hormone treatment and interactions among hormone treatment and time of testing as well as drug (Effect of Hormone F[1,4]=0.002; Hormone X Time F[1,4]=2.72, p<0.03; Drug X Hormone F[1,4]=4.16, p<0.003).
Summary
Behavioral sensitization to cocaine in OVX female rats tested during the dark phase of the cycle was affected by hormone treatment as indicated by the overall analysis, but group comparisons did not indicate a significant effect of treatment with individual hormone protocols. The strongest effects were seen with E and EPS treatment where animals showed the greatest cocaine-induced turning at ZT1600, relative to Oil treated controls. In general, animals turned less at ZT2000, but only for the P treated group was cocaine-induced turning significantly greater at ZT1600 than at ZT2000 (p<0.01). When examining the results of the saline-treated rats, the EPS group at ZT2000 exhibited more turning than at ZT1600 during all conditions, and more turning than all other groups throughout the experiment.
Experiment 2. Cocaine Self-Administration
All groups exhibited acquisition of cocaine self-administration during the first week of testing, so effects of hormone treatment on acquisition were not observed. There were no effects of time of testing on cocaine self-administration, so the groups tested at ZT16000 and ZT2100 were combined for ease of presentation.
Number of Infusions Received
As illustrated in Figure 8, in the first and second weeks of testing (dose of cocaine was 0.3 or 0.4 mg/kg/infusion, respectively), the hormone treatments did not affect the number of infusions of cocaine received. In the third week of testing, when the dose of cocaine was 0.5 mg/kg/infusion, there was a trend for an effect of hormone treatment on the number of infusions of cocaine received, as the EBPS rats received more infusions than did the Oil-treated group (F[1,4]=2.37, P=0.06; Figure 8A). In the fourth week of testing, when animals received 0.75 mg/kg/infusion, there was a main effect of hormone on the number of infusions received (F[1,4]=4.65, p<0.002). During this week, both the EB and EBPS rats received more infusions of cocaine than the Oil-treated group did (p<0.05).
Figure 8. Self-Administration of Cocaine.
A. Mean±SEM number of infusions of cocaine during each 60 min session. The dose of cocaine increased each week as indicated on the figure.
* indicates significantly different from Oil group (p<0.05).
B. Total Cocaine Received each week (mean+SEM) for the 5 groups tested.
* indicates significantly different from Oil group (p<0.05).
Total Cocaine Received
During the third and fourth weeks of testing hormone treatment influenced the amount of cocaine received (Fig. 8B). We found that EB enhanced cocaine intake at 0.75 mg/kg/inf (F[1,4]=4.67, p<0.002). In addition, EBPS enhanced cocaine intake at 0.5 (p<0.056) and 0.75 mg/kg/inf (p<0.005), relative to Oil-treated controls. There were no other differences among the groups.
Discussion
In the present study we found that in OVX rats, treatment with estradiol (the EB group), or estradiol followed by progesterone (the EBPS group) enhanced total cocaine intake at relatively high doses of cocaine. We did not, however, see an effect of hormone treatment on acquisition of cocaine self-administration, as all groups acquired the behavior as rapidly as is possible. In addition, we found that concurrent administration of progesterone with estradiol (as in the EBPC group) counteracted the effect of estradiol on cocaine intake at 0.75 mg/kg/inf, while progesterone alone did not enhance or inhibit cocaine self-administration. These results support and extend our previous finding that concurrent progesterone suppresses the estradiol-induced enhancement of cocaine self-administration [42]. Thus, the effect of progesterone on total cocaine intake during self-administration depends on whether and when progesterone is delivered relative to estradiol.
We have previously hypothesized that the effect of estradiol and progesterone on cocaine self-administration could be mediated by the development of behavioral sensitization. As we showed again here, repeated exposure to cocaine results in behavioral sensitization. Estradiol has been shown to facilitate the development of behavioral sensitization and associated changes in DA when animals are tested during the light part of the light cycle [39, 65]. Intriguingly, we saw only a trend for an effect of estradiol or estradiol followed by progesterone to enhance behavioral sensitization to cocaine, and this was seen only early during the dark phase of the cycle at ZT1600. AT ZT2000 there was no effect of estradiol or estradiol followed by progesterone on behavioral sensitization to cocaine. We conclude that under these conditions, the effects of estradiol on cocaine self-administration vs. behavioral sensitization to cocaine are dissociable, and/or that self-administration is more sensitive to hormonal influences than is behavioral sensitization. Other groups have shown a dissociation of the psychomotor sensitizing effects of cocaine or heroin from self-administration behavior, and self-administration seems to be a more sensitive measure than is behavioral sensitization, at lease in some paradigms [2, 48]
Previous reports from this laboratory have found that estradiol enhances behavioral sensitization to cocaine [39], as well as acquisition of cocaine self-administration [40]. Methodological differences may have produced the in differences in findings across studies. One crucial difference between the cocaine-induced sensitization of rotational behavior in this study, and the results of Hu and Becker [39] is that the present study was done during the dark phase of the light:dark cycle, whereas the previous study was done during the light phase of the rats' light:dark cycle. Sensitization to cocaine has been shown to be influenced by circadian rhythms in recent research in drosophila, mice and rats [1, 3, 56, 82, 85]. For example, a recent study compared cocaine sensitization in male rats at five different ZTs and found an effect of time of day on long-term sensitization [82]. These authors found the greatest sensitization when male rats were tested shortly after lights off when animals were maintained on a 12:12 light:dark cycle [82]. Self-administration of cocaine may also be affected by the time of testing. Another study found that male rats will self-administer at lower doses of cocaine immediately after lights off, and this effect was not due to circadian variation in the pharmacokinetics of cocaine metabolism [6]. So, at least in males, the greatest responses to cocaine are seen early during the dark phase of the light-dark cycle.
In female rats, there are interactions between the hormones of the estrous cycle and circadian cycles. For example, estradiol shortens the period of the circadian activity rhythm and advances the onset of a free-running period [61]. Progesterone, however, antagonizes the effect of estradiol [76]. In addition, estradiol regulates expression of clock-related genes in the brain [64]. It is probably not too surprising that the effects of ovarian hormones on behavioral activation induced by cocaine vary with the time-of-day.
It should be noted that there was considerably more conditioned turning in this experiment compared with Hu and Becker [39], which we believe is due to the difference in the time of the animal's subjective day when testing occurred. Animals are more active during the dark phase of the cycle, and this circadian rhythm of activity may have interacted with spontaneous activity induced by the novel environment to enhance conditioned rotational behavior in the testing apparatus. It is also possible that the endogenous activity rhythm competes with cocaine-induced rotational behavior and thereby modulates behavioral sensitization to cocaine in a time dependent way. Estradiol and progesterone could influence behavioral sensitization by exerting effects on either the neural systems mediating behavioral sensitization or the systems mediating circadian rhythms or both.
During the second and third weeks of cocaine-induced rotational behavior testing there was no additional behavioral sensitization in this study, while in the previous study animals continued to exhibit enhanced rotational behavior through the second week and into the third week of testing with 10 mg/kg cocaine (compare Figure 3 in [39] with Figure 4 in this study). We know from the results of experiments conducted by Robinson and colleagues that the context in which male rats are exposed to cocaine or amphetamine can influence whether or not sensitization occurs [4, 5, 13, 19, 74]. The data presented here suggest that environment is also important for female rats, and that an environment that elicits spontaneous activity for females will enhance conditioned turning and diminish cocaine-induced sensitization, even after an hour in the chamber prior to cocaine. This is also different from the results of Crombag et al [19] who found that an hour of habituation in the test chamber was sufficient to prevent conditioned turning behavior in male rats. There are, of course, a number of things that are different about the two studies: males vs. female rats, testing during the dark phase of the cycle vs. the light phase, and in the present studies animals received an injection of hormone or vehicle 30 min after being placed in the chambers. All of these factors are likely contributors to the conditioned rotational behavior reported here. More importantly, this conditioned rotational behavior was also shown to be influenced by ovarian hormone and the time of day. If conditioned rotational behavior antagonized cocaine induced rotational behavior, it is very possible that the effect of ovarian hormone and the time of day on conditioned rotational behavior also contribute to the development of cocaine sensitization.
The facilitating effect of estradiol on cocaine intake is consistent with the results from a number of previous studies reporting that estradiol facilitates the psychomotor activating and rewarding effects of psychomotor stimulant drugs [8, 11, 39, 52, 55, 71, 80]. This effect of estradiol may be mediated by its well-characterized actions on DA neurotransmission [22, 23, 49, 50, 63, 72, 84, 90, 91]; see [40] for further discussion). It is possible that the failure to see an effect was not due to a lack of an effect of estradiol at the lower doses, but because animals were still acquiring the self-administration behavior. Future studies need to randomly vary the dose of cocaine after self-administration behavior has stabilized to determine if this is the case.
Of particular interest here is the finding that progesterone counteracted the effect of estradiol. The general idea that progesterone can inhibit behaviors previously activated by estradiol is not novel. For example, the activation of progesterone receptors in sexually receptive females is known to inhibit sexual receptivity [70]. Given that motivated behaviors, such as sexual behavior and drug-taking behavior are mediated by overlapping neural systems [32, 33, 46], including DA systems [59, 66, 67, 87], perhaps it is not surprising that progesterone has inhibitory effects on cocaine self-administration behavior. Furthermore, several recent publications have postulated that progesterone attenuates cocaine-induced activation [42, 77, 79]. OVX rats that received chronic estradiol and progesterone administration had both a lower frequency of cocaine-induced stereotyped and locomotor behaviors and lower amphetamine-stimulated DA release from striatal slices in vitro compared with OVX rats that received estradiol alone [65]. In addition, progesterone given concurrently with estradiol prevents the enhancement of cocaine self-administration seen with estradiol [42]. Thus, progesterone may antagonize the facilitatory effect of estradiol on self-administration by antagonizing incentive sensitization.
Importantly, progesterone can both enhance and antagonize estradiol's enhancement of DA. For example, 30 min after progesterone was administered to estradiol-primed rats, amphetamine-stimulated DA release from striatal tissue in vitro was enhanced. However, 24 hours after progesterone administration, amphetamine-stimulated DA release from striatal tissue in vitro was inhibited [25]. This effect of progesterone has been demonstrated in a series of studies by Dluzen and Ramirez [24-28, 69].
Finally, the effects of estradiol and progesterone on the DA system could be mediated indirectly through their effects on other neurons. For example, we have shown that EB inhibits L-type calcium current on medium spiny GABAergic neurons in striatum [60] and we have recently shown that estradiol rapidly inhibits KCl-stimulated GABA overflow in dialysate from striatum [41], while progesterone and its metabolites facilitate GABA activity at the GABA-A receptors [78]. Thus, estradiol and progesterone may act by modulating GABAergic release and GABA receptor activity, thereby modulating dopaminergic function indirectly. Since mesotelencephalic DA systems are thought to mediate reward [89], it is likely that the ability of estradiol to facilitate DA activity is related to estradiol's facilitation of cocaine self-administration behavior in females. Since these effects of estradiol and progesterone vary with the time of testing and the behavioral test employed, ovarian hormones are just one factor that deserves to be considered.
Given the influence of ovarian hormones on responses to cocaine and other drugs of abuse [16], it is important to understand the mechanisms by which these hormones exert their influence. Inherent sex differences in brain organization and sex differences in the effects of circulating ovarian hormones are likely to be important contributing factors to a women's likelihood of becoming addicted. Effects of circulating ovarian hormones may facilitate the acquisition of a cocaine habit for women, while organizational brain differences may keep women vulnerable. Because the likelihood of developing lifetime cocaine dependence is greater for women than men [45], incorporating considerations of gender, hormonal status, and circadian rhythms are critical to designing appropriate prevention, intervention and treatment strategies for women.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse DA012677 to JBB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):9026–30. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:3. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- 3.Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacology, Biochemistry & Behavior. 2004;79(1):37–42. doi: 10.1016/j.pbb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. Journal of Neuroscience. 1998;18(24):10579–93. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badiani A, Oates MM, Fraioli S, Browman KE, Ostrander MM, Xue CJ, Wolf ME, Robinson TE. Environmental modulation of the response to amphetamine: dissociation between changes in behavior and changes in dopamine and glutamate overflow in the rat striatal complex. Psychopharmacology. 2000;151(23):166–174. doi: 10.1007/s002139900359. [DOI] [PubMed] [Google Scholar]
- 6.Baird TJ, Gauvin D. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacology, Biochemistry & Behavior. 2000;65(2):289–99. doi: 10.1016/s0091-3057(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 7.Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacology Biochemistry and Behavior. 1999;64(4):803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 8.Becker JB, Cha J. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- 9.Neurochemical correlates of behavioral changes following intraventricular adrenal medulla grafts: intraventricular microdialysis in freely moving rats. In: Becker JB, Freed WJ, editors; Gash DM, Sladek JR, editors. Progress in Brain Research. Vol. 78. Elsevier; New York: 1988. pp. 527–533. [DOI] [PubMed] [Google Scholar]
- 10.Becker JB, Molenda HA, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine: implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 11.Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80(1):65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- 12.Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: A microdialysis study. Pharmacology Biochemistry and Behavior. 1999;64(1):53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 13.Browman KE, Badiani A, Robinson TE. Environmental influences on the psychomotor response to intravenous cocaine: a dose-effect investigation. Soc Neurosci Absts. 1996;22:926. [Google Scholar]
- 14.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol -17-β throughout the 4-day estrous cycle of the rat. Endocrinol. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 15.Camp DM, Becker JB, Robinson TE. Sex differences in the effects of gonadectomy on amphetamine-induced rotational behavior in rats. Behav Neural Biol. 1986;46(3):491–495. doi: 10.1016/s0163-1047(86)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Carroll M, Lynch W, Roth M, Morgan A, Cosgrove K. Sex and estrogen influence drug abuse. Trends in Pharmacological Sciences. 2004;25(5):273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161(3):304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- 18.Castañeda E, Becker JB, Robinson TE. The long-term effects of repeated amphetamine treatment in vivo on amphetamine, KCl and electrical stimulation evoked striatal dopamine release in vitro. Life Sci. 1988;42:2447–56. doi: 10.1016/0024-3205(88)90343-8. [DOI] [PubMed] [Google Scholar]
- 19.Crombag HS, Badiani A, Chan J, Dell'Orco J, Dineen SP, Robinson TE. The ability of environmental context to facilitate psychomotor sensitization to amphetamine can be dissociated from its effect on acute drug responsiveness and on conditioned responding. Neuropsychopharmacology. 2001;24(6):680–690. doi: 10.1016/S0893-133X(00)00238-4. [DOI] [PubMed] [Google Scholar]
- 20.Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behavioural Brain Research. 2000;116(1):1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- 21.Crombag HS, Mueller H, Browman KE, Badiani A, Robinson TE. A comparison of two behavioral measures of psychomotor activation following intravenous amphetamine or cocaine: dose- and sensitization-dependent changes. Behavioural Pharmacology. 1999;10(2):205–213. doi: 10.1097/00008877-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Di Paolo T, Falardeau P, Morissette M. Striatal D-2 dopamine agonist binding sites fluctuate during the rat estrous cycle. Life Sci. 1988;43(8):665–672. doi: 10.1016/0024-3205(88)90137-3. [DOI] [PubMed] [Google Scholar]
- 23.Di Paolo T, Rouillard C, Bedard P. 17 beta-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117(2):197–203. doi: 10.1016/0014-2999(85)90604-1. [DOI] [PubMed] [Google Scholar]
- 24.Dluzen DE, McDermott JL, Ramirez VD. Changes in dopamine release in vitro from the corpus striatum of young versus aged rats as a function of infusion modes of L-dopa, potassium, and amphetamine. Exp Neurol. 1991;112(2):153–60. doi: 10.1016/0014-4886(91)90065-k. [DOI] [PubMed] [Google Scholar]
- 25.Dluzen DE, Ramirez VD. Bimodal effect of progesterone on in vitro dopamine function of the rat corpus striatum. Neuroendocrinol. 1984;39(2):149–155. doi: 10.1159/000123971. [DOI] [PubMed] [Google Scholar]
- 26.Dluzen DE, Ramirez VD. Intermittent infusion of progesterone potentiates whereas continuous infusion reduces amphetamine-stimulated dopamine release from ovariectomized estrogen-primed rat striatal fragments superfused in vitro. Brain Res. 1987;406(12):1–9. doi: 10.1016/0006-8993(87)90762-1. [DOI] [PubMed] [Google Scholar]
- 27.Dluzen DE, Ramirez VD. Progesterone effects upon dopamine release from the corpus striatum of female rats. II. Evidence for a membrane site of action and the role of albumin. Brain Res. 1989;476(2):338–344. doi: 10.1016/0006-8993(89)91255-9. [DOI] [PubMed] [Google Scholar]
- 28.Dluzen DE, Ramirez VD. In vitro progesterone modulation of amphetamine-stimulated dopamine release from the corpus striatum of ovariectomized estrogen-treated female rats: response characteristics. Brain Res. 1990;517:117–122. doi: 10.1016/0006-8993(90)91016-a. [DOI] [PubMed] [Google Scholar]
- 29.England B, Parsons G, Possley R, McConnell D, Midgley A. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clinical Chemistry. 2002;48:1584–1586. [PubMed] [Google Scholar]
- 30.Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31(3):659–74. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- 31.Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- 32.Everitt BJ, Fuxe K, Hokfelt T. Inhibitory role for dopamine and 5-hydroxytryptamine in the sexual behavior of the female rat. Eur J Pharmacol. 1974;29:187–191. doi: 10.1016/0014-2999(74)90190-3. [DOI] [PubMed] [Google Scholar]
- 33.Everitt BJ, Fuxe K, Hokfelt T, Jonsson G. Role of monoamines in the control of hormones of sexual receptivity in the female rat. J Comp Physiol Psychol. 1975;89:556–572. doi: 10.1037/h0077430. [DOI] [PubMed] [Google Scholar]
- 34.Greenblatt J, Gfroerer J, editors. Self-reported problems associated with drug use. Analysis of substance abuse and treatment need issues. Substance Abuse and Mental Health Services Administration; Rockville, MD: 1998. [Google Scholar]
- 35.Griffin ML, Weiss RD, Lange U. A comparison of male and female cocaine abuse. Arch Gen Psychiatry. 1989;46:122–6. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- 36.Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiology & Behavior. 1997;61(5):755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- 37.Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Developmental Psychobiology. 1999;35(2):136–145. [PubMed] [Google Scholar]
- 38.Henderson SR, Baker C, Fink G. Oestradiol-17β and pituitary responsiveness to luteinizing hormone releasing factor in the rat: a study using rectangular pulses of estradiol-17β monitored by non-chromatographic radioimmunoassay. J Endocrinol. 1977;73:441–453. doi: 10.1677/joe.0.0730441. [DOI] [PubMed] [Google Scholar]
- 39.Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23(2):693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29(1):81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 41.Hu M, Watson C, Kennedy R, Becker J. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59:122–124. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- 42.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 43.Justice AJH, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145(1):67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- 44.Justice AJH, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacology Biochemistry and Behavior. 2000;66(3):509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 45.Kandel DB, Warner MPP, Kessler RC. In: The epidemiology of substance abuse and dependence among women, in Drug Addiction Research and the Health of Women. Wetherington CL, Roman AR, editors. U. S. Department of Health and Human Services; Rockville, MD: 1995. pp. 105–130. [Google Scholar]
- 46.Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. Journal of Neuroscience. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differeces in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–6. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- 48.Lenoir M, Ahmed S. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32(3):616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- 49.Levesque D, Di Paolo T. Rapid conversion of high into low striatal D2-dopamine receptor agonist binding states after an acute physiological dose of 17 beta- estradiol. Neurosci Lett. 1988;88(1):113–118. doi: 10.1016/0304-3940(88)90324-2. [DOI] [PubMed] [Google Scholar]
- 50.Levesque D, Gagnon S, Di Paolo T. Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci Lett. 1989;98:345–350. doi: 10.1016/0304-3940(89)90426-6. [DOI] [PubMed] [Google Scholar]
- 51.Lex BW. Some gender differences in alcohol and polysubstance users. Health Psychology. 1991;10(2):121–32. doi: 10.1037//0278-6133.10.2.121. [DOI] [PubMed] [Google Scholar]
- 52.Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152(2):132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- 53.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 54.Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 55.Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacology Biochemistry and Behavior. 2001;68(4):641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 56.Manev H, Uz T. Clock genes: influencing and being influenced by psychoactive drugs. Trends in Pharmacological Sciences. 2006;27(4):186–9. doi: 10.1016/j.tips.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Maren S, Decola JP, Swain RA, Fanselow MS, Thompson RF. Parallel Augmentation of Hippocampal Long-Term Potentiation, Theta-Rhythm, and Contextual Fear Conditioning in Water- Deprived Rats. Behavioral Neuroscience. 1994;108(1):44–56. doi: 10.1037//0735-7044.108.1.44. [DOI] [PubMed] [Google Scholar]
- 58.McFarlane DK, Martonyi BJ, Becker JB, Robinson TE. An inexpensive automated system for the quantification of rotational behavior in small animals. Soc Neurosci Abstr. 1990;16:754. [Google Scholar]
- 59.Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behavioral Neuroscience. 1995;109:354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- 60.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons through a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196(4287):305–7. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- 62.Morissette M, Di Paolo T. Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J Neurochem. 1993;60(5):1876–83. doi: 10.1111/j.1471-4159.1993.tb13415.x. [DOI] [PubMed] [Google Scholar]
- 63.Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58(1):16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. Journal of Neuroscience Research. 2005;82(5):622–30. doi: 10.1002/jnr.20677. [DOI] [PubMed] [Google Scholar]
- 65.Peris J, Decambre N, Coleman-Hardee M, Simpkins J. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated [3H]dopamine release. Brain Res. 1991;566:255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- 66.Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- 67.Phillips AG, Pfaus JG, Blaha CD. In: Dopamine and motivated behavior: insights provided by in vivo analyses, in The mesolimbic dopamine system: from motivation to action. Willmer P, Scheel-Kruger J, editors. John Wiley & sons Ltd; New York: 1991. pp. 199–224. [Google Scholar]
- 68.Quinones-Jenab V, Perrotti LI, Ho A, Jenab S, Schlussman SD, Franck J, Kreek MJ. Cocaine affects progesterone plasma levels in female rats. Pharmacology Biochemistry and Behavior. 2000;66(2):449–453. doi: 10.1016/s0091-3057(00)00213-6. [DOI] [PubMed] [Google Scholar]
- 69.Ramirez VD, Dluzen DE, Ke FC. Effects of progesterone and its metabolites on neuronal membranes. Ciba Found Symp. 1990;153(125):125–41. doi: 10.1002/9780470513989.ch7. [DOI] [PubMed] [Google Scholar]
- 70.Reading DS, Blaustein JD. The Relationship between Heat Abbreviation and Neural Progestin Receptors in Female Rats. Physiology & Behavior. 1984;32(6):973–981. doi: 10.1016/0031-9384(84)90288-9. [DOI] [PubMed] [Google Scholar]
- 71.Roberts DCS, Bennett SAL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- 72.Robinson TE, Becker JB. Behavioral sensitization is accompanied by an enhancement in amphetamine-stimulated dopamine release from striatal tissue in vitro. Eur J Pharmacol. 1982;85(2):253–4. doi: 10.1016/0014-2999(82)90478-2. [DOI] [PubMed] [Google Scholar]
- 73.Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res. 1982;253(12):231–241. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- 74.Robinson TE, Browman KE, Crombag HS. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- 75.Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- 76.Rodier Wr. Progesterone-estrogen interactions in the control of activity-wheel running in the female rat. J Comp Physiol Psychol. 1971;(74):365–73. doi: 10.1037/h0030568. [DOI] [PubMed] [Google Scholar]
- 77.Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120(2):523–33. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 78.Schumacher M, Coirini H, McEwen BS. Regulation of high-affinity GABAA receptors in the dorsal hippocampus by estradiol and progesterone. Brain Res. 1989;487(1):178–83. doi: 10.1016/0006-8993(89)90955-4. [DOI] [PubMed] [Google Scholar]
- 79.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. Journal of Pharmacology and Experimental Therapeutics. 2000;293(3):879–886. [PubMed] [Google Scholar]
- 80.Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- 81.Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis and Sprague-Dawley rats. J Pharmacol exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- 82.Sleipness EP, Sorg BA, Jansen HT. Time of day alters long-term sensitization to cocaine in rats. Brain Research. 2005;1065(12):132–7. doi: 10.1016/j.brainres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 83.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 84.Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62(5):1750–6. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- 85.Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sciences. 2002;70(25):3069–75. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- 86.Verimer T, Arneric SP, Long JP, Walsh BJ, Abou Zeit-Har MS. Effects of ovariectomy, castration, and chronic lithium chloride treatment on stereotyped behavior in rats. Psychopharmacol. 1981;75:273–276. doi: 10.1007/BF00432437. [DOI] [PubMed] [Google Scholar]
- 87.Vezina P. Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: an in vivo microdialysis study in the rat. Brain Res. 1993;605:332–337. doi: 10.1016/0006-8993(93)91761-g. [DOI] [PubMed] [Google Scholar]
- 88.Weeks JR. In: Long-term intravenous infusions, in Methods in Psychobiology. Meyers RD, editor. Academic Press; London: 1972. pp. 155–168. [Google Scholar]
- 89.Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–63. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 90.Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentrations in male and female rats: effects of estrous cycle and gonadectomy. Neuroscience Letters. 1994;180:155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 91.Xiao L, Jackson LR, Becker JB. The effect of estradiol in the striatum is blocked by ICI 182,780 but not tamoxifen: pharmacological and behavioral evidence. Neuroendocrinology. 2003;77:239–245. doi: 10.1159/000070279. [DOI] [PubMed] [Google Scholar]