Abstract
Inflammation is a major component in the pathology of chronic lung diseases, including asthma. Anti-inflammatory treatment with corticosteroids is not effective in all patients. Thus, new therapeutic options are required to control diverse cellular functions that are currently not optimally targeted by these drugs in order to inhibit inflammation and its sequelae in lung disease. Peroxisome proliferator activated receptors (PPARs), originally characterised as regulators of lipid and glucose metabolism, offer marked potential in this respect. PPARs are expressed in both lung infiltrating and resident immune and inflammatory cells, as well as in resident and structural cells in the lungs, and play critical roles in the regulation of airway inflammation. In vitro, endogenous and synthetic ligands for PPARs regulate expression and release of proinflammatory cytokines and chemoattractants, and cell proliferation and survival. In murine models of allergen-induced inflammation, PPARα and PPARγ ligands reduce the influx of inflammatory cells, cytokine and mucus production, collagen deposition, and airways hyperresponsiveness. The activity profiles of PPAR ligands differ to corticosteroids, supporting the hypothesis that PPARs comprise additional therapeutic targets to mimimise the contribution of inflammation to airway remodelling and dysfunction.
1. INTRODUCTION
Current treatment for chronic lung diseases, including asthma, targets the inflammatory response that is a major contributor to disease pathology. Although inhaled corticosteroids are safe and effective in most patients, a significant proportion of patients with asthma fail to obtain the expected benefits of anti-inflammatory treatment or suffer adverse side effects, and these drugs have not been shown to prevent disease progression. The involvement of diverse cell types and mediators in the inflammatory process provides numerous potential therapeutic options in addition to those targeted by corticosteroids. Novel anti-inflammatory agents with different activity profiles to corticosteroids may minimise persistent inflammation and reduce its contribution to airway remodelling and airways hyperresponsiveness (AHR) in asthma and the loss of pulmonary function in other chronic inflammatory lung diseases.
Peroxisome proliferator activated receptors (PPARs) are ligand-activated transcription factors that have recently been implicated as targets for the regulation of inflammation. PPARs are members of the nuclear hormone receptor family with three isoforms, designated PPARα (NR1C1), PPARβ (PPARδ, NR1C2), and PPARγ (NR1C3). Activation of these receptors has been shown to regulate diverse cellular responses including production of immunomodulatory cytokines, chemotaxis, cell differentiation, proliferation, and survival. This review describes the localisation of these receptors in key cells involved in the pathogenesis of inflammatory diseases in the lung, and presents in vitro and in vivo evidence describing the anti-inflammatory efficacy of PPAR ligands. The identification of complementary or additional actions to those exerted by corticosteroids supports further exploration of the therapeutic potential of PPAR ligands in asthma and chronic lung inflammation.
2. PPARs AND RXRs
The name PPAR derives from the identification of PPARα as the molecular target for the fibrate class of drugs that induce peroxisome proliferation in rodents, a property not shared by the other PPAR isoforms. PPARα, PPARβ, and PPARγ share a common structure of 4 domains consisting of a variable amino terminal activation function-1 domain (AF-1, A/B), a DNA binding domain (C), a hinge region (D), and a highly conserved activation function-2 domain (AF-2, E/F). The large T-shaped ligand-binding domain within the AF-2 region enables PPARs to bind promiscuously to a plethora of structurally diverse endogenous and synthetic ligands [1]. In addition to ligand binding, AF-2 is important for association with coregulators of receptor activity, and for receptor dimerization and nuclear translocation. Unlike glucocorticoid receptors that form homodimers, PPARs exist as heterodimers with retinoid X receptors (RXR). Like PPARs, there are three distinct isoforms of RXR, namely RXRα, RXRβ, and RXRγ, that are all activated by the endogenous ligand 9-cis retinoic acid [2].
3. PROPOSED MECHANISMS OF GENE REGULATION BY PPAR
The molecular mechanisms of gene regulation by PPARs are complex. Heterodimerization of PPAR with RXR may be affected by competition between PPAR isoforms and with other nuclear receptors that are also RXR partners, such as retinoic acid receptors, vitamin D receptors, and liver X receptors. In the absence of ligand, PPAR-RXR forms a complex with corepressor proteins with histone deacetylase activity, including nuclear receptor corepressor (NCoR) and the silencing mediator for retinoid and thyroid hormone receptors (SMRT) that prevents interaction with intracellular targets. Ligand binding causes corepressor dissociation, and ligand-dependent recruitment of coactivators such as steroid receptor coactivator-1 (SRC-1) and the PPAR binding protein (PBP) [3, 4].
Regulation of gene transcription can occur following nuclear translocation of this activated complex. Transcriptional activation or suppression can occur following recognition of PPAR response elements (PPRE) in promoters of target genes and binding to PPRE consensus sequences comprising AGGTCA hexamers separated by a single nucleotide spacer DR-1 (reviewed in [5]). Alternatively, PPAR can negatively regulate gene expression by antagonizing other signal-dependent transcription factors such as nuclear factor κB (NFκB), CAAT/enhancer binding protein (C/EBP), signal transducers and activators of transcription (STAT) or activator protein 1 (AP-1). This may occur via direct binding to cause transrepression [6] or by sequestering coactivators such as the glucocorticoid receptor interacting protein-1/transcriptional intermediary factor (GRIP-1/TIF) required for activity of other transcription factors [7]. PPARγ ligands may also mediate responses via activation of mitogen-associated protein kinase (MAPK) and phosphoinositide-3-kinase (PI3K) pathways [8, 9]. The differential tissue distribution of PPARα, PPARβ, and PPARγ as well as competition between these isoforms and with other nuclear receptors for the accessory proteins that regulate their activity may allow the specific recognition of target genes and other transcription factors to modulate cell function [3].
4. PPAR TISSUE DISTRIBUTION AND LIGANDS
PPARα, PPARβ, and PPARγ are all widely expressed and share some common ligands. However, activation of a specific PPAR can be achieved using selective ligands in tissues where all isoforms are present or by targeting tissues where the isoforms are differentially expressed (Tables 1, 2).
Table 1.
Natural and synthetic ligands for PPAR isoforms.
| Isoform | Natural ligands | Synthetic ligands | Antagonists |
|
| |||
| PPARα | PGD2 | Fibrates | |
| PGI2 | Wy14 643 | MK866 | |
| LTB4 | GW9578, GW7647 | GW6471 | |
| 8S-HETE | NSAIDs | ||
|
| |||
| PPARβ | PGA1 | GW501516, GW0742 | Sulindac |
| PGI2 | L165041 | ||
|
| |||
| PPARγ | PGD2, PGJ2,15-d-PGJ2 | TZDs | BADGE |
| 9-HODE, 13-HODE | GW262570 | GW9662 | |
| 12-HETE, 15-HETE | NSAIDs | T0070907 | |
|
| |||
| PPARα, γ | — | GW2331 | — |
| Ragaglitazar | |||
|
| |||
| PPARα, β, γ | Saturated FAs | — | — |
| PUFAs | |||
BADGE: bisphenol A diglycidyl ether; FA: fatty acid; HETE: hydroxyeicosatetranoic acid; HODE: hydroxyoctadecadienoic acid; NSAID: nonsteroidal anti-inflammatory drug; PUFA: polyunsaturated fatty acid; TZD: thiazolidinedione.
Table 2.
PPAR isoforms in inflammatory cells and lung structural cells.
| Cell type | PPARα | PPARβ | PPARγ |
|
| |||
| Macrophage/monocyte | ✓ [16, 22] | ✓ [22, 33] | ✓ [13, 17, 18, 33] |
| × [33] | — | × [22] | |
| Eosinophil | ✓ [19] | — | ✓ [13, 19, 20] |
| Neutrophils | ✓ [22] | ✓ [21, 22] | ✓ [21] |
| — | — | × [22] | |
| Lymphocytes | ✓ [22, 24, 25] | ✓ [22] | ✓ [24, 25] |
| — | — | × [22] | |
| Dendritic cells | × [14, 23] | — | ✓ [14, 23] |
| Mast cells | × [26, 34] | ✓ [26] | ✓ [26] |
| Epithelial cells | ✓ [28] | ✓ [28, 29] | ✓ [13, 27, 28, 30] |
| Lung fibroblasts | — | — | ✓ [13, 31] |
| Airway smooth muscle | ✓ [32, 35] | × [32, 35] | ✓ [13, 32, 35] |
PPARα is highly expressed predominantly in liver, kidney, skeletal muscle, and heart, and has a role in the catabolism of fatty acids. Structurally diverse ligands for PPARα include naturally occurring fatty acids and eicosanoids such as 8S-hydroxyeicosatetranoic acid (8S-HETE) and leukotriene B4 (LTB4). Among the synthetic PPARα ligands described are the fibrate class of drugs used clinically for the treatment of dyslipidaemia such as clofibrate and fenofibrate, and pharmacological tools such as Wy14 643 [2].
The physiological role of PPARβ is less certain due to its ubiquitous expression. Fatty acids also activate PPARβ, with prostacyclin among other potential endogenous ligands for PPARβ. Recently developed synthetic ligands such as GW501516 and L165041 have been used to support a role for PPARβ in regulation of fatty acid oxidation and cell differentiation in skeletal muscle and adipose tissue [2, 4].
PPARγ was originally characterised as a regulator of adipocyte differentiation, but also plays key roles in glucose and lipid metabolism. Activation of PPARγ also occurs in response to a wide variety of potential endogenous ligands as well as synthetic agonists, such as the thiazolidinedione (TZD) class of insulin-sensitising drugs. Naturally occurring PPARγ ligands include polyunsaturated fatty acids (PUFAs), such as linoleic acid, arachidonic acid, and eicosapentanoic acids, and oxidised lipids such as 9- and 13-hydroxyoctadecadienoic acid (HODE), and 12- and 15-HETE [10]. The arachidonic acid metabolite 15-deoxy-Δ12, 14-prostaglandin-J2 (15-d-PGJ2) has been widely used experimentally to define PPARγ-dependent responses, despite additional actions mediated through PPARα activation [11]. In this context, it is important to also note that it is still uncertain whether this prostaglandin D2 metabolite can be generated in vivo at the micromolar concentrations sufficient to mediate potential PPARγ-dependent effects [12].
The TZD or glitazone class of drugs used in the treatment of type 2 diabetes are believed to exert their insulin-sensitising and hypoglycaemic effects through stimulation of PPARγ [4]. Activation of PPARγ by TZDs results in an alteration in the transcription of several genes involved in glucose and lipid utilisation such as GLUT4 glucose transporter and fatty acid transporter protein [13], and their binding affinity to PPARγ closely parallels their in vivo hypoglycaemic potency [14]. These synthetic ligands include rosiglitazone (RGZ), ciglitazone (CGZ), pioglitazone (PGZ), and troglitazone (TGZ), with RGZ reported to be the most potent [15].
5. PPAR EXPRESSION AND ITS REGULATION IN INFLAMMATORY CELLS AND IN THE LUNG
Recent evidence supports a role for PPARs in the regulation of lung inflammation. Differential expression of PPAR isoforms has been demonstrated in different inflammatory, resident, and structural cells in the airways (see Table 2). Both PPARα and PPARγ are expressed in macrophages and monocytes [16–18], eosinophils [19, 20], with PPARβ also expressed in neutrophils [21, 22]. Dendritic cells express PPARγ but not PPARα [14, 23], but both PPARα and PPARγ are expressed in B and T lymphocytes [24, 25]. PPARβ and PPARγ but not PPARα are expressed in mast cells [26], while all three isoforms have been detected in A549 and BEAS-2B airway epithelial cell lines [27–30]. Mesenchymal expression of PPARs has been demonstrated with PPARγ detected in primary fibroblasts [31], and with PPARα and PPARγ but not PPARβ in airway smooth muscle [13, 32]. The variable patterns of expression of these PPARs in these diverse cell types suggest that receptor activation of different isoforms may specifically modulate both the production of mediators implicated in inflammation and the cellular responses that contribute to tissue remodelling and the development of AHR.
Emerging evidence suggests that PPAR receptor expression is altered in lung disease, with changes in PPARγ levels being the most extensively studied. PPARγ has been localised in mucosal eosinophils and macrophages, airway epithelium and smooth muscle in human airway biopsies, with increased expression in asthmatic patients compared with controls [13]. In addition, in murine models of allergen-induced inflammation, higher levels of PPARγ were evident in total lung extracts [36, 37] and could be localised to airway epithelium and muscle cells, mast cells, and some inflammatory cells [38].
The stimulus for the increased PPARγ levels detected in intact airways is unclear, as is the functional role of this increase. In vitro, PPARγ is inducible by the inflammatory cytokine interleukin-4 (IL-4) in airway epithelial cells and macrophages [27, 39]. PPARγ expression in monocytes is increased with macrophage differentiation and activation [16, 17] and in sensitised mast cells following antigen exposure [26]. Similar changes in the cellular environment in asthmatic airways may contribute to the elevation in PPARγ levels. The lower levels of PPARγ expression in glucocorticoid-treated asthmatics, compared with untreated asthmatics [13], suggest that increased PPARγ expression may be a product of the inflammatory pathways sensitive to steroid therapy. The current hypothesis is that PPARγ expression is upregulated in response to inflammatory cytokines to provide a negative feedback mechanism, whereby endogenous PPARγ ligands could activate these receptors to limit the cellular inflammatory response in the airways. Increases in PPARα and PPARβ during inflammation have not yet been described, although PPARβ expression in the lung has recently been shown to be increased in the lungs of streptozotocin-induced diabetic rats [40].
However, PPAR upregulation does not appear to be a generalised response to inflammation. PPARα and PPARβ were expressed in peripheral blood lymphocytes, monocytes, and neutrophils healthy subjects and in patients with cystic fibrosis (CF). However, relatively lower levels and activity of PPARα, and not PPARβ, were detected in the lymphocytes from CF patients [22]. The authors speculate that this may be associated with changes in levels of endogenous PPARα ligands in CF, but that treatment with synthetic PPARα ligands may increase receptor expression and activity to minimise the immune response [41]. Although ovalbumin sensitisation has been shown to increase PPARγ expression in the lung [36–38], levels of PPARα were decreased in inflamed lungs of allergen-exposed mice [42]. In addition, a recent study has shown that PPARγ mRNA and protein are downregulated in alveolar macrophages following segmental allergen challenge in asthmatic patients, but not healthy controls [43]. It has been suggested that this downregulation could contribute to ongoing pulmonary inflammation, tissue injury, and loss of function. Alternatively, it could accompany the reduction or resolution of inflammation following activation by PPARγ ligands since increases in PPARγ expression induced in a murine model of asthma by allergen sensitisation were inhibited by administration of the synthetic ligand, CGZ [38].
These studies suggest a complex interaction between the initiation and resolution of the inflammatory process and changes in PPAR receptor expression that may be regulated by both inflammatory mediators and the levels of endogenous PPAR ligands. It is important therefore to define receptor-mediated responses at both the cellular level and in integrated animal models of disease to elucidate the role for PPARs in lung inflammation.
Against this translational background, the development of selective ligands for PPAR isoforms has been critical. However, there are marked differences between reported binding affinities and receptor activation potencies for PPAR ligands versus the concentrations required to elicit cellular effects. Multiple approaches are therefore required to support claims for PPAR-dependency of these actions. These include confirming receptor expression in cells of interest and the use of pharmacological antagonists. Several irreversible antagonists for PPARγ have been described including bisphenol A diglycidyl ether (BADGE) [44], and GW9662 [45], although the utility of the former may be compromised by its partial agonist activity [46]. Antagonists for other PPAR isoforms have been described (see Table 1), but have not been utilised in studies outlined in this review.
More recently, molecular techniques have been used to characterise potential PPAR-mediated responses. Adenoviral constructs expressing a dominant negative PPARγ gene that binds to the ligand and the PPRE on DNA but does not initiate transcription have been used in lung fibroblasts [31], while the effects of overexpression of functional PPARγ have been assessed in murine models of asthma [36, 37, 47]. In vivo, transgenic approaches have also characterised the regulatory role of PPARα in inflammation using PPARα-deficient mice [19, 48]. A similar approach for PPARγ is not possible, since complete elimination of this isoform results in embryonic lethality. However, heterozygous knockout mice (PPARγ +/−) have been generated [49] and used to implicate PPARγ in mast cell proliferation [50]. More recently, a developmental study using mice with specific ablation of PPARγ in the airway epithelium showed that these conditionally PPAR−/−-targeted mice had reduced collagen extracellular matrix (ECM) gene expression in the lung [51]. This suggests that PPARγ has a role in the epithelial-mesenchymal interactions necessary for the establishment of normal lung structure [51]. The implications of this finding on inflammation in lung disease have yet to be explored.
6. PPAR FUNCTION IN INFLAMMATORY CELLS AND IN THE LUNG
There is now extensive evidence that PPAR ligands regulate inflammatory and immune processes mediated by cells in which one or more PPAR isoforms is expressed. Many of these actions are in common with corticosteroids, which have been shown to have inhibitory effects on T cell, eosinophil, neutrophil, mast cell/basophil, and macrophage function [52]. PPAR ligands have also been shown to affect cellular responses of resident and structural cells implicated in inflammation and tissue remodelling in chronic lung diseases. Most of these studies have focussed on PPARγ, and some direct comparisons have been made with corticosteroids [32, 53]. In many cases it remains to be determined whether the effects of PPAR ligands are receptor-mediated, to clarify differences in the activities and mechanisms of action of putative endogenous ligands and synthetic agonists, and to determine their potential therapeutic advantages over corticosteroids.
A characteristic feature of airway inflammation in asthma is the predominance of Th2 lymphocytes and their products, which mediate inflammatory cell recruitment of mast cells, eosinophils, and lymphocytes and subsequent release of mediators from these cells. Both 15-d-PGJ2 and CGZ are reported to inhibit T cell proliferation [24, 54]. 15-d-PGJ2 but not CGZ or PPARα agonists induced T cell apoptosis [55]. 15-d-PGJ2 also decreased production of both Th1 and Th2 type cytokines from T cells [56]. In addition, T cells obtained from sensitised mice treated with CGZ showed decreased IFNγ, IL-4, and IL-2 release when exposed to allergen [56]. However, 15-d-PGJ2 also caused a potentially proinflammatory induction of IL-8 gene expression in human T cells and macrophages via a MAPK and/or NFκB-dependent signalling pathway [8, 57].
In activated monocytes, the PPARγ ligands 15-d-PGJ2 and TGZ inhibited production of proinflammatory cytokines tumour necrosis factor α (TNFα), interleukin-1β (IL-1β) and IL-6 [58]. In contrast, natural and synthetic agonists for PPARα were ineffective in these cells [58]. 15-d-PGJ2 and RGZ also reduced TNFα release and the expression of inducible nitric oxide synthase (iNOS) and matrix metalloproteinase (MMP)-9 in activated macrophages, in part by antagonising the activities of AP-1, STAT, and NFκB [17, 33]. Both PPARα and PPARγ agonists induced macrophage apoptosis, in cells stimulated with TNFα and IFNγ [16].
6.1. Eosinophils
Eosinophils are elevated in the airways of asthmatics and can release inflammatory and cytotoxic mediators, cytokines, and growth factors that contribute to tissue remodelling and AHR. IL-5 and eotaxin-induced chemotaxis were reduced by PPARα- and PPARγ-selective ligands Wy14 643 and RGZ at micromolar concentrations, with the effect of RGZ prevented by the PPARγ antagonist, GW9662 [19]. In contrast to these findings, it has recently been shown that both 15-d-PGJ2 and TGZ prime eotaxin-induced chemotaxis in the picomolar to low nanomolar concentration range, and that this effect is also prevented by GW9662 [59]. The possibility that endogenous ligands may have proinflammatory effects via PPARγ at physiological concentrations, but that exogenous ligands may be negative immunomodulators at higher concentrations, will require further investigation. This explanation may also resolve discrepancies in other in vitro studies examining regulation of cytokine release and expression that ascribe both pro- and anti-inflammatory properties to PPARγ ligands.
6.2. Mast cells
Mast cell infiltration of airway smooth muscle (ASM) in the airway wall is associated with impaired function in asthma [60]. In response to antigen stimulation, mast cells release stored mediators such as histamine, and produce arachidonic acid derivatives such as prostaglandins and leukotrienes, and cytokines such as TNFα, IL-4, and granulocyte macrophage-colony stimulating factor (GM-CSF). Several roles for PPARs in regulation of mast cell function have been proposed. The PPARβ ligand carbaprostacyclin and PPARγ ligands 15-d-PGJ2 and TGZ suppressed histamine release and TNFα and GM-CSF production by human basophilic KU812 cells and mast cells [26, 61]. In addition, the increase in cell surface expression of the high affinity IgE receptor FcεRI in response to antigen was reduced by selective PPARβ and PPARγ ligands, namely PGA1 and 15-d-PGJ2 [61]. PPARα ligands had no effect on cytokine release or FcεRI expression in these human cells [26, 61]. Although fenofibrate, Wy14 643, and CGZ inhibited antigen-induced leukotriene production from rat basophilic leukemia (RBL)-2H3 cells, these effects are likely to be PPAR-independent since PPARα mRNA was below detection [34] and the inhibition by CGZ was not prevented by a PPARγ antagonist or associated with nuclear translocation of the receptor [62]. However, proliferation of bone-marrow derived murine mast cells was increased by RGZ in an apparently PPARγ-dependent manner, since it was prevented by the PPARγ antagonist GW9662 and the effect of RGZ was reduced in cells from PPARγ +/− mice [50].
Further studies are required to explore the mechanisms of action of these selective PPARβ and PPARγ ligands, and to clarify the role of both receptors in the modulation of mast cell function. Previous in vivo studies suggest that corticosteroids have a minimal effect on mast cell degranulation and the appearance of mast cell mediators after segmental antigen challenge in subjects with asthma [63].
6.3. Epithelial cells
Epithelial remodelling has been documented early in the development of asthma. Airway epithelial cells can contribute to persistent inflammation through synthesis and secretion of enzymes and mediators that regulate matrix turnover and inflammatory cell influx. Potential anti-inflammatory actions for PPARγ ligands have been described in various epithelial cell lines. RGZ and PGZ decreased TNFα- and phorbol myristate acetate (PMA)-induced MMP-9 activity levels in NL20 and BEAS cells, associated with inhibition of NFκB activation [30]. In A549 cells, TZDs reduced expression of iNOS [27] and decreased secretion of Regulated upon Activation, Normal T-cell Expressed, and Secreted (RANTES) and IL-8 [27, 64]. In contrast to these studies, both RGZ and TGZ potentiated TNFα-induced production of proinflammatory cytokines GM-CSF, IL-6 and IL-8 from A549 cells, independently of PPARγ, NFκB or MAPK activation [65].
There is also a complex relationship between PPARγ and cyclo-oxygenase-2 (COX-2) expression in airway epithelial cells. Both RGZ and CGZ inhibited increases in PMA-induced COX-2 expression by inhibiting AP-1 signalling [66]. However, TGZ increased basal and TNFα-induced COX-2 expression independently of PPARγ and NFκB, but dependent on PI3K and ERK MAPK pathways in A549 cells [9]. In this study, neither 15-d-PGJ2 nor RGZ (PPARγ ligands), GW262570 (PPARγ/α agonist), nor L-165041 (PPARβ agonist) regulated COX-2 expression. Further investigation of these discrepancies will be required to define the effects of PPARγ ligands on airway epithelial cells and the mechanisms by which these pro- and/or anti-inflammatory responses occur.
6.4. Mesenchymal cells
Fibroblasts play an important role in regulation of ECM deposition that contributes to subepithelial fibrosis layer of the airway, and the development of fixed airway obstruction in asthma [67]. Thickening of the ASM layer is another characteristic feature of airway remodelling in asthma. This has been associated with ASM hypertrophy and/or hyperplasia [68, 69] and increased ECM deposition within the ASM bundle [70]. These cells can respond to both mitogens and inflammatory mediators and contribute to further changes in the airway through the production of ECM proteins, cytokines, and chemokines. The PPARγ ligands RGZ, CGZ, and 15dPGJ2 inhibited the differentiation of human lung fibroblasts into myofibroblasts and reduce collagen I production following TGFβ stimulation, although the PPARα ligand Wy14 643 was ineffective [31]. These potential antifibrotic effects may be only partly mediated by PPARγ, since they were suppressed by expression of a dominant negative PPARγ construct, but not by GW9662 [31]. It has been reported that corticosteroids did not prevent TGFβ-induced collagen I production by ASM cells from individuals with or without asthma [71], and this suggests that PPARγ agonists may have a therapeutic advantage over corticosteroids in the regulation of lung fibrosis.
In ASM, the PPARγ agonists 15-d-PGJ2 and CGZ suppressed both GM-CSF and G-CSF release [32, 53]. Interestingly, the profile of inflammatory mediator inhibition differed between CGZ and corticosteroids, as dexamethasone inhibited GM-CSF but not G-CSF levels [32, 53]. In a separate study, 15-d-PGJ2 and TGZ were shown to inhibit TNFα-induced production of eotaxin and monocyte chemotactic protein-1 (MCP-1) but not IL-8 secretion from ASM [53]. The inhibitory effects of 15-d-PGJ2 were additive with fluticasone, offering the intriguing possibility that PPARγ agonists in combination with corticosteroids may provide additional therapeutic benefit in asthma [53]. Although PPARγ heterodimerisation with the retinoid X receptor is well characterized, this study also described additional complexity in the mechanisms of action of PPARγ agonists, with direct physical interactions between PPARγ and GR [53]. In addition, PPARγ agonists were shown to mediate anti-inflammatory effects directly via GR activation, with RGZ and CGZ stimulating GR nuclear translocation in a PPARγ deficient cell line [72]. Assessment of these potential interactions between PPARγ and GR by receptor translocation studies may provide additional insights into mechanisms underlying the relative activities of nuclear receptor agonists in ASM.
PPARγ agonists CGZ and 15-d-PGJ2 increased COX-2 expression in ASM, by binding to the PPRE in the COX-2 promoter [35]. Despite the proposed proinflammatory role for this enzyme, the increased PGE2 levels following induction of COX-2 may act in an autocrine manner to reduce subsequent production of GM-CSF, and to inhibit proliferation of ASM [73, 74].
PPARγ ligands may also exert direct antimitogenic actions that could inhibit airway remodelling. Both 15-d-PGJ2 and TZDs inhibited proliferation of human cultured ASM cells [32, 75]. The effects of 15-d-PGJ2 and RGZ were mitogen-independent, as each ligand decreased FGF2- and thrombin-mediated proliferation with similar potency [75]. However, only the effects of RGZ were reversed by the selective PPARγ antagonist GW9662 [75]. RGZ caused inhibition of cell-cycle progression in late G1 phase, without decreasing mitogen-stimulated cyclin D1 protein levels, a mechanism that differs from dexamethasone [76]. Of additional interest, the degree of inhibition of serum-induced ASM proliferation was greater for CGZ than dexamethasone [32]. It is critical to extend these comparisons to cells derived from asthmatics where the ability of corticosteroids to inhibit proliferation is reduced [77] to determine whether PPARγ provides an additional or alternative therapeutic target to glucocorticoid receptors to regulate remodelling in asthma.
PPARα was also expressed in ASM, but surprisingly, Wy14 643 did not regulate inflammatory cytokine production [32, 53], COX-2 expression [35], or proliferation [32, 77] of these cells.
7. PPAR REGULATION OF ALLERGEN-INDUCED INFLAMMATION IN VIVO
On the basis of these in vitro findings in inflammatory and structural cells, the role of PPARs has now been explored in murine models of allergen-induced bronchial inflammation. Although these models do not recapitulate all the pathophysiological changes seen in asthma, sensitisation and repeated aerosol challenges with ovalbumin (OVA) induce airway eosinophilia, changes in airway structure and increases in airways reactivity. Numerous studies have now utilised synthetic PPAR ligands, adenoviral constructs carrying PPAR cDNA (AdPPAR) and transgenics to support immunomodulatory roles for PPARs in the regulation of inflammation, airway wall remodelling, and hyperresponsiveness. Although the majority of studies have focussed on PPARγ, some comparisons have been made with the other PPAR isoforms.
7.1. PPARα
In PPARα-deficient Balb/c mice sensitized and challenged with OVA, there were greater increases in lung inflammation, airway eosinophilia, and antigen-specific serum IgE levels than in wild-type OVA-treated mice [19]. This was associated with relatively higher IL-6, IL-13, and eotaxin levels in the lung, although IL-4, IL-5, and soluble vascular and cell adhesion molecule-1 (sVCAM-1) were not different [19]. Critically, the PPARα-deficient Balb/c mice also displayed a greater response to MCh after OVA-sensitization and aerosol challenge than wild-type mice, providing a functional correlate to the cellular and humoral changes [19]. It was suggested that this PPARα deletion worsened eosinophilia and asthma-like symptoms by preventing the anti-inflammatory actions mediated by the endogenous PPARα ligand, LTB4, known to be produced abundantly by mast cells and other cell types in asthma. A separate study utilising a selective PPARα ligand provides support for this explanation, since GW9578 inhibited allergen-induced influx of eosinophils and lymphocytes [28]. In the same study, the selective PPARβ agonist GW501516 had no effect on inflammatory cell influx [28].
There are conflicting reports on the role of PPARα in regulating airway inflammation induced by lipopolysaccharide (LPS), which is characterised by infiltration of neutrophils and macrophages, increased chemoattractant levels, and elevated MMP activity in BALF. Although GW8578 had no effect on neutrophil influx or increased levels of keratinocyte derived-chemokine (KC) or TNFα following LPS treatment [28], fenofibrate reduced the increase in BALF neutrophils and macrophages as well as levels of TNFα, KC, macrophage inflammatory protein-2 (MIP-2), monocyte chemoattractant protein-1 (MCP-1) and both MMP-2 and MMP-9 activities [78]. Further studies are required to confirm if PPARα activation may also have a beneficial effect in acute or chronic inflammatory airway disorders involving neutrophils and macrophages.
7.2. PPARγ
7.2.1. Inflammation
In Balb/c mice sensitized and challenged with OVA, PPARγ ligands reduced levels of proinflammatory mediators in the bronchoalveolar lavage (BAL) fluid and lung [19, 28, 36, 37, 56] (see Figure 1). Significantly, cytokines associated with Th2-driven humoral responses were decreased by treatment with synthetic PPARγ ligands. Increased levels of IL-4, IL-5, IL-13, and eosinophil cationic protein were reduced by the administration of RGZ or PGZ [36, 37]. In addition, in vitro studies of T cell obtained from sensitised mice treated with CGZ showed decreased IL-4 release when exposed to allergen [56].
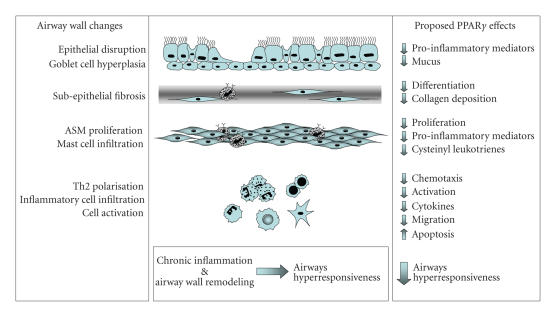
Figure 1.
Proposed effects of PPARγ ligands on inflammatory and remodelling changes in the asthmatic airway that contribute to airways hyperresponsiveness.
Although the antigen sensitisation protocol differed, a common finding in these studies in Balb/c mice has been that PPARγ ligands CGZ, RGZ and PGZ, and administration of AdPPARγ reduced the OVA-induced influx of inflammatory cells, specifically eosinophils [19, 28, 36–38, 47] (see Figure 1). However, in C57BL/6 mice, RGZ treatment had no effect on levels of inflammatory cells in the bronchoalveolar lavage fluid despite inhibiting airways hyperresponsiveness [79]. Whether this is a strain difference in sensitivity to regulation of this measure of airway inflammation, or relates to the ligand and/or route of administration used is yet to be clarified.
7.2.2. Airway remodelling
Airway wall remodelling is characterised by goblet cell metaplasia, collagen deposition and subepithelial fibrosis, and smooth muscle hypertrophy and/or hyperplasia. The role of PPARγ in regulating these changes has been explored. Treatment of Balb/c mice with nebulised CGZ was associated with a reduction in mucous production [38] (see Figure 1). Since orally administered CGZ had no effect [56] and oral RGZ treatment had no impact on goblet cell hyperplasia in C57BL/6 mice [79], the route of administration may be critical to regulate this parameter.
There is now evidence that activation of PPARγ may regulate ECM deposition that occurs in airway wall remodelling. CGZ decreased basement membrane thickness and airways collagen deposition in response to antigen sensitization and challenge, associated with a reduction in TGF-β synthesis [38]. Inhibition of TGF-β signalling has also previously been shown to be inhibited by CGZ in cultured lung fibroblasts [31]. In support of a generalised antifibrotic activity for PPAR ligands, both RGZ and Wy14 643 have been shown to reduce bleomycin-induced pulmonary fibrosis in mice [18, 48, 80].
The antifibrotic effect of CGZ seen in vivo may also be related to regulation of the activity of MMPs or their inhibitors. Although an increase in MMP-2 proteolytic activity in the BALF with OVA treatment was not affected by RGZ [79], it has previously been reported that RGZ inhibits MMP-9 expression in bronchial epithelial cell lines [30].
Further studies are required to assess whether the inhibitory effects of TZDs on ASM proliferation translate to the in vivo setting, where antigen sensitization and challenge leads to inflammation, airway fibrosis, and thickening of the ASM layer. This is critical since the ability of corticosteroids to reduce airway structural changes in murine models is variable [81, 82], and airway remodelling persists despite optimal clinical use of corticosteroids in asthmatic patients [83].
7.2.3. Airways hyperresponsiveness
The impact of regulation of these markers of inflammation and tissue remodelling by PPARγ ligands on AHR has also been explored. Using unrestrained plethysmography, the methacholine (MCh)-induced increase in enhanced pause (Penh) has been used as a measure of AHR following allergen sensitisation and challenge. In Balb/c mice, nebulized CGZ, oral RGZ, or oral PGZ completely prevented the increased response to MCh [19, 36–38, 47]. The effects of the synthetic PPARγ ligands were mimicked by AdPPARγ [36, 37, 47] and abrogated by GW9662 [19, 36–38, 47].
Using invasive measurements of respiratory resistance and compliance, RGZ reduced the increase in airways resistance after OVA challenge in C57BL/6 mice, without affecting the decrease in lung compliance, reflecting an effect on the airways rather than the parenchyma of the lung [79]. This finding provides further support for the proposed role of PPARγ in the regulation of AHR, and is important in light of recent criticism of the use of Penh measurements to draw conclusions about the effects of potential therapeutic agents on AHR [84]. However, since this inhibition by RGZ occurred in the absence of a significant effect on the OVA-induced increase in BAL inflammatory cells, MMP-2 activity, and goblet cell number, it is possible that RGZ may modulate AHR by a mechanism that is independent of inhibition of inflammatory cell recruitment to the airway [79]. Dissociation between inhibition of eosinophilia and reduction in AHR have previously been reported, although the dose of dexamethasone required to inhibit AHR was higher than that needed to inhibit eosinophilia in a murine model of allergic airway inflammation [85].
7.2.4. Potential mechanisms for decreased inflammation and AHR by PPARγ ligands
MCh reactivity was unchanged in saline-challenged mice after oral treatment with RGZ for 7 days [79], suggesting that exposure of ASM to RGZ does not directly inhibit contractile responsiveness. In addition, RGZ did not modulate basal airway tone, or the contraction in response to MCh or histamine in isolated guinea pig tracheal rings [79]. However, CGZ and RGZ have been shown to produce concentration-dependent relaxation smooth muscle of isolated mouse trachea [86]. This effect was not prevented by GW9662, but was inhibited by indomethacin, and suggested that TZDs could act independently of PPARγ to inhibit PGE2 metabolism by 15-hydroxyprostaglandin dehydrogenase leading to a dilator response [86, 87].
A series of studies have shown that inhibitory effects of TZDs and AdPPARγ on both eosinophilia and AHR were prevented by GW9662 and implicate increases in phosphatase and tensin homologue deleted on chromosome ten (PTEN) and IL-10 in the protective roles of PPARγ-activation [36, 37, 47]. This is in agreement with a separate study, in which RGZ inhibited the migration of antigen-loaded dendritic cells in the mediastinal lymph nodes and increased IL-10 production [88].
8. SUMMARY
On the basis of these in vitro and in vivo findings, substantial evidence has emerged to provide proof-of-concept for the future clinical application of PPARγ ligands to treat airway inflammation. Given their long record of use in type 2 diabetes, TZDs appear to be ideally placed for use in the treatment of chronic lung disease. Like corticosteroids, they appear to have broad anti-inflammatory effects and possess potential anti-remodelling efficacy on multiple cell types in the lung and in animal models of asthma. However, PPARγ agonists including RGZ may offer additional therapeutical advantages to current treatment, if they can be proven to exert control over proinflammatory and proasthmatic pathways that are not susceptible to inhibition by corticosteroids in the clinical setting. Further comparative studies are required to explore these novel complementary or additional actions of PPARγ agonists to those already identified.
The efficacy of TZDs in asthma has not yet been evaluated in human clinical studies, although there has been a case report describing the reduction in asthma symptoms in patients treated with PGZ for diabetes [89]. A limited trial examining the effect of RGZ on lung function in comparison with low dose inhaled corticosteroids in steroid naïve smokers with asthma is currently underway in the United Kingdom to determine whether targeting PPARγ may offer therapeutic benefit in the future treatment of asthma and other inflammatory lung diseases.
ABBREVIATIONS
- AHR:
Airways hyperresponsiveness
- AP-1:
Activator protein-1
- ASM:
Airway smooth muscle
- BADGE:
Bisphenol A diglycidyl ether
- BALF:
Bronchoalveolar lavage fluid
- C/EBP:
CAAT/enhancer binding protein
- CF:
Cystic fibrosis
- CGZ:
Ciglitazone
- COX-2:
Cyclo-oxygenase-2
- 15-d-PGJ2 :
15-deoxy-Δ12,14-prostaglandin J2
- ECM:
Extracellular matrix
- ERK:
Extracellular regulated kinase
- FA:
Fatty acid
- FGF-2:
Fibroblast growth factor-2
- G-CSF:
Granulocyte colony stimulating factor
- GM-CSF:
Granulocyte-macrophage colony stimulating factor
- GR:
Glucocorticoid receptor
- GRIP-1:
Glucocorticoid receptor interacting protein-1
- HETE:
Hydroxyeicosatetranoic acid
- HODE:
Hydroxyoctadecadienoic acid
- IFNγ :
Interferonγ
- iNOS:
Inducible nitric oxide synthase
- KC:
Keratinocyte-derived chemokine
- LPS:
Lipopolysaccharide
- LTB4:
Leukotriene B4
- MAPK:
Mitogen-associated protein kinase
- MCh:
Methacholine
- MCP-1:
Monocyte chemotactic protein-1
- MIP-2:
Macrophage inflammatory protein-2
- MMP:
Matrix metalloprotease
- NFκB:
Nuclear factor κB
- NSAID:
Nonsteroidal anti-inflammatory drug
- OVA:
Ovalbumin
- PGZ:
Pioglitazone
- PI3K:
Phosphoinositide-3-kinase
- PMA:
Phorbol myristate acetate
- PPAR:
Peroxisome proliferator activated receptor
- PPRE:
Peroxisome proliferator activated receptor response element
- PUFA:
Polyunsaturated fatty acid
- PTEN:
Phosphatase and tensin homologue deleted on chromosome ten
- RANTES:
Regulated upon activation normal T cell expressed and secreted
- RGZ:
Rosiglitazone
- RXR:
Retinoid X receptor
- STAT:
Signal transducers and activators of transcription
- sVCAM-1:
Soluble vascular and cell adhesion molecule-1
- TGFβ :
Transforming growth factorβ
- TGZ:
Troglitazone
- TIF:
Transcriptional intermediary factor
- TNFα :
Tumour necrosis factorα
- TZD:
Thiazolidinedione
References
- 1.Nolte RT, Wisely GB, Westin S, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ . Nature. 1998;395(6698):137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 2.Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacology and Therapeutics. 2006;110(3):371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Tien ES, Hannon DB, Thompson JT, Vanden Heuvel JP. Examination of ligand-dependent coactivator recruitment by peroxisome proliferator-activated receptor-α (PPARα) PPAR Research. 2006;2006:9 pages. doi: 10.1155/PPAR/2006/69612. Article ID 69612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger J, Moller DE. The mechanisms of action of PPARs. Annual Review of Medicine. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 5.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Reviews. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 6.Straus DS, Pascual G, Li M, et al. 15-deoxyΔ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gervois P, Vu-Dac N, Kleemann R, et al. Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor α agonists via inhibition of CCAAT box/enhancer-binding protein β . Journal of Biological Chemistry. 2001;276(36):33471–33477. doi: 10.1074/jbc.M102839200. [DOI] [PubMed] [Google Scholar]
- 8.Harris SG, Smith RS, Phipps RP. 15-deoxy-Δ12,14-PGJ2 induces IL-8 production in human T cells by a mitogen-activated protein kinase pathway. Journal of Immunology. 2002;168(3):1372–1379. doi: 10.4049/jimmunol.168.3.1372. [DOI] [PubMed] [Google Scholar]
- 9.Patel KM, Wright KL, Whittaker P, Chakravarty P, Watson ML, Ward SG. Differential modulation of COX-2 expression in A549 airway epithelial cells by structurally distinct PPARγ agonists: evidence for disparate functional effects which are independent of NF-κB and PPARγ . Cellular Signalling. 2005;17(9):1098–1110. doi: 10.1016/j.cellsig.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. Journal of Medicinal Chemistry. 2000;43(4):527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 11.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ . Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 12.Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-Δ12,14-PGJ2 and the ligation of PPARγ . Journal of Clinical Investigation. 2003;112(6):945–955. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benayoun L, Letuve S, Druilhe A, et al. Regulation of peroxisome proliferator-activated receptor γ expression in human asthmatic airways: relationship with proliferation, apoptosis, and airway remodeling. American Journal of Respiratory and Critical Care Medicine. 2001;164(8, part 1):1487–1494. doi: 10.1164/ajrccm.164.8.2101070. [DOI] [PubMed] [Google Scholar]
- 14.Faveeuw C, Fougeray S, Angeli V, et al. Peroxisome proliferator-activated receptor γ activators inhibit interleukin-12 production in murine dendritic cells. FEBS Letters. 2000;486(3):261–266. doi: 10.1016/s0014-5793(00)02319-x. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) Journal of Biological Chemistry. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 16.Chinetti G, Griglio S, Antonucci M, et al. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. Journal of Biological Chemistry. 1998;273(40):25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 17.Ricote M, Huang J, Fajas L, et al. Expression of the peroxisome proliferator-activated receptor γ (PPARγ) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(13):7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Standiford TJ, Keshamouni VG, Reddy RC. Peroxisome proliferator-activated receptor-γ as a regulator of lung inflammation and repair. Proceedings of the American Thoracic Society. 2005;2(3):226–231. doi: 10.1513/pats.200501-010AC. [DOI] [PubMed] [Google Scholar]
- 19.Woerly G, Honda K, Loyens M, et al. Peroxisome proliferator-activated receptors α and γ down-regulate allergic inflammation and eosinophil activation. Journal of Experimental Medicine. 2003;198(3):411–421. doi: 10.1084/jem.20021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueki S, Adachi T, Bourdeaux J, et al. Expression of PPARγ in eosinophils and its functional role in survival and chemotaxis. Immunology Letters. 2003;86(2):183–189. doi: 10.1016/s0165-2478(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 21.Greene ME, Blumberg B, McBride OW, et al. Isolation of the human peroxisome proliferator activated receptor γ cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expression. 1995;4(4-5):281–299. [PMC free article] [PubMed] [Google Scholar]
- 22.Reynders V, Loitsch S, Steinhauer C, Wagner T, Steinhilber D, Bargon J. Peroxisome proliferator-activated receptor α (PPARα) down-regulation in cystic fibrosis lymphocytes. Respiratory Research. 2006;7:104. doi: 10.1186/1465-9921-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosset P, Charbonnier A-S, Delerive P, et al. Peroxisome proliferator-activated receptor γ activators affect the maturation of human monocyte-derived dendritic cells. European Journal of Immunology. 2001;31(10):2857–2865. doi: 10.1002/1521-4141(2001010)31:10<2857::aid-immu2857>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 24.Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPARγ and immunoregulation: PPARγ mediates inhibition of helper T cell responses. Journal of Immunology. 2000;164(3):1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 25.Jones DC, Ding X, Daynes RA. Nuclear receptor peroxisome proliferator-activated receptor α (PPARα) is expressed in resting murine lymphocytes. The PPARα in T and B lymphocytes is both transactivation and transrepression competent. Journal of Biological Chemistry. 2002;277(9):6838–6845. doi: 10.1074/jbc.M106908200. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama H, Nonaka T, Kishimoto T, Komoriya K, Tsuji K, Nakahata T. Peroxisome proliferator-activated receptors are expressed in human cultured mast cells: a possible role of these receptors in negative regulation of mast cell activation. European Journal of Immunology. 2000;30(12):3363–3370. doi: 10.1002/1521-4141(2000012)30:12<3363::AID-IMMU3363>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Wang ACC, Dai X, Luu B, Conrad DJ. Peroxisome proliferator-activated receptor-γ regulates airway epithelial cell activation. American Journal of Respiratory Cell and Molecular Biology. 2001;24(6):688–693. doi: 10.1165/ajrcmb.24.6.4376. [DOI] [PubMed] [Google Scholar]
- 28.Trifilieff A, Bench A, Hanley M, Bayley D, Campbell E, Whittaker P. PPAR-α and -γ but not -δ agonists inhibit airway inflammation in a murine model of asthma: in vitro evidence for an NF-κB-independent effect. British Journal of Pharmacology. 2003;139(1):163–171. doi: 10.1038/sj.bjp.0705232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold R, König W. Peroxisome-proliferator-activated receptor-γ agonists inhibit the release of proinflammatory cytokines from RSV-infected epithelial cells. Virology. 2006;346(2):427–439. doi: 10.1016/j.virol.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Hetzel M, Walcher D, Grüb M, Bach H, Hombach V, Marx N. Inhibition of MMP-9 expression by PPARγ activators in human bronchial epithelial cells. Thorax. 2003;58(9):778–783. doi: 10.1136/thorax.58.9.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgess HA, Daugherty LE, Thatcher TH, et al. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. American Journal of Physiology. 2005;288(6 32-6):L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 32.Patel HJ, Belvisi MG, Bishop-Bailey D, Yacoub MH, Mitchell JA. Activation of peroxisome proliferator-activated receptors in human airway smooth muscle cells has a superior anti-inflammatory profile to corticosteroids: relevance for chronic obstructive pulmonary disease therapy. Journal of Immunology. 2003;170(5):2663–2669. doi: 10.4049/jimmunol.170.5.2663. [DOI] [PubMed] [Google Scholar]
- 33.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita M. Peroxisome proliferator-activated receptor α-independent effects of peroxisome proliferators on cysteinyl leukotriene production in mast cells. European Journal of Pharmacology. 2007;556(1–3):172–180. doi: 10.1016/j.ejphar.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 35.Pang L, Nie M, Corbett L, Knox AJ. Cyclooxygenase-2 expression by nonsteroidal anti-inflammatory drugs in human airway smooth muscle cells: role of peroxisome proliferator-activated receptors. Journal of Immunology. 2003;170(2):1043–1051. doi: 10.4049/jimmunol.170.2.1043. [DOI] [PubMed] [Google Scholar]
- 36.Kim SR, Lee KS, Park HS, et al. Involvement of IL-10 in peroxisome proliferator-activated receptor γ-mediated anti-inflammatory response in asthma. Molecular Pharmacology. 2005;68(6):1568–1575. doi: 10.1124/mol.105.017160. [DOI] [PubMed] [Google Scholar]
- 37.Lee KS, Park SJ, Hwang PH, et al. PPAR-γ modulates allergic inflammation through up-regulation of PTEN. The FASEB Journal. 2005;19(8):1033–1035. doi: 10.1096/fj.04-3309fje. [DOI] [PubMed] [Google Scholar]
- 38.Honda K, Marquillies P, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptor γ is expressed in airways and inhibits features of airway remodeling in a mouse asthma model. Journal of Allergy and Clinical Immunology. 2004;113(5):882–888. doi: 10.1016/j.jaci.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 39.Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400(6742):378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 40.Huang C-J, Liu I-M, Cheng J-T. Increase of peroxisome proliferator-activated receptor δ gene expression in the lungs of streptozotocin-induced diabetic rats. Pulmonary Pharmacology & Therapeutics. 2007;20(1):69–74. doi: 10.1016/j.pupt.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Inoue I, Shino K, Noji S, Awata T, Katayama S. Expression of peroxisome proliferator-activated receptor α (PPARα) in primary cultures of human vascular endothelial cells. Biochemical and Biophysical Research Communications. 1998;246(2):370–374. doi: 10.1006/bbrc.1998.8622. [DOI] [PubMed] [Google Scholar]
- 42.Becker J, Delayre-Orthez C, Frossard N, Pons F. Regulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases? Fundamental and Clinical Pharmacology. 2006;20(5):429–447. doi: 10.1111/j.1472-8206.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi M, Thomassen MJ, Rambasek T, et al. An inverse relationship between peroxisome proliferator-activated receptor γ and allergic airway inflammation in an allergen challenge model. Annals of Allergy, Asthma & Immunology. 2005;95(5):468–473. doi: 10.1016/S1081-1206(10)61173-8. [DOI] [PubMed] [Google Scholar]
- 44.Wright HM, Clish CB, Mikami T, et al. A synthetic antagonist for the peroxisome proliferator-activated receptor γ inhibits adipocyte differentiation. Journal of Biological Chemistry. 2000;275(3):1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]
- 45.Leesnitzer LM, Parks DJ, Bledsoe RK, et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41(21):6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 46.Bishop-Bailey D, Hla T, Warner TD. Bisphenol A diglycidyl ether (BADGE) is a PPARγ agonist in an ECV304 cell line. British Journal of Pharmacology. 2000;131(4):651–654. doi: 10.1038/sj.bjp.0703628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee KS, Kim SR, Park SJ, et al. Peroxisome proliferator activated receptor-γ modulates reactive oxygen species generation and activation of nuclear factor-κB and hypoxia-inducible factor 1α in allergic airway disease of mice. Journal of Allergy and Clinical Immunology. 2006;118(1):120–127. doi: 10.1016/j.jaci.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Genovese T, Mazzon E, Di Paola R, et al. Role of endogenous and exogenous ligands for the peroxisome proliferator-activated receptor α in the development of bleomycin-induced lung injury. Shock. 2005;24(6):547–555. doi: 10.1097/01.shk.0000190825.28783.a4. [DOI] [PubMed] [Google Scholar]
- 49.Miles PDG, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-γ deficiency. Journal of Clinical Investigation. 2000;105(3):287–292. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeyama K, Emi M, Tachibana M. Nuclear receptors as targets for drug development: peroxisome proliferator-activated receptor γ in mast cells: its roles in proliferation and differentiation. Journal of Pharmacological Sciences. 2005;97(2):190–194. doi: 10.1254/jphs.fmj04008x6. [DOI] [PubMed] [Google Scholar]
- 51.Simon DM, Arikan MC, Srisuma S, et al. Epithelial cell PPARγ contributes to normal lung maturation. The FASEB Journal. 2006;20(9):1507–1509. doi: 10.1096/fj.05-5410fje. [DOI] [PubMed] [Google Scholar]
- 52.Belvisi MG. Regulation of inflammatory cell function by corticosteroids. Proceedings of the American Thoracic Society. 2004;1(3):207–214. doi: 10.1513/pats.200402-002MS. [DOI] [PubMed] [Google Scholar]
- 53.Nie M, Corbett L, Knox AJ, Pang L. Differential regulation of chemokine expression by peroxisome proliferator-activated receptor γ agonists: interactions with glucocorticoids and β 2-agonists. Journal of Biological Chemistry. 2005;280(4):2550–2561. doi: 10.1074/jbc.M410616200. [DOI] [PubMed] [Google Scholar]
- 54.Cunard R, Ricote M, DiCampli D, et al. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. Journal of Immunology. 2002;168(6):2795–2802. doi: 10.4049/jimmunol.168.6.2795. [DOI] [PubMed] [Google Scholar]
- 55.Harris SG, Phipps RP. Prostaglandin D2, its metabolite 15-d-PGJ2, and peroxisome proliferator activated receptor-γ agonists induce apoptosis in transformed, but not normal, human T lineage cells. Immunology. 2002;105(1):23–34. doi: 10.1046/j.0019-2805.2001.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller C, Weaver V, Vanden Heuvel JP, August A, Cantorna MT. Peroxisome proliferator-activated receptor γ ligands attenuate immunological symptoms of experimental allergic asthma. Archives of Biochemistry and Biophysics. 2003;418(2):186–196. doi: 10.1016/j.abb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Fu Y, Luo N, Lopes-Virella MF. Upregulation of interleukin-8 expression by prostaglandin D2 metabolite 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2) in human THP-1 macrophages. Atherosclerosis. 2002;160(1):11–20. doi: 10.1016/s0021-9150(01)00541-x. [DOI] [PubMed] [Google Scholar]
- 58.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi Y, Ueki S, Mahemuti G, et al. Physiological levels of 15-deoxy-Δ12,14-prostaglandin J2 prime eotaxin-induced chemotaxis on human eosinophils through peroxisome proliferator-activated receptor-γ ligation. Journal of Immunology. 2005;175(9):5744–5750. doi: 10.4049/jimmunol.175.9.5744. [DOI] [PubMed] [Google Scholar]
- 60.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. New England Journal of Medicine. 2002;346(22):1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 61.Fujimura Y, Tachibana H, Yamada K. Peroxisome proliferator-activated receptor ligands negatively regulate the expression of the high-affinity IgE receptor FcεRI in human basophilic KU812 cells. Biochemical and Biophysical Research Communications. 2002;297(2):193–201. doi: 10.1016/s0006-291x(02)02139-3. [DOI] [PubMed] [Google Scholar]
- 62.Okuyama K, Yamashita M, Kitabatake Y, Kawamura S, Takayanagi M, Ohno I. Ciglitazone inhibits the antigen-induced leukotrienes production independently of PPARγ in RBL-2H3 mast cells. European Journal of Pharmacology. 2005;521(1–3):21–28. doi: 10.1016/j.ejphar.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Liu MC, Proud D, Lichtenstein LM, et al. Effects of prednisone on the cellular responses and release of cytokines and mediators after segmental allergen challenge of asthmatic subjects. Journal of Allergy and Clinical Immunology. 2001;108(1):29–38. doi: 10.1067/mai.2001.116004. [DOI] [PubMed] [Google Scholar]
- 64.Momoi A, Murao K, Imachi H, et al. Thiazolidinedione inhibits production of RANTES in a cytokine-treated human lung epithelial cell line. FEBS Letters. 1999;452(3):301–304. doi: 10.1016/s0014-5793(99)00678-x. [DOI] [PubMed] [Google Scholar]
- 65.Desmet C, Warzée B, Gosset P, et al. Pro-inflammatory properties for thiazolidinediones. Biochemical Pharmacology. 2005;69(2):255–265. doi: 10.1016/j.bcp.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Subbaramaiah K, Lin DT, Hart JC, Dannenberg AJ. Peroxisome proliferator-activated receptor γ ligands suppress the transcriptional activation of cyclooxygenase-2. Evidence for involvement of activator protein-1 and CREB-binding protein/p300. Journal of Biological Chemistry. 2001;276(15):12440–12448. doi: 10.1074/jbc.M007237200. [DOI] [PubMed] [Google Scholar]
- 67.Bousquet J, Chanez P, Lacoste JY, et al. Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. Journal of Allergy and Clinical Immunology. 1991;88(4):649–660. doi: 10.1016/0091-6749(91)90159-l. [DOI] [PubMed] [Google Scholar]
- 68.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. American Review of Respiratory Disease. 1993;148(3):720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- 69.Woodruff PG, Dolganov GM, Ferrando RE, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. American Journal of Respiratory and Critical Care Medicine. 2004;169(9):1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 70.Thomson RJ, Bramley AM, Schellenberg RR. Airway muscle stereology: implications for increased shortening in asthma. American Journal of Respiratory and Critical Care Medicine. 1996;154(3, part 1):749–757. doi: 10.1164/ajrccm.154.3.8810615. [DOI] [PubMed] [Google Scholar]
- 71.Burgess JK, Oliver BGG, Poniris MH, et al. A phosphodiesterase 4 inhibitor inhibits matrix protein deposition in airways in vitro. Journal of Allergy and Clinical Immunology. 2006;118(3):649–657. doi: 10.1016/j.jaci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Ialenti A, Grassia G, Di Meglio P, Maffia P, Di Rosa M, Ianaro A. Mechanism of the anti-inflammatory effect of thiazolidinediones: relationship with the glucocorticoid pathway. Molecular Pharmacology. 2005;67(5):1620–1628. doi: 10.1124/mol.104.004895. [DOI] [PubMed] [Google Scholar]
- 73.Saunders MA, Mitchell JA, Seldon PM, et al. Release of granulocyte-macrophage colony stimulating factor by human cultured airway smooth muscle cells: suppression by dexamethasone. British Journal of Pharmacology. 1997;120(4):545–546. doi: 10.1038/sj.bjp.0700998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belvisi MG, Saunders M, Yacoub M, Mitchell JA. Expression of cyclo-oxygenase-2 in human airway smooth muscle is associated with profound reductions in cell growth. British Journal of Pharmacology. 1998;125(5):1102–1108. doi: 10.1038/sj.bjp.0702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward JE, Gould H, Harris T, Bonacci JV, Stewart AG. PPARγ ligands, 15-deoxy-Δ2,14-prostaglandin J2 and rosiglitazone regulate human cultured airway smooth muscle proliferation through different mechanisms. British Journal of Pharmacology. 2004;141(3):517–525. doi: 10.1038/sj.bjp.0705630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernandes D, Guida E, Koutsoubos V, et al. Glucocorticoids inhibit proliferation, cyclin D1 expression, and retinoblastoma protein phosphorylation, but not activity of the extracellular-regulated kinases in human cultured airway smooth muscle. American Journal of Respiratory Cell and Molecular Biology. 1999;21(1):77–88. doi: 10.1165/ajrcmb.21.1.3396. [DOI] [PubMed] [Google Scholar]
- 77.Johnson PRA, Burgess JK, Underwood PA, et al. Extracellular matrix proteins modulate asthmatic airway smooth muscle cell proliferation via an autocrine mechanism. Journal of Allergy and Clinical Immunology. 2004;113(4):690–696. doi: 10.1016/j.jaci.2003.12.312. [DOI] [PubMed] [Google Scholar]
- 78.Delayre-Orthez C, Becker J, Guenon I, et al. PPARα downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respiratory Research. 2005;6:91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ward JE, Fernandes DJ, Taylor CC, Bonacci JV, Quan L, Stewart AG. The PPARγ ligand, rosiglitazone, reduces airways hyperresponsiveness in a murine model of allergen-induced inflammation. Pulmonary Pharmacology & Therapeutics. 2006;19(1):39–46. doi: 10.1016/j.pupt.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Genovese T, Cuzzocrea S, Di Paola R, et al. Effect of rosiglitazone and 15-deoxy-Δ12,14-prostaglandin J2 on bleomycin-induced lung injury. European Respiratory Journal. 2005;25(2):225–234. doi: 10.1183/09031936.05.00049704. [DOI] [PubMed] [Google Scholar]
- 81.Trifilieff A, El-Hashim A, Bertrand C. Time course of inflammatory and remodeling events in a murine model of asthma: effect of steroid treatment. American Journal of Physiology. 2000;279(6):L1120–L1128. doi: 10.1152/ajplung.2000.279.6.L1120. [DOI] [PubMed] [Google Scholar]
- 82.Lee SY, Kim JS, Lee JM, et al. Inhaled corticosteroid prevents the thickening of airway smooth muscle in murine model of chronic asthma. doi: 10.1016/j.pupt.2006.10.009. to appear in Pulmonary Pharmacology & Therapeutics. [DOI] [PubMed] [Google Scholar]
- 83.Ward C, Walters H. Airway wall remodelling: the influence of corticosteroids. Current Opinion in Allergy and Clinical Immunology. 2005;5(1):43–48. doi: 10.1097/00130832-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 84.Bates J, Irvin C, Brusasco V, et al. The use and misuse of Penh in animal models of lung disease. American Journal of Respiratory Cell and Molecular Biology. 2004;31(3):373–374. doi: 10.1165/ajrcmb.31.3.1. [DOI] [PubMed] [Google Scholar]
- 85.Birrell MA, Battram CH, Woodman P, McCluskie K, Belvisi MG. Dissociation by steroids of eosinophilic inflammation from airway hyperresponsiveness in murine airways. Respiratory Research. 2003;4:3. doi: 10.1186/rr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henry PJ, D'Aprile A, Self G, Hong T, Mann TS. Inhibitors of prostaglandin transport and metabolism augment protease-activated receptor-2-mediated increases in prostaglandin E2 levels and smooth muscle relaxation in mouse isolated trachea. Journal of Pharmacology and Experimental Therapeutics. 2005;314(3):995–1001. doi: 10.1124/jpet.105.086124. [DOI] [PubMed] [Google Scholar]
- 87.Cho H, Tai H-H. Thiazolidinediones as a novel class of NAD+-dependent 15-hydroxyprostaglandin dehydrogenase inhibitors. Archives of Biochemistry and Biophysics. 2002;405(2):247–251. doi: 10.1016/s0003-9861(02)00352-1. [DOI] [PubMed] [Google Scholar]
- 88.Hammad H, de Heer HJ, Soullié T, et al. Activation of peroxisome proliferator-activated receptor-γ in dendritic cells inhibits the development of eosinophilic airway inflammation in a mouse model of asthma. American Journal of Pathology. 2004;164(1):263–271. doi: 10.1016/s0002-9440(10)63116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hashimoto Y, Nakahara K. Improvement of asthma after administration of pioglitazone. Diabetes Care. 2002;25(2):401. doi: 10.2337/diacare.25.2.401. [DOI] [PubMed] [Google Scholar]