Abstract
Successful long term parenteral nutrition has transformed the prognosis for children with irreversible intestinal failure in the last three decades, but has also highlighted the long term complications: intestinal failure associated liver disease; recurrent catheter sepsis; and impaired venous access. Recent advances in small bowel transplantation and non‐transplant surgical techniques now offer hope of sustained survival in the future without parenteral nutrition.
Keywords: intestinal failure, parenteral nutrition, intestinal transplantation, short bowel syndrome
The concept of intestinal failure (IF), defined as “a reduction in the functioning intestinal mass below the amount necessary for adequate absorption to allow for growth” has emerged in the last two decades, due to the success of long term parenteral nutrition (PN).1 The majority of children with IF are weaned from PN within 12 weeks and only a small proportion of children require long term PN.
Intestinal failure
Epidemiology
The precise epidemiology and incidence of children with IF is not known. Using children on home PN as a proxy, the incidence in the early 1990s was estimated to be 1–2 per million population.2 More recently, a multicentre European survey conducted in 2001 estimated the incidence of children on home PN to be 2–6.8 per million population.3 The British Artificial Nutrition Survey registered 81 children for home PN between1996 and 1999, giving a point prevalence of 2 children per million.4 On the basis of a survey conducted in West Yorkshire, the authors estimated that around 200 children in the UK need PN for more than 28 days.5 Advances in neonatal intensive care, paediatric surgical techniques, and improved PN, will almost certainly result in an increase in the incidence of long term survival of children with IF.
Current national scene
A UK based IF registry has been established by the British Society of Paediatric Gastroenterology and Nutrition with the support of the Department of Health and the British Association of Paediatric Surgeons to identify the number of children with IF on long term PN within the UK.
Children with IF are managed at regional gastroenterology centres in the UK and children with severe complications are referred to Birmingham Children's Hospital (BCH), the only designated paediatric intestinal transplantation centre in the UK, for consideration of intestinal transplantation.
Aetiology
This is subdivided into three categories (table 1):
Table 1 Causes of intestinal failure.
| Category | Disorder |
|---|---|
| Short bowel syndrome | Neonatal period |
| Gastroschisis | |
| Necrotising enterocolitis | |
| Small bowel atresia | |
| Malrotation with volvulus | |
| Total aganglionosis | |
| Older children | |
| Crohn's disease | |
| Mesenteric infarction | |
| Radiation enteritis | |
| Tumours | |
| Trauma | |
| Motility disorders | Hirschprung's disease |
| Hollow visceral myopathy | |
| Neuronal intestinal dysplasia | |
| Megacystis‐microcolon‐hypoperistalsis syndrome | |
| Abnormalities of interstitial cells of Cajal | |
| Mucosal disorders | Primary epithelial abnormalities |
| Microvillous inclusion disease | |
| Tufting enteropathy (primary epithelial dysplasia) | |
| Congenital disorders of glycosylation | |
| Immune mediated | |
| Underlying immunodeficiency states, e.g. severe combined immunodeficiency, pan‐hypogammaglobulinaemia | |
| Autoimmune | |
| Autoimmune enteropathy with nephropathy (AIE‐75/harmonin antibody) | |
| Unclassified autoimmune enteropathy | |
| Syndromic intractable diarrhoea |
Short bowel syndrome
Motility disorders
Primary mucosal disorders.
Short bowel syndrome
The small intestine has a vast functional reserve and can tolerate 40–50% resection without major metabolic or nutritional sequelae.6 The residual length of short bowel, an intact ileo‐caecal valve, preservation of at least the right colon, and functioning intestinal continuity are all considered important factors for survival and adaptation. In addition, the degree of cholestasis, age at the time of resection, type of the small bowel remaining (ileum adapts better than jejunum), and the functional integrity of the remaining small intestine are all factors which determine long term prognosis.7 Following resection, the remaining bowel undergoes structural and functional changes for which PN provides a crucial “time bridge” to allow this process of intestinal adaptation to occur.
Enteral nutrition
Enteral nutrition is the most significant single factor in promoting intestinal adaptation and may play a part in reducing the frequency of intestinal failure associated liver disease (IFALD). Detailed evidence on the management of short bowel syndrome (SBS) has recently been published.8 Breast milk may be the best choice in the first few months, because of the presence of trophic factors such as epidermal growth factor.9 Conceptually, a protein hydrolysate or amino acid based formula feed would seem to be more appropriate in children with SBS due to the decreased luminal contact time. However, there is no convincing evidence to support their use in preference to a polymeric feed.10 Amino acid based formulas may be beneficial in weaning children from PN, perhaps due to less antigenic load.9 Many infants have some degree of disaccharide intolerance, and a glucose polymer based formula feed may be used. A high fat diet (60% of the calories as fat) may be beneficial in providing a good source of energy and does not have a major impact on the stool volume and losses in children with an end‐jejunostomy.11 When glucose polymer intolerance is a problem, a modular feed allows the individual components of the feeds to be varied independently and tailored to the tolerances of the individual patient. The availability of skilled dietetic advice before embarking on modular feeds is of paramount importance.8 Continuous nasogastric (NG) feeding initially, followed by overnight NG feeding and bolus feeding during the day, is recommended in order to utilise the existing small bowel function and to encourage oral feeding. Maintaining a urinary sodium/potassium ratio of at least 2:1 with an absolute urinary sodium concentration of over 10–20 mmol/l is important in children with ongoing fluid and electrolyte losses.12 Currently, insufficient evidence exists in the literature to support the routine use of pectin, glutamine, growth hormone, IGF‐1, or Saccharomyces boulardii as trophic factors in the process of adaptation.13
One of the most promising agents in the promotion of post‐resection adaptation is glucagon‐like peptide‐2 (GLP‐2). GLP‐2 is one of a number of pro‐glucagon derived peptides secreted from the ileal and colonic mucosa after food. In the experimental animal, post‐resection plasma concentrations co‐relate with the extent of resection, crypt cell proliferation, and nutrient malabsorption.14 GLP‐2 induces marked proliferation of the small intestine epithelium and in a small study in humans with SBS led to improvement in body weight and nutrient absorption.15,16 The effects of growth hormone administration remain controversial, with differing effects according to dosage and concerns about side effects.17
Small bowel bacterial overgrowth
Following intestinal resection sufficient to cause SBS, the residual intestine may become dilated and dysmotile, leading to stasis of intestinal nutrients, thereby promoting small bowel bacterial overgrowth (SBBO). The SBBO and bacterial translocation may lead to malabsorption and potentially systemic sepsis. The diagnosis of SBBO is difficult to establish and the gold standard is aerobic and anaerobic culture of jejunal fluid. Usually the diagnosis is made empirically on the basis of a dilated dysmotile small bowel in the presence of delayed adaptation. Differing antibiotic regimens have been used for treatment and are usually given for 7–14 days with a 14–28 day rest period.18 The alteration of the enteric bacterial flora by probiotics, although documented in isolated case reports, has not been shown to have conclusive benefit in big multicentre studies in children with SBBO.19,20
Non‐transplant surgery
The surgical options aim to provide maximum mucosal contact without disturbing motility or reducing total absorptive mass. The entire length of the bowel available should be used—that is, stomas should be closed and any stricture or localised area of diseased bowel resected. The Bianchi (bowel lengthening) procedure, intestinal tapering or plication, and recently, the serial transverse enteroplasty procedure (STEP) have been tried with varying success.21 A favourable outcome following a lengthening procedure was seen in children with at least 40 cm of bowel and little hepatic dysfunction, although the observations were uncontrolled.22 The STEP procedure may appear to have some advantages over the Bianchi procedure: (1) the mesenteric blood supply remains undisturbed; (2) it can be performed on smaller dilated segments; and (3) it can be performed on dilated bowel segments after a previous Bianchi procedure. However, since the procedure has been introduced only recently, the long term outcome is still unknown. Deciding on the optimal timing for these procedures is difficult. Moreover, they have not been shown to be of benefit in children with significant liver disease and signs of portal hypertension.23
The outcome of children with SBS on long term PN support has been recently described in two separate reports.24,25 The favourable factors for intestinal adaptation are a short bowel length of >15 cm, presence of the ileocaecal valve, preservation of the colon, and early establishment of intestinal continuity in selected cases. The children who discontinued PN and achieved intestinal autonomy exhibited a normal growth pattern in the long term.24,25
Primary motility disorders
Chronic intestinal pseudo‐obstruction (CIP) is a heterogeneous group of rare disorders, presenting with symptoms and signs of intestinal obstruction, but without a mechanical basis. They result from a variety of abnormalities in the enteric nervous system or musculature and can affect variable segments of the gastrointestinal tract, sometimes with other hollow viscera involved such as the bladder. Most patients present in infancy. The diagnosis is usually established by careful history, physical examination, exclusion of a mechanical obstruction radiologically, and exclusion of Hirschprung's disease or intestinal neuronal dysplasia by rectal biopsy and manometric studies. Symptoms mimicking intestinal pseudo‐obstruction can be fabricated and the clinician should have a high index of suspicion for factitious illness. The presence of daily abdominal pain, the involvement of more than three organ systems, a disease process which is rapidly progressive, preterm delivery, and the absence of dilated gut on x ray examination with normal motility studies are all suggestive of factitious illness.26 Isolated reports exist in the literature about children with factitious illness mimicking pseudo‐obstruction showing improvement of symptoms following separation from their parents.27 In a recent report, manometric studies, along with histochemical techniques and ultrastructural studies performed on full thickness biopsy specimens, helped in categorisation of the causes into myopathic (abnormal smooth muscle) or neuropathic (abnormal neuronal function). Exploratory laparotomy should be avoided unless and until there is clear evidence of mechanical obstruction. A low fibre, low lactose, low residue, low fat feed should be considered. Children with CIP may need a gastrostomy, gastro‐jejunal tube, or a jejunostomy for advancing feeds. Indicators of poor prognosis are considered to be: onset of the symptoms at less than 1 year of age; involvement of the urinary tract; midgut malrotation; or a myopathic histology.28 The prognosis of children with CIP has improved substantially over recent years with the advent of home PN and meticulous care of central venous catheters.7
Primary mucosal disorders
Recent advances in molecular biology and immunology have led to a better understanding of the enteropathies associated with intractable diarrhoea.29 In microvillous inclusion disease, a probable disturbance in the apical targeting mechanism for microvilli results in the characteristic appearances seen on electron microscopy: microvillous inclusion bodies and apical secretory granules. Two variants of this disorder are recognised: an early variant, presenting in the first week of life and needing lifelong PN; and a late variant presenting at a few months of life in which variable amounts of enteral nutrition are tolerated. Due to the autosomal recessive inheritance pattern, multiple siblings in a family may be affected. The identification of the gene remains elusive.29
Tufting enteropathy or primary epithelial dysplasia usually presents in the first few months of life and is characterised by the presence of focal tufts on the epithelium comprising of closely packed enterocytes with rounding of the apical cell membrane resembling a “tear drop” configuration.29 A primary genetic defect, as yet unidentified, is likely, based on the high frequency of the disorder in Malta. Molecular studies in individuals with tufting enteropathy have raised the question of a defect in the cell matrix:basement membrane interaction, due to the presence of desmosomes on ultra‐structural studies. Clinically, tufting enteropathy is often characterised by a mixed picture of osmotic and secretory diarrhoea. Children have specific defects in the absorption of carbohydrate suggesting that there may be a down‐regulation of sodium‐glucose transport.
Most of the children in this subgroup of disorders are candidates for long term PN and will ultimately need intestinal transplantation.
Parenteral nutrition in the management of IF
The North American Home Parenteral and Enteral Nutrition patient registry indicates a four year survival on home parenteral nutrition of 80% for SBS patients and 70% for motility disorders.30 The quality of life (QOL) of home PN patients of all ages is reported to be not significantly different from the scores in a reference population of healthy children and adolescents.31 The QOL is significantly impaired in parents, especially in mothers for items related to work, inner life, and freedom. A multidisciplinary nutritional care team (NCT) which includes a gastroenterologist, paediatric surgeon, dietician, PN pharmacist, social worker, and nutritional nurse is necessary in preventing complications and improving long term survival of children on PN.32
The main complications commonly associated with long term use of PN are:
Central venous catheter (CVC) related infections
Thrombosis of the vessels leading to impaired venous access
Intestinal failure associated liver disease (IFALD).
Central venous catheter related infections
Episodes of line infection can cause a greater than 30% rise in bilirubin level, and cholestasis may develop in 90% of infants after the first line infection.33,34 The reduction in the overall incidence of CVC infection is crucial to sustained good health. Failure to prevent CVC infection greatly contributes to progression of the liver disease. Involvement of a multidisciplinary nutritional care team and early discharge on home PN has been shown to reduce the incidence of CVC infection.35,36
Thrombosis of the vessels leading to impaired venous access
The repeated episodes of line infections with multiple surgical procedures to remove and replace new catheters may predispose to thrombosis of the major vessels, leading to impaired venous access (defined as loss of two vascular sites in the neck to thrombosis).37 Percutaneous vascular insertion techniques using Doppler ultrasound to guide the tip of the needle into the vein with a single pass and minimal trauma may have a significant role to play in preservation of the functional venous access and prevention of thrombotic occlusion of central veins.38 Despite meticulous care and aggressive strategies to prevent line infections, some children may develop end stage loss of venous access and need referral for intestinal transplantation. There is little evidence that the use of anticoagulants prevents thrombosis of the vessels in children on long term PN.39
Pulmonary thromboembolism is another potentially fatal complication of long term venous access, occurring in 39% of the children.40 In asymptomatic children, yearly echocardiography and ventilation‐perfusion scanning are recommended, unless there is clinical suspicion or the child is exhibiting symptoms suggestive of pulmonary embolism.
Intestinal failure associated liver disease
The development of liver disease is seen in 40–60% of the children with IF on long term parenteral nutrition.41 In a multivariate analysis of post‐surgical neonates developing cholestasis, the significant factors associated with IFALD were prematurity (median gestational age 34 weeks) and CVC line infections. Several speculative mechanisms are postulated:
Direct hepatotoxicity due to the hepatic immaturity in disposing of potentially toxic substances41
Deficiencies of taurine, choline, glutamine, and methyl donor molecules such as serine and methionine have been proposed, but no strong evidence exists in the literature41
Absence of enteral nutrition, leading to lack of stimulation of the enterohepatic circulation and accumulation of toxic bile acids causing cholestasis
Small bowel bacterial overgrowth and line infections release endotoxin and inducible pro‐inflammatory mediators such as tumour necrosis factor, which probably stimulate de novo fatty acid and triglyceride synthesis, resulting in steatosis and liver disease
The amount of lipid infused has been suggested to be a causative factor in a retrospective study, and limiting the load of lipid has been suggested to prevent progression of liver disease.42 However, of the 10 children reported in the study, six were considered for combined liver and small bowel transplantation, and prolonged lipid removal resulted in essential fatty acid deficiency and growth retardation.42 Various combinations of medium and long chain triglyceride based lipid emulsions are proposed in an attempt to prevent progression of liver disease. Although a combination of medium and long chain triglyceride based lipid emulsions has been proved to be safe and effective, the results of a long term randomised double blind controlled trial in the prevention or reversal of cholestatic disease are urgently needed.43 Elevation of plant sterols reported previously may be secondary to cholestasis rather than a cause for cholestasis.44 In interpreting all the trials it is important to remember that IFALD was seen before the use of lipid in nutritional support.
The role of using PN with elevated glucose concentrations to compensate for the limited lipid load is not favoured as it may induce insulin resistance and lead to liver steatosis.
Abnormalities in hepatic enzymes are often seen within four weeks of onset of PN in children. Treatment with ursodeoxycholic acid reduces transaminase levels, but does not alter the course of IFALD.45,46 In children developing progressive liver disease, lipid intake in PN should be restricted to 2 g/kg/day, and all efforts should be directed towards increasing enteral feed tolerance. Early referral for intestinal transplantation is recommended because rapid progression of liver disease is well recognised. Children referred with a plasma bilirubin in excess of 200 μmol/l have a life expectancy without transplantation of around six months.47 In children with established cirrhosis, the 12 month survival is 30%, while the development of portal hypertension with varices and coagulopathy reduces survival to less than eight weeks.23
Isolated liver transplantation in children with SBS
Some infants and young children under the age of 3 years with SBS and adequate length of bowel fail to achieve maximal adaptation because of end stage liver disease, and may benefit from isolated liver transplantation (ILTx).48 The fate of the transplanted liver depends on the ability of the residual bowel to tolerate at least some enteral feeding after ILTx while receiving PN; thus ILTx is not an option in children with ultra short bowel (residual bowel length of less than 25 cm), nor is it an option in children with an underlying primary motility disorder or mucosal disorder. It is our current practice to offer ILTx for children with SBS who have both tolerated at least 50% of estimated daily requirements enterally and demonstrated adequate weight gain, and in whom at least 30 cm residual small bowel remains intact, with or without the ileo‐caecal valve. Following liver transplantation, weaning from PN to full enteral feeds may take several months to years.48,49
The selection of candidates for ILTx should be done jointly by paediatric gastroenterologists, paediatric hepatologists, and transplant surgeons at a centre experienced in the management of intestinal failure. The role of paediatric hepatology centres (involved in liver transplantation for primary liver diseases) should be limited to assessment of the severity of liver disease and referral to an appropriate centre experienced in the management of such complex children.
Intestinal transplantation
Intestinal transplantation (ITx) has evolved from being an experimental procedure in the late 1980s to its current status of a life saving option for children who develop major complications of intestinal failure. The international ITx registry reported in July 2005 that more than 1300 ITx have been performed worldwide in 65 centres in 19 countries.
Indications and contraindications
The indications for ITx are irreversible intestinal failure and one of the following: impaired venous access (reduced to two suitable veins for placement of feeding catheters); progressive liver disease with coagulopathy, ascites, and encephalopathy; and life threatening episodes of catheter sepsis.30
With the improvement of post‐transplant immunosuppression and better control of infections resulting in improved survival, the contraindications can be further subdivided as follows:
-
Absolute contraindications
-
-
Profound neurological disabilities
-
-
Life threatening and other irreversible disease not related to the digestive system
-
-
Non‐resectable malignancies
-
-
-
Relative contraindications
-
-
Severe congenital or acquired immunological deficiencies
-
-
Multi‐system autoimmune diseases
-
-
Insufficient vascular patency to guarantee vascular access for up to six months after transplant
-
-
Chronic lung disease of prematurity.
-
-
Types of operation
The complete multi‐visceral allograft has been likened to a grape cluster with a double central stem consisting of the coeliac axis and the superior mesenteric artery.50 The individual organs can be removed and transplanted like grapes of a vine. The severity of the liver disease determines the organs to be transplanted, so that patients with mild liver disease (no evidence of portal hypertension, mild hepatic fibrosis on liver biopsy) can be offered an isolated intestinal, or a modified multi‐visceral graft, including stomach if dysmotility of the foregut is a prominent clinical problem.
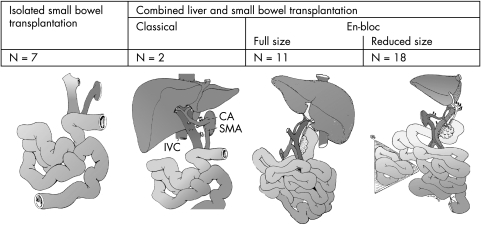
However, in patients with moderate to severe liver disease, a liver and small bowel transplant is recommended. The precise method of implantation of the organs can be varied. The preferred technique is the composite graft where the liver and intestine with bile ducts, duodenum, and head of pancreas can be implanted en bloc with minimal disruption to the vascular and other structures connecting the organs; or the organs can be retrieved from the donor, separated, and implanted individually, which is known as non‐composite combined liver and small bowel transplantation. Multi‐visceral transplantation is the term applied to any intestinal transplant in which more than the liver and small bowel are transplanted (usually stomach and whole pancreas); it is offered to patients with extensive disease, for example, motility disorders or desmoid tumour (fig 1).
Figure 1 The different types of intestinal transplantation performed at Birmingham Children's Hospital (n = 38). CA, coeliac axis; IVC, inferior vena cava; SMA, superior mesenteric artery. (Reproduced by permission of Prof. Jean de Ville de Goyet.)
Due to the lack of availability of size matched organs, 50–60% of the children die on the ITx waiting list, the majority of these being the under 1 year of age and less than 10 kg weight. The technique of en bloc reduction, using liver and small bowel from much larger adult donors for small children by excision of usually the right lobe of the liver and mid‐section of the small bowel graft, has been successfully used to overcome this problem.51 Living related ITx is beneficial in mononzygotic twins, but does not seem to confer any immunological benefit in other situations.52,53
Long term outcome
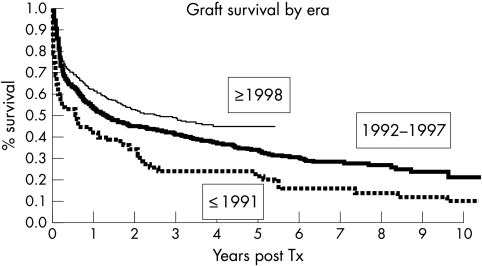
Complications were common in the early years following ITx and included significant surgical morbidity, moderate to severe acute rejection, and opportunistic infections. The incidence and severity of rejection has improved considerably after the advent of IL‐2 blockers and other immunosuppression strategies.54 With the use of an appropriate cytomegalovirus prophylaxis regimen and Epstein‐Barr virus polymerase chain reaction monitoring techniques for prevention of quasi‐neoplastic post‐transplant lymphoproliferative disease, the incidence of these dreaded complications is significantly less.55 The advances in surgical techniques and use of newer immunosuppressive regimens have resulted in improved outcomes following intestinal transplantation and increased optimism that it offers a real opportunity for cure (fig 2).55
Figure 2 Graft survival rates after intestine transplantation have significantly improved over time (p<0.001) as per data from the intestinal transplant registry. (Reproduced, with permission, from Grant et al, Ann Surg 2005;241:607–13.)
The improving long term success has raised issues regarding quality of life and growth in children following ITx. Sudan et al reported on quality of life in 10–16 year old small bowel transplant recipients. The recipients rated their quality of life as equivalent to healthy children of the same age, although their parents remained more anxious than the parents of healthy children.56 The catch‐up growth seen in children following liver transplantation has not been demonstrated in children with ITx.57 This is probably related to the severity of the illness at the time of presentation and the use of high intensity immunosuppression, including the use of long term steroids.
BCH experience
Two hundred and twelve children with IF were assessed for intestinal transplantation (ITx) at BCH between 1989 and 2005. Following the assessment, children were categorised into the three prognostic groups: (1) stable on PN (n = 82) and further follow up with the referring paediatric gastroenterologist; (2) unsuitable for transplantation (n = 43) due to end stage liver disease and/or other co‐morbid conditions; and (3) recommended for transplantation (n = 87). Of the 87 children recommended for transplantation, nine families declined transplantation following counselling, 22 children died on the waiting list, two children improved and were removed from the transplant list, and 38 ITx (median age 2.3 years, median weight 11 kg) and 16 ILTx (median age 0.8 years, median weight 7.8 kg) have been performed. At the time of reporting, 18/38 ITx children and 9/14 isolated liver transplant children are alive, with a overall five year survival rate of 52%.
Summary
No effort should be spared in promoting intestinal adaptation in children with SBS, including the option of surgery where appropriate. A multidisciplinary NCT management strategy needs to be evolved by each regional centre to minimise the complications associated with long term PN and to facilitate early discharge to home PN whenever possible. Complications should be recognised and earlier referral (serum bilirubin >100 μmol/l sustained over a period of 3–4 weeks) to an intestinal transplant centre is recommended. ITx is still reserved for children developing complications related to PN. However, with improving results of ITx worldwide, it may have to be considered earlier, both for economic reasons and to improve quality of life.
Footnotes
Competing interests: none declared
References
- 1.Irving M. Intestinal failure. J Gastroenterol Hepatol 200015(suppl)G26–G29. [DOI] [PubMed] [Google Scholar]
- 2.Ingham Clark C L, Lear P A, Wood S.et al Potential candidates for small bowel transplantation. Br J Surg 199279676–679. [DOI] [PubMed] [Google Scholar]
- 3.Van Gossum A, Vahedi K, Abdel M.et al Clinical, social and rehabilitation status of long term home parenteral nutrition patients: results of a European multicentre survey. Clin Nutr 200120205–210. [DOI] [PubMed] [Google Scholar]
- 4.Holden C. Review of home paediatric parenteral nutrition on the UK. Br J Nurs 200110782–788. [DOI] [PubMed] [Google Scholar]
- 5.Koeglmeier J, Martin H, Day C.et al A survey of children with intestinal failure in West Yorkshire. Pediatr Transplant 20059(suppl 6)77 [Google Scholar]
- 6.Jeejeebhoy K N. Therapy of the short‐gut syndrome. Lancet 198311427–1430. [DOI] [PubMed] [Google Scholar]
- 7.Goulet O, Ruemmele F, Lacaille F.et al Irreversible intestinal failure. J Pediatr Gastroenterol Nutr 200438250–269. [DOI] [PubMed] [Google Scholar]
- 8.Booth I W, Lander A D. Short bowel syndrome. Baillieres Clin Gastroenterol 199812739–773. [DOI] [PubMed] [Google Scholar]
- 9.Andorsky D J, Lund D P, Lillehei C W.et al Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr 200113927–33. [DOI] [PubMed] [Google Scholar]
- 10.Ksiazyk J, Piena M, Kierkus J.et al Hydrolyzed versus nonhydrolyzed protein diet in short bowel syndrome in children. J Pediatr Gastroenterol Nutr 200235615–618. [DOI] [PubMed] [Google Scholar]
- 11.Woolf G M, Miller C, Kurian R.et al Diet for patients with a short bowel: high fat or high carbohydrate? Gastroenterology 198384823–828. [PubMed] [Google Scholar]
- 12.Uchiyama M, Otsuka T, Shibuya Y.et al Electrolyte excretion in 12‐hour urine and in spot urine. Relationship to plasma renin activity and aldosterone concentration in older children. Acta Paediatr Scand 198574394–399. [DOI] [PubMed] [Google Scholar]
- 13.Vanderhoof J A. New and emerging therapies for short bowel syndrome in children. J Pediatr Gastroenterol Nutr 200439(suppl 3)769–771. [DOI] [PubMed] [Google Scholar]
- 14.Martin G R, Wallace L E, Sigalet D L. Glucagon‐like peptide‐2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2004286G964–G972. [DOI] [PubMed] [Google Scholar]
- 15.Jeppesen P B, Hartmann B, Thulesen J.et al Glucagon‐like peptide 2 improves nutrient absorption and nutritional status in short‐bowel patients with no colon. Gastroenterology 2001120806–815. [DOI] [PubMed] [Google Scholar]
- 16.Estall J L, Drucker D J. Tales beyond the crypt: glucagon‐like peptide‐2 and cytoprotection in the intestinal mucosa. Endocrinology 200514619–21. [DOI] [PubMed] [Google Scholar]
- 17.Szkudlarek J, Jeppesen P B, Mortensen P B. Effect of high dose growth hormone with glutamine and no change in diet on intestinal absorption in short bowel patients: a randomised, double blind, crossover, placebo controlled study. Gut 200047199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kocoshis S. Small intestinal failure in children. Curr Treat Options Gastroenterol 20014423–432. [DOI] [PubMed] [Google Scholar]
- 19.Matarese L E, Seidner D L, Steiger E. The role of probiotics in gastrointestinal disease. Nutr Clin Pract 200318507–516. [DOI] [PubMed] [Google Scholar]
- 20.Kanamori Y, Hashizume K, Sugiyama M.et al Combination therapy with Bifidobacterium breve, Lactobacillus casei, and galactooligosaccharides dramatically improved the intestinal function in a girl with short bowel syndrome: a novel synbiotics therapy for intestinal failure. Dig Dis Sci 2001462010–2016. [DOI] [PubMed] [Google Scholar]
- 21.Sudan D, Dibaise J, Torres C.et al A multidisciplinary approach to the treatment of intestinal failure. J Gastrointest Surg 20059165–177. [DOI] [PubMed] [Google Scholar]
- 22.Bianchi A. Experience with longitudinal intestinal lengthening and tailoring. Eur J Pediatr Surg 19999256–259. [DOI] [PubMed] [Google Scholar]
- 23.Bueno J, Ohwada S, Kocoshis S.et al Factors impacting the survival of children with intestinal failure referred for intestinal transplantation. J Pediatr Surg 19993427–32. [DOI] [PubMed] [Google Scholar]
- 24.Goulet O, Baglin‐Gobet S, Talbotec C.et al Outcome and long term growth after extensive small bowel resection in the neonatal period: a survey of 87 children. Eur J Pediatr Surg 20051595–101. [DOI] [PubMed] [Google Scholar]
- 25.Quiros‐Tejeira R E, Ament M E, Reyen L.et al Long term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: a 25‐year experience. J Pediatr 2004145157–163. [DOI] [PubMed] [Google Scholar]
- 26.Hyman P E, Bursch B, Beck D.et al Discriminating pediatric condition falsification from chronic intestinal pseudo‐obstruction in toddlers. Child Maltreat 20027132–137. [DOI] [PubMed] [Google Scholar]
- 27.Ginies J L, Goulet O, Champion G.et al [Munchausen's syndrome by proxy and chronic intestinal pseudo‐obstruction]. Arch Fr Pediatr 198946267–269. [PubMed] [Google Scholar]
- 28.Heneyke S, Smith V V, Spitz L.et al Chronic intestinal pseudo‐obstruction: treatment and long term follow up of 44 patients. Arch Dis Child 19998121–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murch S H. Toward a molecular understanding of complex childhood enteropathies. J Pediatr Gastroenterol Nutr 200234(suppl 1)S4–10. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman S S, Atkinson J B, Bianchi A.et al Indications for pediatric intestinal transplantation: a position paper of the American Society of Transplantation. Pediatr Transplant 2001580–87. [DOI] [PubMed] [Google Scholar]
- 31.Gottrand F, Staszewski P, Colomb V.et al Satisfaction in different life domains in children receiving home parenteral nutrition and their families. J Pediatr 2005146793–797. [DOI] [PubMed] [Google Scholar]
- 32.Agostoni C, Axelson I, Colomb V.et al The need for nutrition support teams in pediatric units: a commentary by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr 2005418–11. [DOI] [PubMed] [Google Scholar]
- 33.Sondheimer J M, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resection and long term parenteral nutrition. J Pediatr Gastroenterol Nutr 199827131–137. [DOI] [PubMed] [Google Scholar]
- 34.Beath S V, Davies P, Papadopoulou A.et al Parenteral nutrition‐related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg 199631604–606. [DOI] [PubMed] [Google Scholar]
- 35.Knafelz D, Gambarara M, Diamanti A.et al Complications of home parenteral nutrition in a large pediatric series. Transplant Proc 2003353050–3051. [DOI] [PubMed] [Google Scholar]
- 36.Beath S V, Booth I W, Murphy M S.et al Nutritional care and candidates for small bowel transplantation. Arch Dis Child 199573348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juno R J, Knott A W, Racadio J.et al Reoperative venous access. Semin Pediatr Surg 200312132–139. [DOI] [PubMed] [Google Scholar]
- 38.McBride K D, Fisher R, Warnock N.et al A comparative analysis of radiological and surgical placement of central venous catheters. Cardiovasc Intervent Radiol 19972017–22. [DOI] [PubMed] [Google Scholar]
- 39.Newall F, Barnes C, Savoia H.et al Warfarin therapy in children who require long term total parenteral nutrition. Pediatrics 2003112e386. [DOI] [PubMed] [Google Scholar]
- 40.Dollery C M, Sullivan I D, Bauraind O.et al Thrombosis and embolism in long term central venous access for parenteral nutrition. Lancet 19943441043–1045. [DOI] [PubMed] [Google Scholar]
- 41.Kelly D A. Liver complications of pediatric parenteral nutrition—epidemiology. Nutrition 199814153–157. [DOI] [PubMed] [Google Scholar]
- 42.Colomb V, Jobert‐Giraud A, Lacaille F.et al Role of lipid emulsions in cholestasis associated with long term parenteral nutrition in children. JPEN J Parenter Enteral Nutr 200024345–350. [DOI] [PubMed] [Google Scholar]
- 43.Goulet O, de Potter S, Antebi H.et al Long term efficacy and safety of a new olive oil‐based intravenous fat emulsion in pediatric patients: a double‐blind randomized study. Am J Clin Nutr 199970338–345. [DOI] [PubMed] [Google Scholar]
- 44.Clayton P T, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition 199814158–164. [DOI] [PubMed] [Google Scholar]
- 45.Chen C Y, Tsao P N, Chen H L.et al Ursodeoxycholic acid (UDCA) therapy in very‐low‐birth‐weight infants with parenteral nutrition‐associated cholestasis. J Pediatr 2004145317–321. [DOI] [PubMed] [Google Scholar]
- 46.Spagnuolo M I, Iorio R, Vegnente A.et al Ursodeoxycholic acid for treatment of cholestasis in children on long term total parenteral nutrition: a pilot study. Gastroenterology 1996111716–719. [DOI] [PubMed] [Google Scholar]
- 47.Beath S V, Needham S J, Kelly D A.et al Clinical features and prognosis of children assessed for isolated small bowel or combined small bowel and liver transplantation. J Pediatr Surg 199732459–461. [DOI] [PubMed] [Google Scholar]
- 48.Horslen S P, Sudan D L, Iyer K R.et al Isolated liver transplantation in infants with end‐stage liver disease associated with short bowel syndrome. Ann Surg 2002235435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottrand F, Michaud L, Bonnevalle M.et al Favorable nutritional outcome after isolated liver transplantation for liver failure in a child with short bowel syndrome. Transplantation 199967632–634. [DOI] [PubMed] [Google Scholar]
- 50.Kato T, Tzakis A G, Selvaggi G.et al Surgical techniques used in intestinal transplantation. Curr Opin Organ Transplant 20049207–213. [Google Scholar]
- 51.de Ville D G, Mitchell A, Mayer A D.et al En block combined reduced‐liver and small bowel transplants: from large donors to small children. Transplantation 200069555–559. [DOI] [PubMed] [Google Scholar]
- 52.Holterman M J, Holterman A L, Carrol R.et al Living‐related bowel transplantation to treat short bowel syndrome in a four‐year‐old child: a case report. J Pediatr Surg 2003381763–1765. [DOI] [PubMed] [Google Scholar]
- 53.Fujimoto Y, Uemoto S, Inomata Y.et al Small bowel transplantation using grafts from living‐related donors. Two case reports. Transpl Int 200013(suppl 1)S179–S184. [DOI] [PubMed] [Google Scholar]
- 54.Sudan D L, Chinnakotla S, Horslen S.et al Basiliximab decreases the incidence of acute rejection after intestinal transplantation. Transplant Proc 200234940–941. [DOI] [PubMed] [Google Scholar]
- 55.Grant D, Abu‐Elmagd K, Reyes J.et al 2003 report of the intestine transplant registry: a new era has dawned. Ann Surg 2005241607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sudan D, Horslen S, Botha J.et al Quality of life after pediatric intestinal transplantation: the perception of pediatric recipients and their parents. Am J Transplant 20044407–413. [DOI] [PubMed] [Google Scholar]
- 57.Iyer K, Horslen S, Iverson A.et al Nutritional outcome and growth of children after intestinal transplantation. J Pediatr Surg 200237464–466. [DOI] [PubMed] [Google Scholar]