Abstract
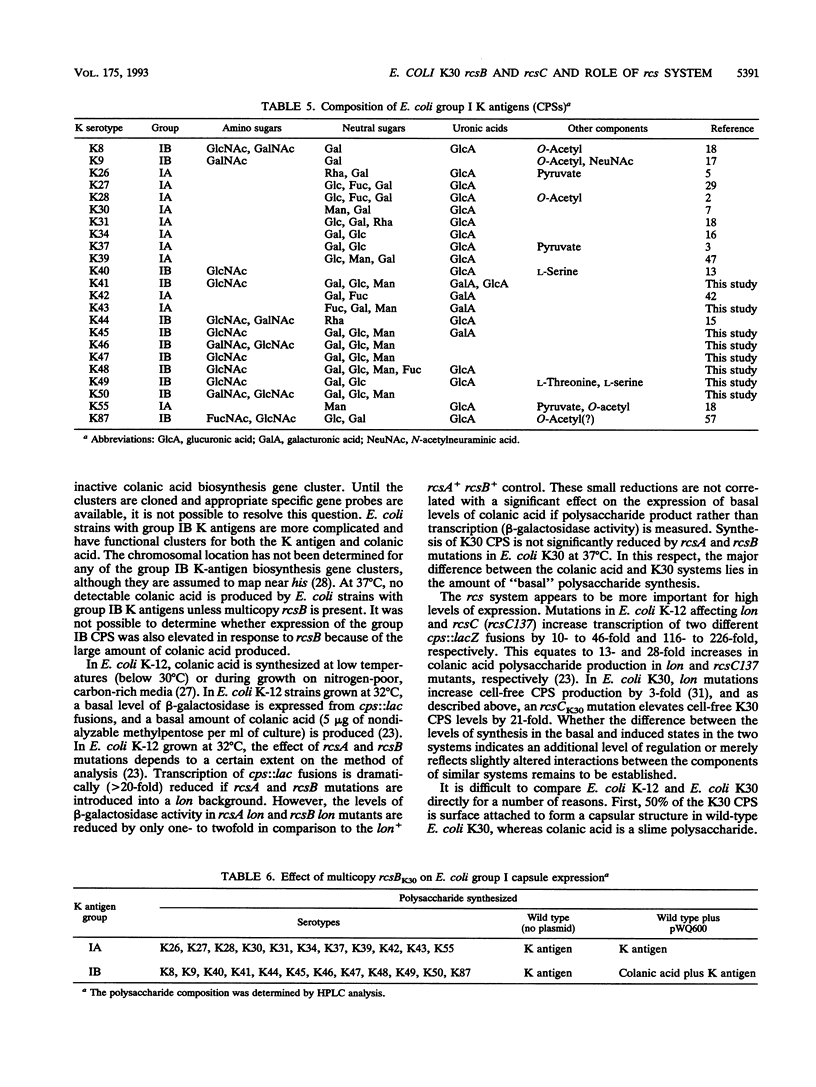
In Escherichia coli K-12, RcsC and RcsB are thought to act as the sensor and effector components, respectively, of a two-component regulatory system which regulates expression of the slime polysaccharide colanic acid (V. Stout and S. Gottesman, J. Bacteriol. 172:659-669, 1990). Here, we report the cloning and DNA sequence of a 4.3-kb region containing rcsC and rcsB from E. coli O9:K30:H12. This strain does not produce colanic acid but does synthesize a K30 (group I) capsular polysaccharide. The rcsB gene from E. coli K30 (rcsBK30) is identical to the rcsB gene from E. coli K-12 (rcsBK-12). rcsCK30 has 16 nucleotide changes, resulting in six amino acid changes in the predicted protein. To examine the function of the rcs regulatory system in expression of the K30 capsular polysaccharide, chromosomal insertion mutations were constructed in E. coli O9:K30:H12 to independently inactivate rcsBK30 and the auxiliary positive regulator rcsAK30. Strains with these mutations maintained wild-type levels of K30 capsular polysaccharide expression and still produced a K30 capsule, indicating that the rcs system is not essential for expression of low levels of the group I capsular polysaccharide in lon+ E. coli K30. However, K30 synthesis is increased by introduction of a multicopy plasmid carrying rcsBK30. K30 polysaccharide expression is also markedly elevated in an rcsBK30-dependent fashion by a mutation in rcsCK30, suggesting that the rcs system is involved in high levels of synthesis. To determine whether the involvement of the rcs system in E. coli K30 expression is typical of group I (K antigen) capsules, multicopy rcsBK30 was introduced into 22 additional strains with structurally different group I capsules. All showed an increase in mucoid phenotype, and the polysaccharides produced in the presence and absence of multicopy rcsBK30 were examined. It is has been suggested that E. coli strains with group I capsules can be subdivided based on K antigen structure. For the first time, we show that strains with group I capsules can also be subdivided by the ability to produce colanic acid. Group IA contains capsular polysaccharides (including K30) with repeating-unit structures lacking amino sugars, and expression of group IA capsular polysaccharides is increased by multicopy rcsBK30. Group IB capsular polysaccharides all contain amino sugars. In group IB strains, multicopy rcsBK30 activates synthesis of colanic acid.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P., Hart C. A., Saunders J. R. Isolation from Klebsiella and characterization of two rcs genes that activate colanic acid capsular biosynthesis in Escherichia coli. J Gen Microbiol. 1987 Feb;133(2):331–340. doi: 10.1099/00221287-133-2-331. [DOI] [PubMed] [Google Scholar]
- Altman E., Dutton G. G. Chemical and structural analysis of the capsular polysaccharide from Escherichia coli O9:K28(A):H- (K28 antigen). Carbohydr Res. 1985 May 15;138(2):293–303. doi: 10.1016/0008-6215(85)85112-0. [DOI] [PubMed] [Google Scholar]
- Anderson A. N., Parolis H., Parolis L. A. Structural investigation of the capsular polysaccharide from Escherichia coli O9:K37 (A 84a). Carbohydr Res. 1987 Jun 1;163(1):81–90. doi: 10.1016/0008-6215(87)80167-2. [DOI] [PubMed] [Google Scholar]
- Bernhard F., Poetter K., Geider K., Coplin D. L. The rcsA gene from Erwinia amylovora: identification, nucleotide sequence, and regulation of exopolysaccharide biosynthesis. Mol Plant Microbe Interact. 1990 Nov-Dec;3(6):429–437. doi: 10.1094/mpmi-3-429. [DOI] [PubMed] [Google Scholar]
- Beynon L. M., Dutton G. G., Richards J. C. Structure of the amino acid-containing capsular polysaccharide from Escherichia coli O8:K49:H21. Carbohydr Res. 1990 Sep 19;205:347–359. doi: 10.1016/0008-6215(90)80152-s. [DOI] [PubMed] [Google Scholar]
- Beynon L. M., Dutton G. G. Structural studies of E. coli K26 capsular polysaccharide, using g.l.c.-c.i.-m.s. Carbohydr Res. 1988 Aug 15;179:419–423. doi: 10.1016/0008-6215(88)84137-5. [DOI] [PubMed] [Google Scholar]
- Chakraborty A. K., Friebolin H., Stirm S. Primary structure of the Escherichia coli serotype K30 capsular polysaccharide. J Bacteriol. 1980 Feb;141(2):971–972. doi: 10.1128/jb.141.2.971-972.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R., Acres S. D., Costerton J. W. Use of specific antibody to demonstrate glycocalyx, K99 pili, and the spatial relationships of K99+ enterotoxigenic Escherichia coli in the ileum of colostrum-fed calves. Infect Immun. 1982 Sep;37(3):1170–1180. doi: 10.1128/iai.37.3.1170-1180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. J., Sarabia V., Keenleyside W., MacLachlan P. R., Whitfield C. The compositional analysis of bacterial extracellular polysaccharides by high-performance anion-exchange chromatography. Anal Biochem. 1991 Nov 15;199(1):68–74. doi: 10.1016/0003-2697(91)90270-4. [DOI] [PubMed] [Google Scholar]
- Coleman M., Pearce R., Hitchin E., Busfield F., Mansfield J. W., Roberts I. S. Molecular cloning, expression and nucleotide sequence of the rcsA gene of Erwinia amylovora, encoding a positive regulator of capsule expression: evidence for a family of related capsule activator proteins. J Gen Microbiol. 1990 Sep;136(9):1799–1806. doi: 10.1099/00221287-136-9-1799. [DOI] [PubMed] [Google Scholar]
- Dengler T., Jann B., Jann K. Structure of the serine-containing capsular polysaccharide K40 antigen from Escherichia coli O8:K40:H9. Carbohydr Res. 1986 Aug 1;150:233–240. doi: 10.1016/0008-6215(86)80019-2. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton G. G., Karunaratne D. N., Lim A. V. Escherichia coli serotype K44: an acidic capsular polysaccharide containing two 2-acetamido-2-deoxyhexoses. Carbohydr Res. 1988 Nov 15;183(1):111–122. doi: 10.1016/0008-6215(88)80050-8. [DOI] [PubMed] [Google Scholar]
- Dutton G. G., Kuma-Mintah A. Structure of Escherichia coli capsular antigen K34. Carbohydr Res. 1987 Nov 15;169:213–220. doi: 10.1016/0008-6215(87)80252-5. [DOI] [PubMed] [Google Scholar]
- Dutton G. G., Parolis H., Parolis L. A. The structure of the neuraminic acid-containing capsular polysaccharide of Escherichia coli serotype K9. Carbohydr Res. 1987 Dec 15;170(2):193–206. doi: 10.1016/s0008-6215(00)90904-2. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais F. G., Drapeau G. R. Identification, cloning, and characterization of rcsF, a new regulator gene for exopolysaccharide synthesis that suppresses the division mutation ftsZ84 in Escherichia coli K-12. J Bacteriol. 1992 Dec;174(24):8016–8022. doi: 10.1128/jb.174.24.8016-8022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais F. G., Phoenix P., Drapeau G. R. The rcsB gene, a positive regulator of colanic acid biosynthesis in Escherichia coli, is also an activator of ftsZ expression. J Bacteriol. 1992 Jun;174(12):3964–3971. doi: 10.1128/jb.174.12.3964-3971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Stout V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Mol Microbiol. 1991 Jul;5(7):1599–1606. doi: 10.1111/j.1365-2958.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Trisler P., Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985 Jun;162(3):1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad J. J., Gyles C. L. The role of K antigens of enteropathogenic Escherichia coli in colonization of the small intestine of calves. Can J Comp Med. 1982 Jan;46(1):21–26. [PMC free article] [PubMed] [Google Scholar]
- Homonylo M. K., Wilmot S. J., Lam J. S., MacDonald L. A., Whitfield C. Monoclonal antibodies against the capsular K antigen of Escherichia coli (O9:K30(A):H12): characterisation and use in analysis of K antigen organisation on the cell surface. Can J Microbiol. 1988 Oct;34(10):1159–1165. doi: 10.1139/m88-204. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houng H. S., Noon K. F., Ou J. T., Baron L. S. Expression of Vi antigen in Escherichia coli K-12: characterization of ViaB from Citrobacter freundii and identity of ViaA with RcsB. J Bacteriol. 1992 Sep;174(18):5910–5915. doi: 10.1128/jb.174.18.5910-5915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann B., Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- Jann K., Jann B., Schneider K. F., Orskov F., Orskov I. Immunochemistry of K antigens of Escherichia coli. 5. The K antigen of E. coli 08:K27(A):H negative. Eur J Biochem. 1968 Sep 24;5(4):456–465. doi: 10.1111/j.1432-1033.1968.tb00392.x. [DOI] [PubMed] [Google Scholar]
- Keenleyside W. J., Jayaratne P., MacLachlan P. R., Whitfield C. The rcsA gene of Escherichia coli O9:K30:H12 is involved in the expression of the serotype-specific group I K (capsular) antigen. J Bacteriol. 1992 Jan;174(1):8–16. doi: 10.1128/jb.174.1.8-16.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Kuhn H. M., Meier-Dieter U., Mayer H. ECA, the enterobacterial common antigen. FEMS Microbiol Rev. 1988 Sep;4(3):195–222. doi: 10.1111/j.1574-6968.1988.tb02743.x. [DOI] [PubMed] [Google Scholar]
- Laakso D. H., Homonylo M. K., Wilmot S. J., Whitfield C. Transfer and expression of the genetic determinants for O and K antigen synthesis in Escherichia coli O9:K(A)30 and Klebsiella sp. O1:K20, in Escherichia coli K12. Can J Microbiol. 1988 Aug;34(8):987–992. doi: 10.1139/m88-173. [DOI] [PubMed] [Google Scholar]
- McCallum K. L., Laakso D. H., Whitfield C. Use of a bacteriophage-encoded glycanase enzyme in the generation of lipopolysaccharide O side chain deficient mutants of Escherichia coli O9:K30 and Klebsiella O1:K20: role of O and K antigens in resistance to complement-mediated serum killing. Can J Microbiol. 1989 Nov;35(11):994–999. doi: 10.1139/m89-166. [DOI] [PubMed] [Google Scholar]
- McCallum K. L., Whitfield C. The rcsA gene of Klebsiella pneumoniae O1:K20 is involved in expression of the serotype-specific K (capsular) antigen. Infect Immun. 1991 Feb;59(2):494–502. doi: 10.1128/iai.59.2.494-502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine small intestine by Escherichia coli: ileal colonization and adhesion by pig enteropathogens that lack K88 antigen and by some acapsular mutants. Infect Immun. 1976 Apr;13(4):1214–1220. doi: 10.1128/iai.13.4.1214-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H., Chakraborty A. K., Friebolin H., Stirm S. Primary structure of the Escherichia coli serotype K42 capsular polysaccharide and its serological identity with the Klebsiella K63 polysaccharide. J Bacteriol. 1978 Jan;133(1):390–391. doi: 10.1128/jb.133.1.390-391.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Jann B., Jann K. Immunoelectrophoretic patterns of extracts from all Escherichia coli O and K antigen test strains: correlation with pathogenicity. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):142–152. doi: 10.1111/j.1699-0463.1971.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. T., Kloser A. W., Schnaitman C. A., Stein M. A., Gottesman S., Gibson B. W. Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J Bacteriol. 1992 Apr;174(8):2525–2538. doi: 10.1128/jb.174.8.2525-2538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolis H., Parolis L. A., Venter R. D. Escherichia coli serotype-39 capsular polysaccharide: primary structure and depolymerisation by a bacteriophage-associated glycanase. Carbohydr Res. 1989 Feb 1;185(2):225–232. doi: 10.1016/0008-6215(89)80037-0. [DOI] [PubMed] [Google Scholar]
- Poetter K., Coplin D. L. Structural and functional analysis of the rcsA gene from Erwinia stewartii. Mol Gen Genet. 1991 Sep;229(1):155–160. doi: 10.1007/BF00264225. [DOI] [PubMed] [Google Scholar]
- Runnels P. L., Moon H. W. Capsule reduces adherence of enterotoxigenic Escherichia coli to isolated intestinal epithelial cells of pigs. Infect Immun. 1984 Sep;45(3):737–740. doi: 10.1128/iai.45.3.737-740.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K., Orskov I., Orskov F. Genetic determinants of the synthesis of the polysaccharide capsular antigen K27(A) of Escherichia coli. J Gen Microbiol. 1977 Jun;100(2):355–361. doi: 10.1099/00221287-100-2-355. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The influence of plasmid-determined and other characteristics of enteropathogenic Escherichia coli on their ability to proliferate in the alimentary tracts of piglets, calves and lambs. J Med Microbiol. 1978 Nov;11(4):471–492. doi: 10.1099/00222615-11-4-471. [DOI] [PubMed] [Google Scholar]
- Stout V., Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990 Feb;172(2):659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout V., Torres-Cabassa A., Maurizi M. R., Gutnick D., Gottesman S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J Bacteriol. 1991 Mar;173(5):1738–1747. doi: 10.1128/jb.173.5.1738-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarcsay L., Jann B., Jann K. Immunochemistry of the K antigens of Escherichia coli. The K87 antigen from Escherichia coli 08:K87(B?):H19. Eur J Biochem. 1971 Dec 10;23(3):505–514. doi: 10.1111/j.1432-1033.1971.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Cabassa A. S., Gottesman S. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J Bacteriol. 1987 Mar;169(3):981–989. doi: 10.1128/jb.169.3.981-989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Cabassa A., Gottesman S., Frederick R. D., Dolph P. J., Coplin D. L. Control of extracellular polysaccharide synthesis in Erwinia stewartii and Escherichia coli K-12: a common regulatory function. J Bacteriol. 1987 Oct;169(10):4525–4531. doi: 10.1128/jb.169.10.4525-4531.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisler P., Gottesman S. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1984 Oct;160(1):184–191. doi: 10.1128/jb.160.1.184-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacharotayankun R., Arakawa Y., Ohta M., Hasegawa T., Mori M., Horii T., Kato N. Involvement of rcsB in Klebsiella K2 capsule synthesis in Escherichia coli K-12. J Bacteriol. 1992 Feb;174(3):1063–1067. doi: 10.1128/jb.174.3.1063-1067.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C., Schoenhals G., Graham L. Mutants of Escherichia coli O9:K30 with altered synthesis and expression of the capsular K30 antigen. J Gen Microbiol. 1989 Oct;135(10):2589–2599. doi: 10.1099/00221287-135-10-2589. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]