Abstract
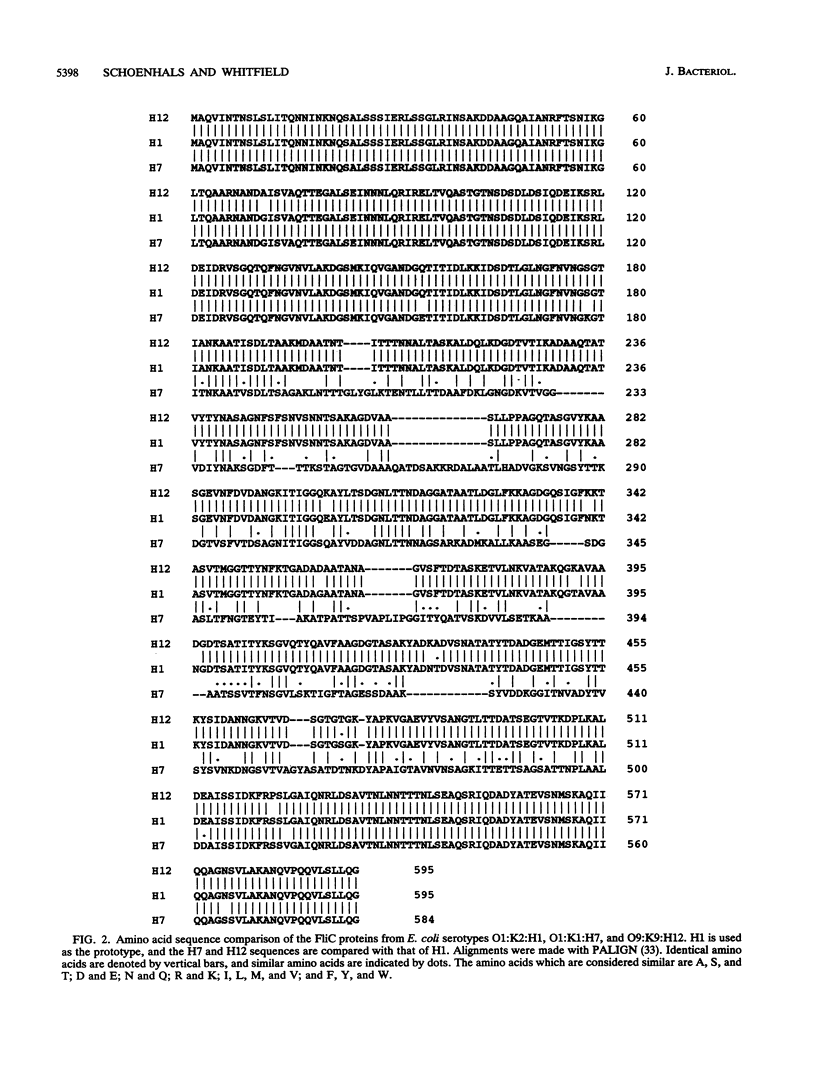
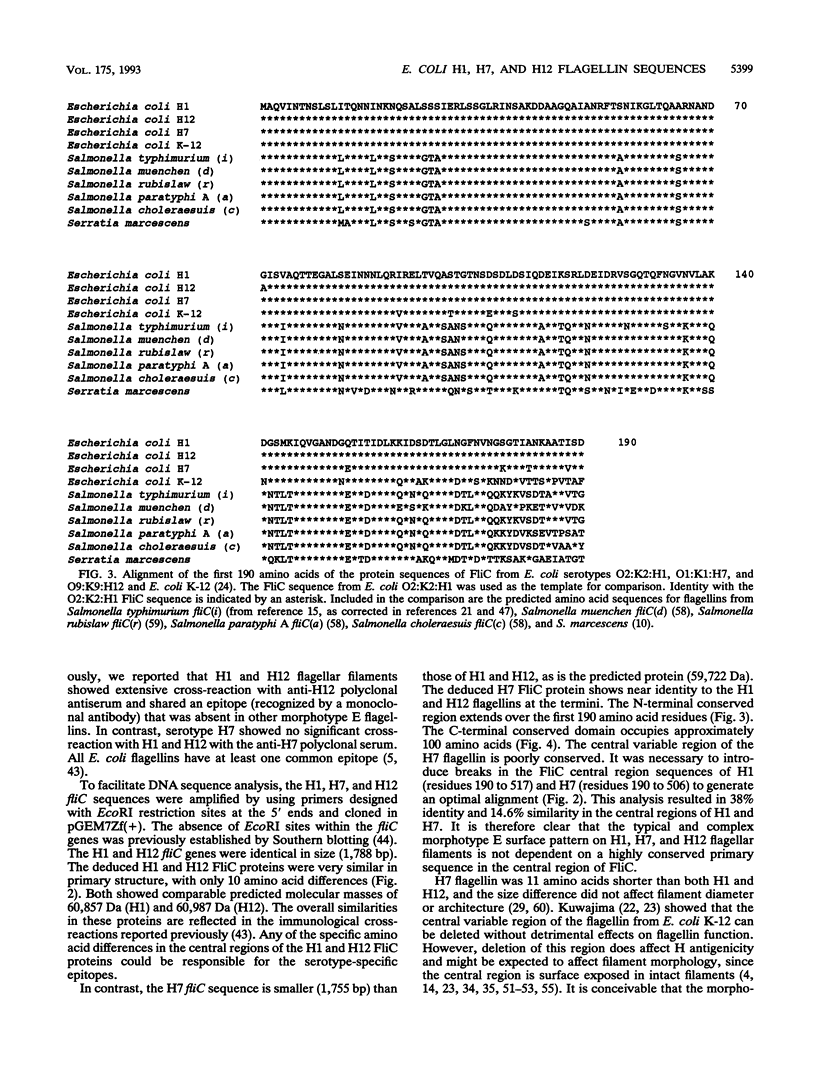
Escherichia coli morphotype E flagellar filaments have a characteristic surface pattern of short-pitch loops when examined by electron microscopy. Seven of the 50 known E. coli H (flagellar antigen) serotypes (H1, H7, H12, H23, H45, H49, and H51) produce morphotype E filaments. Polymerase chain reaction was used to amplify flagellin structural (fliC) genes from E. coli strains producing morphotype E flagellar filaments and from strains with flagellar filaments representing other morphotypes. A single DNA fragment was obtained from each strain, and the size of the amplified DNA correlated with the molecular mass of the corresponding flagellin protein. This finding and hybridization data suggest that these bacteria are monophasic. fliC genes from three E. coli serotypes (H1, H7, and H12) possessing morphotype E flagellar filaments were sequenced in order to assess the contribution of conserved flagellin primary sequence to the characteristic filament architecture. The H1 and H12 fliC sequences were identical in length (1,788 bp), while the H7 fliC sequence was shorter (1,755 bp). The deduced molecular masses of the FliC proteins were 60,857 Da (H1), 59,722 Da (H7), and 60,978 Da (H12). The H1, H7, and H12 flagellins demonstrated 98 to 99% identity over the amino-terminal region (190 amino acid residues) and 89% (H7) to 99% (H1 and H12) identity in the carboxy-terminal region (100 amino acid residues). The complete primary amino acid sequences for H1 and H12 flagellins differed by only 10 amino acids, accounting for previously reported serological cross-reactions. However, the central region of H7 flagellin had only 38% identity with H1 and H12 flagellins.The characteristic morphology of morphotype E flagellar filaments is therefore not dependent on a highly conserved primary sequence within the exposed central region. Comparison of morphotype E E. coli flagellins with those from E. coli K-12, Serratia marcescens, and several Salmonella serovars supported the established concept of highly conserved terminal regions flanking a variable central region.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fedorov O. V., Kostyukova A. S., Pyatibratov M. G. Architectonics of a bacterial flagellin filament subunit. FEBS Lett. 1988 Dec 5;241(1-2):145–148. doi: 10.1016/0014-5793(88)81048-2. [DOI] [PubMed] [Google Scholar]
- Feng P., Sugasawara R. J., Schantz A. Identification of a common enterobacterial flagellin epitope with a monoclonal antibody. J Gen Microbiol. 1990 Feb;136(2):337–342. doi: 10.1099/00221287-136-2-337. [DOI] [PubMed] [Google Scholar]
- Frankel G., Newton S. M., Schoolnik G. K., Stocker B. A. Intragenic recombination in a flagellin gene: characterization of the H1-j gene of Salmonella typhi. EMBO J. 1989 Oct;8(10):3149–3152. doi: 10.1002/j.1460-2075.1989.tb08468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerl L., Sumper M. Halobacterial flagellins are encoded by a multigene family. Characterization of five flagellin genes. J Biol Chem. 1988 Sep 15;263(26):13246–13251. [PubMed] [Google Scholar]
- Gill P. R., Agabian N. The nucleotide sequence of the Mr = 28,500 flagellin gene of Caulobacter crescentus. J Biol Chem. 1983 Jun 25;258(12):7395–7401. [PubMed] [Google Scholar]
- Guerry P., Alm R. A., Power M. E., Logan S. M., Trust T. J. Role of two flagellin genes in Campylobacter motility. J Bacteriol. 1991 Aug;173(15):4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakura A., Morimoto K., Sofuni T., Nohmi T. Cloning and characterization of the Salmonella typhimurium ada gene, which encodes O6-methylguanine-DNA methyltransferase. J Bacteriol. 1991 Jun;173(12):3663–3672. doi: 10.1128/jb.173.12.3663-3672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshey R. M., Estepa G., Yanagi H. Cloning and nucleotide sequence of a flagellin-coding gene (hag) from Serratia marcescens 274. Gene. 1989 Jun 30;79(1):1–8. doi: 10.1016/0378-1119(89)90087-5. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim G. F., Fleet G. H., Lyons M. J., Walker R. A. Immunological relationships between Salmonella flagella and their potential application for salmonellae detection by immunoassay. Med Microbiol Immunol. 1985;174(2):87–99. doi: 10.1007/BF02123230. [DOI] [PubMed] [Google Scholar]
- Ibrahim G. F., Fleet G. H., Lyons M. J., Walker R. A. Immunological relationships between Salmonella flagellins and between these and flagellins from other species of Enterobacteriaceae. Med Microbiol Immunol. 1985;174(2):101–113. doi: 10.1007/BF02123231. [DOI] [PubMed] [Google Scholar]
- Iino T. Genetics of structure and function of bacterial flagella. Annu Rev Genet. 1977;11:161–182. doi: 10.1146/annurev.ge.11.120177.001113. [DOI] [PubMed] [Google Scholar]
- Joys T. M. Identification of an antibody binding site in the phase-1 flagellar protein of Salmonella typhimurium. Microbios. 1976;15(61-62):221–228. [PubMed] [Google Scholar]
- Joys T. M., Martin J. F. Identification of amino acid changes in serological mutants of the i flagellar antigen of Salmonella typhimurium. Microbios. 1973;7(25):71–73. [PubMed] [Google Scholar]
- Joys T. M., Schödel F. Epitope mapping of the d flagellar antigen of Salmonella muenchen. Infect Immun. 1991 Sep;59(9):3330–3332. doi: 10.1128/iai.59.9.3330-3332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joys T. M., Stocker B. A. Isolation and serological analysis of mutant forms of flagellar antigen i of Salmonella typhimurium. J Gen Microbiol. 1966 Jul;44(1):121–138. doi: 10.1099/00221287-44-1-121. [DOI] [PubMed] [Google Scholar]
- Joys T. M. The covalent structure of the phase-1 flagellar filament protein of Salmonella typhimurium and its comparison with other flagellins. J Biol Chem. 1985 Dec 15;260(29):15758–15761. [PubMed] [Google Scholar]
- Joys T. M. The flagellar filament protein. Can J Microbiol. 1988 Apr;34(4):452–458. doi: 10.1139/m88-078. [DOI] [PubMed] [Google Scholar]
- Kanto S., Okino H., Aizawa S., Yamaguchi S. Amino acids responsible for flagellar shape are distributed in terminal regions of flagellin. J Mol Biol. 1991 Jun 5;219(3):471–480. doi: 10.1016/0022-2836(91)90187-b. [DOI] [PubMed] [Google Scholar]
- Kuwajima G., Asaka J., Fujiwara T., Fujiwara T., Node K., Kondo E. Nucleotide sequence of the hag gene encoding flagellin of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1479–1483. doi: 10.1128/jb.168.3.1479-1483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima G. Construction of a minimum-size functional flagellin of Escherichia coli. J Bacteriol. 1988 Jul;170(7):3305–3309. doi: 10.1128/jb.170.7.3305-3309.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima G. Flagellin domain that affects H antigenicity of Escherichia coli K-12. J Bacteriol. 1988 Jan;170(1):485–488. doi: 10.1128/jb.170.1.485-488.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima G., Kawagishi I., Homma M., Asaka J., Kondo E., Macnab R. M. Export of an N-terminal fragment of Escherichia coli flagellin by a flagellum-specific pathway. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4953–4957. doi: 10.1073/pnas.86.13.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVallie E. R., Stahl M. L. Cloning of the flagellin gene from Bacillus subtilis and complementation studies of an in vitro-derived deletion mutation. J Bacteriol. 1989 Jun;171(6):3085–3094. doi: 10.1128/jb.171.6.3085-3094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman R. E. The occurrence of antigenic determinants common to flagella of different salmonella strains. Eur J Immunol. 1972 Dec;2(6):582–586. doi: 10.1002/eji.1830020620. [DOI] [PubMed] [Google Scholar]
- Lawn A. M. Comparison of the flagellins from different flagellar morphotypes of Escherichia coli. J Gen Microbiol. 1977 Jul;101(1):112–130. doi: 10.1099/00221287-101-1-121. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Orskov I., Orskov F. Morphological distinction between different H serotypes of Escherichia coli. J Gen Microbiol. 1977 Jul;101(1):111–119. doi: 10.1099/00221287-101-1-111. [DOI] [PubMed] [Google Scholar]
- Martinez R. J., Ichiki A. T., Lundh N. P., Tronick S. R. A single amino acid substitution responsible for altered flagellar morphology. J Mol Biol. 1968 Jun 28;34(3):559–564. doi: 10.1016/0022-2836(68)90180-0. [DOI] [PubMed] [Google Scholar]
- Mirel D. B., Chamberlin M. J. The Bacillus subtilis flagellin gene (hag) is transcribed by the sigma 28 form of RNA polymerase. J Bacteriol. 1989 Jun;171(6):3095–3101. doi: 10.1128/jb.171.6.3095-3101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E. W., Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988 Mar;4(1):11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Namba K., Yamashita I., Vonderviszt F. Structure of the core and central channel of bacterial flagella. Nature. 1989 Dec 7;342(6250):648–654. doi: 10.1038/342648a0. [DOI] [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- Newton S. M., Wasley R. D., Wilson A., Rosenberg L. T., Miller J. F., Stocker B. A. Segment IV of a Salmonella flagellin gene specifies flagellar antigen epitopes. Mol Microbiol. 1991 Feb;5(2):419–425. doi: 10.1111/j.1365-2958.1991.tb02124.x. [DOI] [PubMed] [Google Scholar]
- Okazaki N., Matsuo S., Saito K., Tominaga A., Enomoto M. Conversion of the Salmonella phase 1 flagellin gene fliC to the phase 2 gene fljB on the Escherichia coli K-12 chromosome. J Bacteriol. 1993 Feb;175(3):758–766. doi: 10.1128/jb.175.3.758-766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleier E., Schmitt R. Identification and sequence analysis of two related flagellin genes in Rhizobium meliloti. J Bacteriol. 1989 Mar;171(3):1467–1475. doi: 10.1128/jb.171.3.1467-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri A., Ghosh S., Upadhyay S., Talwar G. P. Monoclonal antibodies against flagellar antigen of Salmonella typhi. Hybridoma. 1989 Jun;8(3):353–360. doi: 10.1089/hyb.1989.8.353. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhals G., Whitfield C. Monoclonal antibodies against serotype specific and conserved epitopes in morphotype E Escherichia coli flagellins. FEMS Microbiol Lett. 1990 Oct;60(1-2):117–122. doi: 10.1016/0378-1097(90)90356-u. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Smith N. H., Li J., Beltran P., Ferris K. E., Kopecko D. J., Rubin F. A. Molecular evolutionary genetics of the cattle-adapted serovar Salmonella dublin. J Bacteriol. 1992 Jun;174(11):3587–3592. doi: 10.1128/jb.174.11.3587-3592.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakihara Y., Wakabayashi T. Three-dimensional image reconstruction of straight flagella from a mutant Salmonella typhimurium. J Mol Biol. 1979 Jul 5;131(3):485–507. doi: 10.1016/0022-2836(79)90004-4. [DOI] [PubMed] [Google Scholar]
- Smith N. H., Beltran P., Selander R. K. Recombination of Salmonella phase 1 flagellin genes generates new serovars. J Bacteriol. 1990 May;172(5):2209–2216. doi: 10.1128/jb.172.5.2209-2216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N. H., Selander R. K. Molecular genetic basis for complex flagellar antigen expression in a triphasic serovar of Salmonella. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):956–960. doi: 10.1073/pnas.88.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N. H., Selander R. K. Sequence invariance of the antigen-coding central region of the phase 1 flagellar filament gene (fliC) among strains of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):603–609. doi: 10.1128/jb.172.2.603-609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Lory S. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J Bacteriol. 1990 Dec;172(12):7188–7199. doi: 10.1128/jb.172.12.7188-7199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J. A molecular switch: subunit rotations involved in the right-handed to left-handed transitions of Salmonella typhimurium flagellar filaments. J Mol Biol. 1991 Jul 5;220(1):67–77. doi: 10.1016/0022-2836(91)90381-f. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J. Conformational switching in the flagellar filament of Salmonella typhimurium. J Mol Biol. 1992 Jul 20;226(2):447–454. doi: 10.1016/0022-2836(92)90959-n. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J. Three-dimensional reconstruction of the flagellar filament of Caulobacter crescentus. A flagellin lacking the outer domain and its amino acid sequence lacking an internal segment. J Mol Biol. 1988 Aug 20;202(4):787–808. doi: 10.1016/0022-2836(88)90559-1. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J. Three-dimensional structure of the frozen-hydrated flagellar filament. The left-handed filament of Salmonella typhimurium. J Mol Biol. 1987 Jun 5;195(3):581–601. doi: 10.1016/0022-2836(87)90184-7. [DOI] [PubMed] [Google Scholar]
- Vonderviszt F., Aizawa S., Namba K. Role of the disordered terminal regions of flagellin in filament formation and stability. J Mol Biol. 1991 Oct 20;221(4):1461–1474. doi: 10.1016/0022-2836(91)90946-4. [DOI] [PubMed] [Google Scholar]
- Vonderviszt F., Kanto S., Aizawa S., Namba K. Terminal regions of flagellin are disordered in solution. J Mol Biol. 1989 Sep 5;209(1):127–133. doi: 10.1016/0022-2836(89)90176-9. [DOI] [PubMed] [Google Scholar]
- Vonderviszt F., Uedaira H., Kidokoro S., Namba K. Structural organization of flagellin. J Mol Biol. 1990 Jul 5;214(1):97–104. doi: 10.1016/0022-2836(90)90149-g. [DOI] [PubMed] [Google Scholar]
- Wei L. N., Joys T. M. Covalent structure of three phase-1 flagellar filament proteins of Salmonella. J Mol Biol. 1985 Dec 20;186(4):791–803. doi: 10.1016/0022-2836(85)90397-3. [DOI] [PubMed] [Google Scholar]
- Wei L. N., Joys T. M. The nucleotide sequence of the H-1r gene of Salmonella rubislaw. Nucleic Acids Res. 1986 Oct 24;14(20):8227–8227. doi: 10.1093/nar/14.20.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C., Walker S. G., Atkinson C. F., Lam J. S., MacDonald L. A., Beveridge T. J., Orskov I., Orskov F. Serotype-specific monoclonal antibodies against the H12 flagellar antigen of Escherichia coli. J Gen Microbiol. 1988 Jul;134(7):1747–1753. doi: 10.1099/00221287-134-7-1747. [DOI] [PubMed] [Google Scholar]




