Abstract
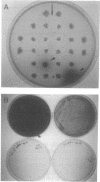
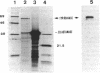
Melanin production by Rhizobium meliloti GR4 is linked to nonsymbiotic plasmid pRmeGR4b (140 MDa). Transfer of this plasmid to GR4-cured derivatives or to Agrobacterium tumefaciens enables these bacteria to produce melanin. Sequence analysis of a 3.5-kb PstI fragment of plasmid pRmeGR4b has revealed the presence of a open reading frame 1,481-bp that codes for a protein whose sequence shows strong homology to two conserved regions involved in copper binding in tyrosinases and hemocyanins. In vitro-coupled transcription-translation experiments showed that this open reading frame codes for a 55-kDa polypeptide. Melanin production in GR4 is not under the control of the RpoN-NifA regulatory system, unlike that in R. leguminosarum bv. phaseoli 8002. The GR4 tyrosinase gene could be expressed in Escherichia coli under the control of the lacZ promoter. For avoiding confusion with mel genes (for melibiose), a change of the name of the previously reported mel genes of R. leguminosarum bv. phaseoli and other organisms to mep genes (for melanin production) is proposed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batut J., Daveran-Mingot M. L., David M., Jacobs J., Garnerone A. M., Kahn D. fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 1989 Apr;8(4):1279–1286. doi: 10.1002/j.1460-2075.1989.tb03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur D., Lamb J. W., Johnston A. W. Identification of two classes of Rhizobium phaseoli genes required for melanin synthesis, one of which is required for nitrogen fixation and activates the transcription of the other. Mol Gen Genet. 1987 Apr;207(1):155–160. doi: 10.1007/BF00331503. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Casadesús J., Olivares J. Rough and fine linkage mapping of the Rhizobium meliloti chromosome. Mol Gen Genet. 1979 Jul 13;174(2):203–209. doi: 10.1007/BF00268356. [DOI] [PubMed] [Google Scholar]
- Coyne V. E., al-Harthi L. Induction of melanin biosynthesis in Vibrio cholerae. Appl Environ Microbiol. 1992 Sep;58(9):2861–2865. doi: 10.1128/aem.58.9.2861-2865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubo M. T., Buendia-Claveria A. M., Beringer J. E., Ruiz-Sainz J. E. Melanin production by Rhizobium strains. Appl Environ Microbiol. 1988 Jul;54(7):1812–1817. doi: 10.1128/aem.54.7.1812-1817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Daveran M. L., Batut J., Dedieu A., Domergue O., Ghai J., Hertig C., Boistard P., Kahn D. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell. 1988 Aug 26;54(5):671–683. doi: 10.1016/s0092-8674(88)80012-6. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins F. K., Johnston A. W. Transcription of a Rhizobium leguminosarum biovar phaseoli gene needed for melanin synthesis is activated by nifA of Rhizobium and Klebsiella pneumoniae. Mol Microbiol. 1988 May;2(3):331–337. doi: 10.1111/j.1365-2958.1988.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Huber M., Lerch K. Identification of two histidines as copper ligands in Streptomyces glaucescens tyrosinase. Biochemistry. 1988 Jul 26;27(15):5610–5615. doi: 10.1021/bi00415a032. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. W., Downie J. A., Johnston A. W. Cloning of the nodulation (nod) genes of Rhizobium phaseoli and their homology to R. leguminosarum nod DNA. Gene. 1985;34(2-3):235–241. doi: 10.1016/0378-1119(85)90132-5. [DOI] [PubMed] [Google Scholar]
- Lang W. H., van Holde K. E. Cloning and sequencing of Octopus dofleini hemocyanin cDNA: derived sequences of functional units Ode and Odf. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):244–248. doi: 10.1073/pnas.88.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz S. H., Murthy V. V. Purification and properties of tyrosinases from Vibrio tyrosinaticus. Arch Biochem Biophys. 1974 Jan;160(1):73–82. doi: 10.1016/s0003-9861(74)80010-x. [DOI] [PubMed] [Google Scholar]
- Robertsen B. K., Aman P., Darvill A. G., McNeil M., Albersheim P. Host-Symbiont Interactions : V. THE STRUCTURE OF ACIDIC EXTRACELLULAR POLYSACCHARIDES SECRETED BY RHIZOBIUM LEGUMINOSARUM AND RHIZOBIUM TRIFOLII. Plant Physiol. 1981 Mar;67(3):389–400. doi: 10.1104/pp.67.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Albright L. M., Ausubel F. M. Rhizobium meliloti ntrA (rpoN) gene is required for diverse metabolic functions. J Bacteriol. 1987 Jun;169(6):2424–2431. doi: 10.1128/jb.169.6.2424-2431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan J., Olivares J. Implication of nifA in regulation of genes located on a Rhizobium meliloti cryptic plasmid that affect nodulation efficiency. J Bacteriol. 1989 Aug;171(8):4154–4161. doi: 10.1128/jb.171.8.4154-4161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. High frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-Mob transposon. Mol Gen Genet. 1984;196(3):413–420. doi: 10.1007/BF00436188. [DOI] [PubMed] [Google Scholar]
- Soto M. J., Zorzano A., Mercado-Blanco J., Lepek V., Olivares J., Toro N. Nucleotide sequence and characterization of Rhizobium meliloti nodulation competitiveness genes nfe. J Mol Biol. 1993 Jan 20;229(2):570–576. doi: 10.1006/jmbi.1993.1060. [DOI] [PubMed] [Google Scholar]
- Szeto W. W., Zimmerman J. L., Sundaresan V., Ausubel F. M. A Rhizobium meliloti symbiotic regulatory gene. Cell. 1984 Apr;36(4):1035–1043. doi: 10.1016/0092-8674(84)90053-9. [DOI] [PubMed] [Google Scholar]
- WHITE L. P. Melanin: a naturally occurring cation exchange material. Nature. 1958 Nov 22;182(4647):1427–1428. doi: 10.1038/1821427a0. [DOI] [PubMed] [Google Scholar]
- Weber G., Reiländer H., Pühler A. Mapping and expression of a regulatory nitrogen fixation gene (fixD) of Rhizobium meliloti. EMBO J. 1985 Nov;4(11):2751–2756. doi: 10.1002/j.1460-2075.1985.tb03999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]