Abstract
Aims
To identify prevalence of delayed detection of cleft palate, and associated factors that could lead to improved identification at neonatal clinical examination.
Methods
Audit of hospital notes, parental questionnaire incorporating open ended questions, and telephone questionnaire of junior doctors in the referring hospitals incorporating fixed choice questions.
Results
Of 344 cleft palate patients without cleft lip or submucous cleft palate, the day the cleft was detected was recorded in 92%. Delayed detection, after the first day, was 28% overall, distributed as 37% with isolated cleft palate and 23% with syndromic cleft palate. Narrow V shaped clefts were more likely to be delayed in detection compared with broad U shaped clefts, as were soft palate clefts compared with hard palate clefts. Five with isolated cleft palates were not detected until after the first year. Babies born at home were unlikely to be detected on day 1. Symptoms were significantly increased in the delayed detection group for feeding problems and nasal regurgitation. A telephone questionnaire of trainee paediatricians in referring units revealed that digital examination was more commonly practised than visual inspection, and few recalled receiving specific instruction on examination of the palate.
Conclusion
Delayed detection of cleft palate was not uncommon, and the features of those more likely to be missed suggested digital examination was related. Trainee doctors and midwives should be instructed to inspect visually using a light and tongue depressor, then digitally if submucous cleft palate is suspected.
Keywords: audit, cleft palate, examination, neonatal, palate
In the UK, clinical examination of each newborn for congenital abnormalities, including healthcare advice informed by it, is accepted as standard care.1 One of the commonest malformations is cleft lip and palate with an incidence of 1 in 700.2 Up to 44% have cleft palate,3 and 47% of them have other major abnormalities.3
Identification of an open cleft palate by inspection at first examination would be predicted as extremely likely compared with congenital heart defects and hip dysplasias in which 30% are detected in the newborn examination because of delay in appearance of symptoms and signs.4,5 A patient support group questionnaire found that delay in diagnosing cleft palate occurred in 10%.6 Our experience as a cleft referral centre suggested delay was even more common, and in addition to the effects on the baby, has led some parents to seek legal redress. We set out to determine the frequency of delay, and associated clinical features and pre‐diagnosis symptoms, and to identify factors that could lead to improved detection. Our findings prompted a telephone survey of the examination techniques used to look at the palate by junior hospital doctors.
Methods
All open cleft palate patients without cleft lip referred from 1988 to 2001 to a tertiary centre, Great Ormond Street Hospital for Children, and a district general hospital Plastic Surgery Centre, St Andrew's, Billericay were audited. These hospitals' cleft surgery services are contiguous over northeast London and the adjoining county of Essex.
Selected for retrospective evaluation from the records were:
Age at detection in days
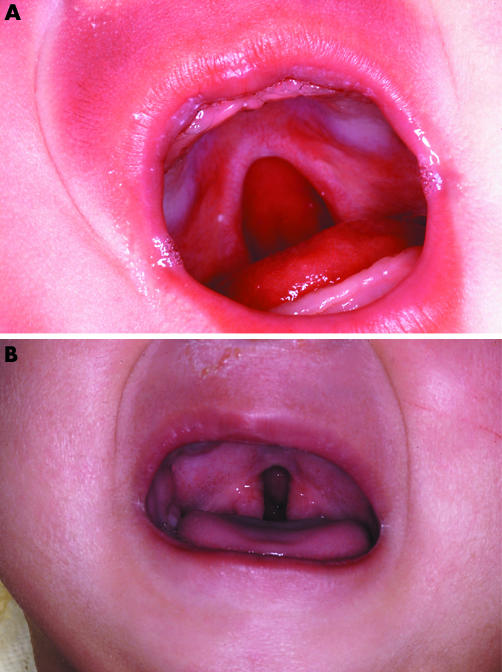
Shape, whether broad U shaped, or narrow V shaped (fig 1)
Extent of the cleft from the surgical findings at operation by one operator (BS)
Associated congenital anomalies. Their absence was categorised as isolated cleft palate (isolated CP), and presence as syndromic cleft palate (syndromic CP); Pierre Robin sequence (PRS) (cleft palate, small jaw, and retro‐positioned tongue) was specifically identified.
Figure 1 (A) Broad U shaped cleft palate extending a third into the hard palate. (B) Narrow V shaped cleft palate at the junction of soft and hard palate.
The recollections of parents were elicited by retrospective questionnaire of symptoms at the time of detection of the cleft palate. They were asked about feeding difficulties, nasal regurgitation of fluids, and breathing difficulties. There was also space for them to add their personal experience of the events surrounding detection.
Statistical analysis
Statistical analysis was carried out using SPSS 12, 95% confidence intervals, χ2 for differences between groups. Where the data were not normally distributed, 95% confidence intervals were applied using log transformation. Permission for the audit project and questionnaire were obtained from the respective hospital ethics committees.
Results
The total number of referrals of cleft palate (CP) was 344, of which 92% (316) were informative about day of detection; 61% were female.
Age at detection of CP
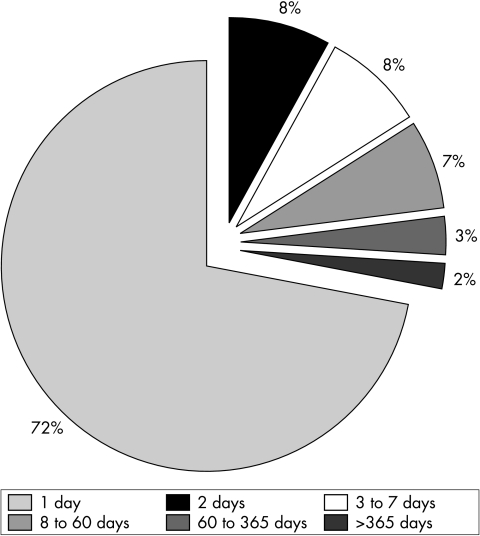
Failure to detect in 96 (28%) on the first day fell to 70 (20%) on the second day (fig 2). The proportion fell progressively over the first year: days 3–7 = 26 (8%), days 8–60 = 22 (7%), days 61–365 = 9 (3%), over 1 year = 5 (2%). The oldest was 5 years old.
Figure 2 Age in days at detection of cleft palate.
Finding of other congenital abnormalities and age at detection of CP
A total of 254 cases of isolated cleft palate and syndromic cleft palate had detailed evaluation. One hundred and sixty eight (66%) were isolated CP, of which 62 (37%) were not detected on day 1, compared with 58 (23%) syndromic CP (χ2 8.7, df 1, p < 0.05, 95% CI ratio between isolated CP:syndromic CP mean age at detection 1.2 to 2.7). All five detected after the first year of life were isolated CP and soft palate only.
Shape and extent of the cleft and age at detection of CP
The shape of the cleft (fig 1) was assessed at operation in 207. The broad U shaped cleft was more often detected on day 1: in 91 of 115 (79%, 95% CI 71 to 85%) compared with 39 of 92 (42%, 95% CI 33 to 53%) with a narrow V shaped cleft (χ2 52.8, df 5, p < 0.001).
The anteroposterior extent of the cleft in 226 subjects was associated with delay in 47/68 (69%) soft palate compared with 36/158 (22%) hard palate (χ2 18.3, df 1, p < 0.001). The smallest clefts were at least a third of the soft palate. The smaller the cleft the more delay was likely.
Birth unit and delay in diagnosis
On day one, 1 of 13 (8%) delivered at home were detected, compared with 66 of 98 (67%) in tertiary hospitals and 150 of 205 (73%) in district general hospitals, (χ2 24.9, df 2, p < 0.001).
Symptoms contributing to early detection
Parents returned 204 questionnaires (59%). The presence of symptoms, nasal regurgitation (χ2 40.8, df 1, p < 0.001), and feeding difficulties (χ2 19.9, df 1, p < 0.001) were each associated with delayed detection after day 1. Breathing difficulties were associated with presence of a cleft on day 1 (χ2 5.8, df 1, p = 0.015).
Survey of junior hospital doctors' practice
All 23 hospital neonatal units referring to the centre were contacted by one of the authors (NE); the junior doctor on duty that day for neonatal care was asked how they examined the palate, which method was advised locally, and whether specific training had been given. Responses were digital examination alone, performed and advised in 14 units, combined visual and digital examination in 9 units. Specific training was recalled by four trainees; two were advised digital only, and two to visually inspect. Each hospital aimed to examine all newborns within the first 24 hours.
Discussion
Delay in detection of CP was more likely in narrow, isolated clefts of the soft palate, though it occurred in all cleft sizes. As digital examination was the most frequent method of inspection reported by hospital doctors, in our opinion omitting to visually inspect was the most likely explanation. None of the infants had a “hidden” or submucous cleft palate, the prime reason for digital palatal examination.
Possible explanations for failure of digital examination include: the digital sensation of a cleft was difficult to appreciate; or the vomer was palpated in the cleft and interpreted as confirming palatal integrity.
Alternatives for failed visual detection to consider are: failure to depress the tongue adequately to gain an adequate view of the mouth, or failure by junior doctors to visually recognise the appearance of even relatively large clefts despite apparently adequate examination technique, as was reported by some parents.
Factors contributing to this underperformance may have included initial wellbeing masking isolated CP more than syndromic CP, the feeding difficulties and nasal regurgitation eventually drawing attention to a physical origin. Even allowing for delayed examination to day 2 still led to 20% non‐detection.
What is already known on this topic
The UK Cleft Lip and Palate Association parental survey reported that 10% of cleft palates were delayed in detection
We are concerned that the practice of palpation alone for integrity of the palate may be widespread, advocated on the grounds that it is less invasive, and hence less upsetting for infants and their parents. Advice to visually inspect7 has been interpreted as an opportunistic process performed during routine newborn examination.8 However, encouraging gagging by digital examination allowed only 14% of palates to be inspected. Counselling the use a laryngoscope8 to improve detection is invasive and unnecessary in our opinion. The World Health Organisation9 recommends direct visualisation in developing countries, yet it seems that many medical trainees, and probably midwifery staff judging by the number undetected among home deliveries, in our part of the UK are not being instructed in this simple screening procedure.
Doctors and midwives in training for routine newborn examination should be instructed in the correct techniques, preferably using an assistant,10 and shown visual material of clefts of various sizes to aid recognition. Direct inspection of the mouth with a flashlight, and applying sufficient pressure with a spatula or sterile disposable 1 ml syringe to depress the tongue in order to visualise the posterior palate and the uvula should identify all cases of open CP. In addition, features may be seen of submucous cleft palate comprising diastasis of muscles of the soft palate, and bifid uvula. It is then that digital examination should be done to identify absence of the posterior nasal spine or notching of the posterior hard palate, and midline tissue deficiency due to palatal muscle diastasis, although in the infant such signs can be subtle and easily missed.
This guidance should be included in the proposed Royal College of General Practitioners postnatal care guidelines, commissioned by the National Institute for Clinical Excellence, due later this year. The resultant parental satisfaction, improved professional standards, and avoidance of occasional litigation (personal communications: Risk Management Adviser for the Medical Defence Union, 2001; NHS Litigation Authority, 2004) should be achievable.
What this study adds
Delayed detection of cleft palate after the first day of life occurred in 28% of referrals. Detection was more common in isolated cleft palate (37%) than syndromic cleft palate (23%), and narrow V shaped than broad U shaped clefts
The appropriate technique is visual inspection of all newborn palates, followed by digital examination where submucous cleft palate is suspected
Acknowledgements
The continuing help and cooperation of junior staff in referring units is gratefully acknowledged.
Footnotes
Competing interests: none
References
- 1.Hall D M B.Health for all children, 3rd edn. Oxford: Oxford University Press 1966
- 2.Clinical Standards Advisory Group Report on cleft lip and/or palate. London: The Stationery Office, 199842
- 3.Stoll C, Alembik Y, Dott B.et al Associated malformations in cases with oral clefts. Cleft Palate Craniofac J 2000374147. [DOI] [PubMed] [Google Scholar]
- 4.Ainsworth S, Wylie J P, Wren C. Prevalence and clinical significance of cardiac murmurs in neonates. Arch Dis Child Fetal Neontal Ed 199980F43–F45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dezateux C, Godward S. A national survey of screening for congenital dislocation of the hip. Arch Dis Child 199674445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical Standards Advisory Group Report on cleft lip and/or palate. London: The Stationery Office, 199834
- 7.Rennie J M, Gandy G M. Examination of the newborn. In: Roberton NRS, Rennie JM, eds. Textbook of neonatology. Edinburgh: Churchill Livingstone, 1999274
- 8.Armstrong H, Simpson R M. Examination of the neonatal palate. Arch Dis Child Fetal Neonatal Ed 200286F210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organisation WHO initiative on the prevention and control of congenital and genetic diseases in developing countries. WHO/HGNWG/00. 1, Appendix II
- 10.James C S, Todd P J. Examination of the neonatal palate. Arch Dis Child Fetal Neonatal Ed Online, 14 May 2002 [DOI] [PMC free article] [PubMed]