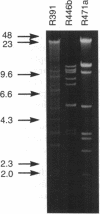
Abstract
Genetic and physiological experiments have demonstrated that the products of the umu-like operon are directly required for mutagenic DNA repair in enterobacteria. To date, five such operons have been cloned and studied at the molecular level. Given the apparent wide occurrence of these mutagenic DNA repair genes in enterobacteria, it seems likely that related genes will be identified in other bacterial species and perhaps even in higher organisms. We are interested in identifying such genes. However, standard methods based on either DNA or protein cross-hybridization are laborious and, given the overall homology between previously identified members of this family (41 to 83% at the protein level), would probably have limited success. To facilitate the rapid identification of more diverse umu-like genes, we have constructed two Escherichia coli strains that allow us to identify umu-like genes after phenotypic complementation assays. With these two strains, we have cloned novel umu-like genes from three R plasmids, the IncJ plasmid R391 and two IncL/M plasmids, R446b and R471a.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailone A., Sommer S., Knezević J., Dutreix M., Devoret R. A RecA protein mutant deficient in its interaction with the UmuDC complex. Biochimie. 1991 Apr;73(4):479–484. doi: 10.1016/0300-9084(91)90115-h. [DOI] [PubMed] [Google Scholar]
- Bezzubova O., Shinohara A., Mueller R. G., Ogawa H., Buerstedde J. M. A chicken RAD51 homologue is expressed at high levels in lymphoid and reproductive organs. Nucleic Acids Res. 1993 Apr 11;21(7):1577–1580. doi: 10.1093/nar/21.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Park D., Xu L., Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992 May 1;69(3):439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Woodgate R. Mutagenic repair in Escherichia coli: products of the recA gene and of the umuD and umuC genes act at different steps in UV-induced mutagenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4193–4197. doi: 10.1073/pnas.82.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D. P., Traxler B. A., Holt S. M., Crose L. L. Enhancement of UV survival, UV- and MMS-mutability, precise excision of Tn10 and complementation of umuC by plasmids of different incompatibility groups. Mutat Res. 1986 Jul;166(1):29–37. doi: 10.1016/0167-8817(86)90038-6. [DOI] [PubMed] [Google Scholar]
- Burckhardt S. E., Woodgate R., Scheuermann R. H., Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti H., Osman M., Grandoni P., Jagendorf A. T. A homolog of Escherichia coli RecA protein in plastids of higher plants. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8068–8072. doi: 10.1073/pnas.89.17.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti D., Ibeanu G. C., Tano K., Mitra S. Cloning and expression in Escherichia coli of a human cDNA encoding the DNA repair protein N-methylpurine-DNA glycosylase. J Biol Chem. 1991 Aug 25;266(24):15710–15715. [PubMed] [Google Scholar]
- Churchward G., Belin D., Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984 Nov;31(1-3):165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Demple B., Herman T., Chen D. S. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11450–11454. doi: 10.1073/pnas.88.24.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutreix M., Moreau P. L., Bailone A., Galibert F., Battista J. R., Walker G. C., Devoret R. New recA mutations that dissociate the various RecA protein activities in Escherichia coli provide evidence for an additional role for RecA protein in UV mutagenesis. J Bacteriol. 1989 May;171(5):2415–2423. doi: 10.1128/jb.171.5.2415-2423.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Goodman M. F. Mutation induced by DNA damage: a many protein affair. Mutat Res. 1990 Sep-Nov;236(2-3):301–311. doi: 10.1016/0921-8777(90)90013-u. [DOI] [PubMed] [Google Scholar]
- Hauser J., Levine A. S., Ennis D. G., Chumakov K. M., Woodgate R. The enhanced mutagenic potential of the MucAB proteins correlates with the highly efficient processing of the MucA protein. J Bacteriol. 1992 Nov;174(21):6844–6851. doi: 10.1128/jb.174.21.6844-6851.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Coetzee J. N., Dennison S. R factors from Proteus morganii. J Gen Microbiol. 1973 Aug;77(2):249–259. doi: 10.1099/00221287-77-2-249. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Rodriguez-Lemoine V., Datta N. R factors from Serratia marcescens. J Gen Microbiol. 1975 Jan;86(1):88–92. doi: 10.1099/00221287-86-1-88. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Khidhir M. A., Casaregola S., Holland I. B. Mechanism of transient inhibition of DNA synthesis in ultraviolet-irradiated E. coli: inhibition is independent of recA whilst recovery requires RecA protein itself and an additional, inducible SOS function. Mol Gen Genet. 1985;199(1):133–140. doi: 10.1007/BF00327522. [DOI] [PubMed] [Google Scholar]
- Kitagawa Y., Akaboshi E., Shinagawa H., Horii T., Ogawa H., Kato T. Structural analysis of the umu operon required for inducible mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4336–4340. doi: 10.1073/pnas.82.13.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W. H., Cebula T. A., Foster P. L., Eisenstadt E. UV mutagenesis in Salmonella typhimurium is umuDC dependent despite the presence of samAB. J Bacteriol. 1992 May;174(9):2809–2815. doi: 10.1128/jb.174.9.2809-2815.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. J., Perry K. L., Walker G. C. Complementation of a pKM101 derivative that decreases resistance to UV killing but increases susceptibility to mutagenesis. Mutat Res. 1985 Jun-Jul;150(1-2):147–158. doi: 10.1016/0027-5107(85)90112-5. [DOI] [PubMed] [Google Scholar]
- Lodwick D., Owen D., Strike P. DNA sequence analysis of the imp UV protection and mutation operon of the plasmid TP110: identification of a third gene. Nucleic Acids Res. 1990 Sep 11;18(17):5045–5050. doi: 10.1093/nar/18.17.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L., Walker G. C. Cold sensitivity induced by overproduction of UmuDC in Escherichia coli. J Bacteriol. 1985 Apr;162(1):155–161. doi: 10.1128/jb.162.1.155-161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi T., Battista J. R., Dodson L. A., Walker G. C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi T., Hakura A., Nakai Y., Watanabe M., Murayama S. Y., Sofuni T. Salmonella typhimurium has two homologous but different umuDC operons: cloning of a new umuDC-like operon (samAB) present in a 60-megadalton cryptic plasmid of S. typhimurium. J Bacteriol. 1991 Feb;173(3):1051–1063. doi: 10.1128/jb.173.3.1051-1063.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi T., Yamada M., Watanabe M., Murayama S. Y., Sofuni T. Roles of Salmonella typhimurium umuDC and samAB in UV mutagenesis and UV sensitivity. J Bacteriol. 1992 Nov;174(21):6948–6955. doi: 10.1128/jb.174.21.6948-6955.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent M. E. A conjugative 'plasmid' lacking autonomous replication. J Gen Microbiol. 1981 Oct;126(2):305–310. doi: 10.1099/00221287-126-2-305. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Yu X., Shinohara A., Egelman E. H. Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science. 1993 Mar 26;259(5103):1896–1899. doi: 10.1126/science.8456314. [DOI] [PubMed] [Google Scholar]
- Pembroke J. T., Stevens E., Brandsma J. A., Van de Putte P. Location and cloning of the ultraviolet-sensitizing function from the chromosomally associated IncJ group plasmid, R391. Plasmid. 1986 Jul;16(1):30–36. doi: 10.1016/0147-619x(86)90076-4. [DOI] [PubMed] [Google Scholar]
- Perry K. L., Elledge S. J., Mitchell B. B., Marsh L., Walker G. C. umuDC and mucAB operons whose products are required for UV light- and chemical-induced mutagenesis: UmuD, MucA, and LexA proteins share homology. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4331–4335. doi: 10.1073/pnas.82.13.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry K. L., Walker G. C. Identification of plasmid (pKM101)-coded proteins involved in mutagenesis and UV resistance. Nature. 1982 Nov 18;300(5889):278–281. doi: 10.1038/300278a0. [DOI] [PubMed] [Google Scholar]
- Pinney R. J. Distribution among incompatibility groups of plasmids that confer UV mutability and UV resistance. Mutat Res. 1980 Aug;72(1):155–159. doi: 10.1016/0027-5107(80)90232-8. [DOI] [PubMed] [Google Scholar]
- Ramotar D., Popoff S. C., Demple B. Complementation of DNA repair-deficient Escherichia coli by the yeast Apn1 apurinic/apyrimidinic endonuclease gene. Mol Microbiol. 1991 Jan;5(1):149–155. doi: 10.1111/j.1365-2958.1991.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Samson L., Derfler B., Boosalis M., Call K. Cloning and characterization of a 3-methyladenine DNA glycosylase cDNA from human cells whose gene maps to chromosome 16. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9127–9131. doi: 10.1073/pnas.88.20.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Survival, mutation and capacity to repair single-strand DNA breaks after gamma irradiation in different Exr - strains of Escherichia coli. Mol Gen Genet. 1972;119(2):93–102. doi: 10.1007/BF00269129. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Ho C., Woodgate R. Mutagenic DNA repair in enterobacteria. J Bacteriol. 1991 Sep;173(18):5604–5611. doi: 10.1128/jb.173.18.5604-5611.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H., Iwasaki H., Kato T., Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H., Kato T., Ise T., Makino K., Nakata A. Cloning and characterization of the umu operon responsible for inducible mutagenesis in Escherichia coli. Gene. 1983 Aug;23(2):167–174. doi: 10.1016/0378-1119(83)90048-3. [DOI] [PubMed] [Google Scholar]
- Shinohara A., Ogawa H., Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992 May 1;69(3):457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- Steinborn G. Uvm mutants of Escherichia coli K12 deficient in UV mutagenesis. I. Isolation of uvm mutants and their phenotypical characterization in DNA repair and mutagenesis. Mol Gen Genet. 1978 Sep 20;165(1):87–93. doi: 10.1007/BF00270380. [DOI] [PubMed] [Google Scholar]
- Story R. M., Bishop D. K., Kleckner N., Steitz T. A. Structural relationship of bacterial RecA proteins to recombination proteins from bacteriophage T4 and yeast. Science. 1993 Mar 26;259(5103):1892–1896. doi: 10.1126/science.8456313. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., Roegner-Maniscalco V., Sweasy J. B., McCall J. O. Recovery from ultraviolet light-induced inhibition of DNA synthesis requires umuDC gene products in recA718 mutant strains but not in recA+ strains of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6805–6809. doi: 10.1073/pnas.84.19.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgate R. Construction of a umuDC operon substitution mutation in Escherichia coli. Mutat Res. 1992 Mar;281(3):221–225. doi: 10.1016/0165-7992(92)90012-7. [DOI] [PubMed] [Google Scholar]
- Woodgate R., Levine A. S., Koch W. H., Cebula T. A., Eisenstadt E. Induction and cleavage of Salmonella typhimurium UmuD protein. Mol Gen Genet. 1991 Sep;229(1):81–85. doi: 10.1007/BF00264216. [DOI] [PubMed] [Google Scholar]
- Woodgate R., Sedgwick S. G. Mutagenesis induced by bacterial UmuDC proteins and their plasmid homologues. Mol Microbiol. 1992 Aug;6(16):2213–2218. doi: 10.1111/j.1365-2958.1992.tb01397.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y., Morita T., Yamamoto A., Matsushiro A. Cloning and sequence of the human RecA-like gene cDNA. Nucleic Acids Res. 1993 Apr 11;21(7):1665–1665. doi: 10.1093/nar/21.7.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]