Abstract
Background
Prevalence rates for both overweight and asthma have been increasing among children in developed countries over the past two decades. Some recent studies have postulated a causal relation between these but have lacked power to form a definitive conclusion.
Aim
To estimate the effect of high body weight in childhood on the future risk of asthma.
Methods
Medline search (1966 to October 2004), supplemented by manual search of reference lists and grey literature. Cohort studies that examined high body weight at birth or during childhood and future outcome of asthma were included. Data from each study were extracted on exposure status, clinical outcome, and study characteristics.
Results
A total of 402 studies were initially identified, of which 12 met the inclusion criteria. The combined results from four studies that examined the effect of high body weight during middle childhood on the outcome of subsequent asthma showed a 50% increase in relative risk (RR 1.5, 95% CI 1.2 to 1.8). The combined results from nine studies that examined the effect of high birth weight on subsequent asthma had a pooled RR of 1.2 (95% CI 1.1 to 1.3). There was consistency among the results in sensitivity analyses examining studies containing only estimates of odds ratios, studies containing only the outcome of physician diagnosis of asthma, and studies including all definitions of high body weight.
Conclusions
Children with high body weight, either at birth or later in childhood, are at increased risk for future asthma. Potential biological mechanisms include diet, gastro‐oesophageal reflux, mechanical effects of obesity, atopy, and hormonal influences. Further research might elucidate the causal pathway, which could improve our understanding of the pathophysiology of asthma and perhaps lead to knowledge of potential preventive interventions.
Keywords: asthma, high birth weight, meta‐analysis, overweight
Asthma is a major cause of morbidity and mortality among children, and the prevalence of asthma has been steadily rising over the past two decades.1 This increase has been attributed to air pollution, environmental tobacco smoke, smaller family size, and decreased exposure to infectious agents, but has yet to be fully explained.2,3,4 The prevalence of overweight has also been increasing among children in the developed world,5 and an association between high weight and asthma has been found in both in adult6,7,8,9 and paediatric populations.10,11,12,13,14 Preliminary research has indicated several plausible biological mechanisms for this relation. Some researchers have identified dietary components that might provide a link between overweight and asthma.15,16,17,18,19 Long chain fatty acids and antioxidants show some tendency towards reducing the risk of asthma, whereas polyunsaturated fats have been postulated to increase risk.17,18 Gastro‐oesophageal reflux may also be a potential cause of association between high body weight and asthma in both adults and children.20,21 Other causal pathways may involve hormonal influences,22,23 atopy,24,25 and the mechanical effects of obesity.10
Several recent longitudinal studies on the effect of high weight during childhood on asthma have not had entirely consistent results, in part because some cohorts were designed for other purposes and therefore lacked power to assess the association between high body weight and future risk of asthma. We present here a systematic review and meta‐analysis of cohort studies to estimate the direction and magnitude of the association between high body weight and asthma.
Methods
Literature search
We searched Medline (US National Library of Medicine) from January 1966 to October 2004 to obtain all publications with longitudinal studies which reported a numerical calculation of the effect of high body weight during childhood on future risk of asthma. The Medline search used the Medical Subject Headings (MeSH) term “asthma” or textword term “asthma” and the MeSH terms “overweight”, “obesity”, and “body weight”, as well as the textwords “overweight”, “obesity”, and “body weight”. These were searched using limits that included all children using MeSH terms “infant”, “child”, and “adolescent”. The search was followed by manual examination of the reference lists of included articles and review articles and a manual review of the grey literature, including conference proceedings and abstracts. There were no language restrictions. If an author published more than one study on this topic, then more than one study was included only if the study populations were independent. If the populations were not independent, the more recent analysis was used. One reviewer judged whether identified studies met our inclusion criterion and abstracted data from the included studies.
Our primary predictor variable was high body weight, measured by either body mass index (BMI), Ponderal index, or birth weight alone (table 1). We defined high body weight as BMI ⩾85th centile for age and gender, Ponderal index ⩾2.5 g/cm3 or ⩾27 kg/m3, and birth weight ⩾3.8 kg. The primary outcome variable was the development of asthma not present at baseline. We included studies that defined the outcome of asthma as physician diagnosis of asthma, hospitalisation for asthma, use of asthma medications, or frequent wheezing in a subject past infancy (table 1). Both predictor and outcome variables were dichotomous.
Table 1 Included studies.
| Reference | Primary exposure measure | Place of study, n | Effect size (95% CI) | Primary outcome measure | Age at follow up | Notes |
|---|---|---|---|---|---|---|
| Bolte, 200435 | Birth weight ⩾4 kg | Munich, n = 534 | OR 1.52 (0.51 to 4.51) | Physician diagnosed asthma | 5–7 y | |
| Sin, 200436 | Birth weight ⩾4.5 kg | Alberta, n = 83 595 | RR 1.16 (1.04 to 1.29) | Asthma Emergency Department visit | 10 y | |
| Yuan, 200237 | Ponderal index ⩾2.5 g/cm3 | Denmark, n = 10 440 | RR 1.53 (1.11 to 2.13) | Asthma hospitalisation | 12 y | |
| Gilliland, 200331 | BMI in 4th, 7th, and 10th grades | Southern California, n = 3792 | RR 1.52 (1.14 to 2.03) | Physician diagnosed asthma | 11–22 y (4 y after enrolled) | |
| Xu, 200224 | Ponderal index ⩾2.83 g/cm3 | Finland, n = 4719 | Effect of birth weight: OR 1.26 (0.88 to 1.82) | Physician diagnosed asthma | 31 y | Effect of high childhood BMI: OR 1.32 (0.82 to 2.4) |
| Chinn, 200132 | BMI age 5–6 | UK, n = 6744 | OR 1.24 (0.82 to 1.88) | Physician diagnosed asthma | 9–10 y | Results for boys and girls were pooled to get total. Also contained info on risk per additional gram of birth weight, but this continuous outcome was not able to be combined with the dichotomous outcomes of the other studies |
| Castro‐Rodriguez, 200133 | BMI age 11 | Tucson, n = 448 (222 boys and 226 girls) | OR 2.7 (0.9 to 8.2) for boys; OR 3.2 (1.1 to 8.8) for girls | Frequent wheeze at age 13 | 13 y | Not enough incidence information given to pool results for gender; each gender entered separately into meta‐analysis |
| Rasanen, 200038 | Ponderal index ⩾27 kg/m3 | Finland, n = 4578 | OR 1.86 (1.13 to 3.07) | Physician diagnosed asthma | 16 y | |
| Leadbitter, 199939 | Birth weight ⩾4 kg | Dunedin, New Zealand, n = 735 | OR 1.0 (0.4 to 2.4) | Physician diagnosed asthma | 13 y | |
| Gregory, 199940 | Birth weight ⩾4 kg | Southampton, UK, n = 249 | OR 1.3 (0.4 to 4.4) | Current wheeze or current use of asthma medication | 11–14 y | |
| Fergusson, 199741 | Birth weight ⩾4 kg | Christchurch, New Zealand, n = 891 | RR 0.89 (0.51 to 1.6) | Physician diagnosed asthma | 16 y | |
| Schwartz42 | Birth weight ⩾3.8 kg | USA, n = 4661 | RR 0.92 (0.62 to 1.37) | Physician diagnosed asthma | 6 m–11 y | Data on childhood BMI cross‐sectional |
CI, confidence interval; OR, odds ratio; RR, relative risk.
Statistical methods and analysis
We abstracted odds ratios (OR), RR, and 95% confidence intervals (CI) from all studies. To convert CIs from the various studies into estimates of the variance of the log(RR), we transformed the interval to a log scale and then calculated standard error. To combine data we used the meta‐analysis routine in Stata 8.2 (Stata Corp., College Station, TX). We calculated a pooled summary relative risk according to both a fixed effects model, which used the inverse of the variance as the weight, and a random effects model, which uses the sum of the inverse of the variance and the moment estimator of the variance as the weight.26 We evaluated the possibility of publication bias with a Begg's funnel plot and with Egger's test.27,28
We analysed the effect of high birth weight and high body weight during middle childhood on the risk of future outcome of asthma. We conducted three sensitivity analyses to examine some of the assumptions of our models. In one, we analysed the estimated effect of any measure of high childhood weight combined on the outcome of asthma. In the second, in order to examine the effect of using odds ratios as estimates of the relative risk, we looked separately at those studies reporting odds ratios. In the third, in order to examine the effect of differences in outcome measures, we looked separately at those studies with an outcome of physician diagnosis of asthma. We used the cumulative meta‐analytic technique proposed by Lau and colleagues29 to check homogeneity of results.
Results
Of the 402 papers found by the search, we identified 16 articles that presented original research with unique populations showing the effect of high body weight either at birth or during childhood on the future outcome of asthma. From these, 12 articles expressed future risk of asthma as a dichotomous outcome compared with children of average or normal body weight (table 1). One study was excluded because the effect of body weight was expressed as a continuous outcome, and no method is available to combine continuous results with the results of dichotomous studies. Two studies were excluded because the only available baseline was low body weight, and low body weight in newborns and children may be associated with other risk factors for respiratory disease. One study was excluded because birth weight as an exposure was adjusted for subsequent BMI (table 2). All 12 included studies had medium‐to‐high quality scores (median score 7; range 6–8 (of 9 possible points)).30 All 12 were cohort studies, either prospective (n = 8) or retrospective (n = 4). Seven studies reported their results as odds ratios, and five reported relative risks.
Table 2 Excluded studies.
| Reference | Reason for exclusion | Results |
|---|---|---|
| Laerum, 200445 | No dichotomous analysis of effect of high weight on asthma | Continuous outcome reported by 500 g from lowest baseline: OR 0.92 (0.62 to 1.35) |
| Gold, 200346 | Baseline was lowest quintile | Overweight boys 1.04 (0.60 to 1.82) compared with lowest quintile; middle quintile was 1.01 compared with lowest quintile. Overweight girls 2.24 (1.14 to 4.40) compared with lowest quintile; middle quintile was 2.18 (1.07 to 4.44) compared with lowest quintile |
| Katz, 200347 | Baseline was lowest quintile | 0.92 (0.62 to 1.35) compared with lowest quintile of birth weight |
| Shaheen, 199948 | Birth weight as exposure was adjusted for adult BMI | OR for effect of birth weight adjusted by BMI was 0.81 (0.55 to 1.18) |
Childhood BMI
We analysed the four studies reporting estimates of effect of high body weight in school aged children on future outcome of asthma.24,31,32,33 The studies all used BMI ⩾85th centile for age and gender as the predictor variable. In one of the studies eligible for inclusion, data were presented separately for boys and girls without pooling, and we used incidence information from the study to recalculate a pooled summary statistic and a pooled variance.32,34 In another eligible study, data were presented separately for boys and girls without sufficient incidence information to allow pooling. For this study we entered results for boys and girls separately into the meta‐analysis.33 RR in these four studies ranged from 1.24 to 3.2. The studies had good homogeneity (p = 0.392). The der Simonian and Laird estimate of between studies variance was 0.002. The pooled summary RR was 1.5, with both fixed and random effects models giving a 95% CI of 1.2 to 1.8. Thus, studies with an estimate of effect of high body weight in school aged children estimated that those with high body weight have a future risk of asthma equal to 1.5 times the future risk of children without high body weight (fig 1). Analysis for metabias by Begg's test showed general symmetry around the median line, and Egger's test revealed no evidence of publication bias.
Figure 1 Meta‐analysis of effect of high body weight during childhood on subsequent development of asthma; Forrest plot (size of square indicates weight given to study in the summary statistic).
High birth weight
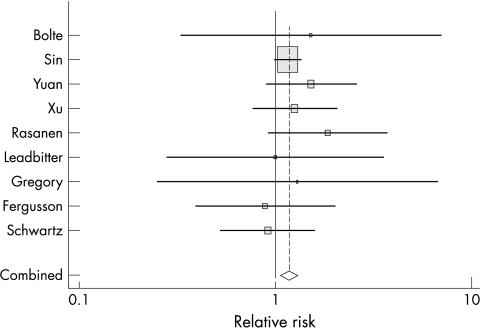
Nine studies reported estimates of the effect of high body weight at birth on risk of future asthma.24,35,36,37,38,39,40,41,42 In these studies, high body weight was identified using either elevated Ponderal index or elevated birth weight. RR for high body weight at birth ranged from 0.89 to 1.86. These nine studies had a low level of heterogeneity (p = 0.440). The Der Simonian and Laird estimate of between studies variance was <0.001. Fixed effects and random effects models gave the same pooled summary RR and CI (RR 1.2, 95% CI 1.1 to 1.3) (fig 2). Analysis for metabias by Begg's test showed general symmetry around the median line, and Egger's test revealed no evidence for publication bias.
Figure 2 Meta‐analysis of effect of high birth weight on subsequent development of asthma; Forrest plot (size of square indicates weight given to study in the summary statistic).
Sensitivity analyses
Combined model
We also created a model in which studies of both high birth weight and high BMI during childhood were combined to determine an estimate of the summary effect of increased body weight. All 12 studies were used in this model. Of note, one of the included studies contained information for both high birth weight and childhood BMI ⩾85%, and in the combined model the estimate for the effect of birth weight was used.24 The range of RR in this combined model was 0.89 to 3.2, with borderline evidence of heterogeneity (p = 0.206). The der Simonian and Laird estimate of between studies variance was 0.012. This level of heterogeneity suggests that the two different exposures of high birth weight and high weight during childhood might be related but distinct. The combined model had a summary relative risk of 1.2 (95% CI 1.1 to 1.3) for the fixed effects estimate and 1.3 (1.1 to 1.5) for the random effects estimate, consistent with the observed borderline heterogeneity.
Odds ratios only
Seven studies reported data for the effect of high weight on risk of future asthma as odds ratios (OR). All of these studies used multivariate logistic regression to obtain results. The effect of high weight on asthma in studies reporting OR was analysed in a subgroup analysis. The range of OR varied from 1.00 to 3.2, with a low level of heterogeneity (p = 0.529). The fixed effects and random effects models gave the same results, with a summary odds ratio of 1.4 (95% CI 1.2 to 1.7).
Physician diagnosis only
Eight studies reported data for the effect of high weight on risk of future asthma diagnosed by a physician, which may be a more precise measure of outcome. The effect of high weight on asthma in these studies was examined in a subgroup analysis. The range of RR varied from 0.89 to 1.86, with an acceptable level of homogeneity (p = 0.329). The fixed effects and random effects models gave the same results, with a summary odds ratio of 1.3 (95% CI 1.1 to 1.5).
Cumulative meta‐analysis
Cumulative meta‐analysis of all studies showed that results of studies from earlier years were somewhat inconsistent with our current findings (fig 3). Early studies of the effect of high weight on asthma showed a negative association. Studies published by the year 2000 showed a positive association, but with a p value greater than 0.05. Not until 2001 did the p value fall below 0.05, and not until 2002 did the p value fall below 0.01 (table 3).
Figure 3 Cumulative meta‐analysis of effect of high weight in childhood on subsequent development of asthma, in order of year of publication.
Table 3 Cumulative meta‐analysis of all studies estimating an effect of high weight in childhood on outcome of asthma.
| Summary estimate | (95% CI) | p value | |
|---|---|---|---|
| Schwartz42 (1990) | 0.92 | (0.62 to 1.37) | 0.682 |
| Fergusson41 (1997) | 0.91 | (0.66 to 1.27) | 0.577 |
| Leadbitter39 (1999) | 0.92 | (0.68 to 1.26) | 0.600 |
| Gregory40 (1999) | 0.941 | (0.70 to 1.27) | 0.692 |
| Rasanen38 (2000) | 1.128 | (0.87 to 1.46) | 0.358 |
| Chinn32 (2001) | 1.158 | (0.93 to 1.44) | 0.188 |
| Castro‐Rodriguez33 (2001) | 1.242 | (1.01 to 1.53) | 0.043 |
| Yuan37 (2002) | 1.304 | (1.08 to 1.57) | 0.005 |
| Xu24 (2002) | 1.295 | (1.10 to 1.53) | 0.002 |
| Gilliland31 (2003) | 1.347 | (1.17 to 1.55) | 0.000 |
| Bolte35 (2004) | 1.349 | (1.17 to 1.55) | 0.000 |
| Sin36 (2004) | 1.227 | (1.13 to 1.34) | 0.000 |
Discussion
By conducting a systematic review and meta‐analysis of the effect of high weight on future risk of asthma, we have obtained an estimate that suggests that high body weight among school aged children increases the risk of future asthma by approximately 50%. The effect of high birth weight appears to be less pronounced but still quite significant, with an RR of 1.2 compared to babies without high birth weight. The association between high weight and asthma remains significant when combining estimates of all age groups for analysis and also remains strong when results reported as ORs are analysed separately from those reported as RRs and when the definition of outcome is narrowed to include physician diagnosis only.
What is already known on this topic
Some studies have found that high birth weight and high BMI in childhood are predictive of future asthma, while others have shown no association
These findings have important implications for public health. The past 20 years have seen a dramatic increase in both high BMI and asthma, and it seems possible that increasing weight may have contributed to increasing asthma. The prevalence of overweight among 6–11 year olds in the United States has recently been estimated to be 15.3%, while that among 12–19 year olds was estimated at 15.5%.43 The population attributable risk could therefore be estimated at 0.066, meaning that 6.6% of all cases of childhood asthma are due to overweight. The 2000 census counted 41.1 million Americans between the ages of 5 and 14 years. Given a prevalence rate for paediatric asthma of 57.8 per 1000,1 the results of this meta‐analysis suggest that over 100 000 American children in this age group suffer from asthma each year as a result of being overweight. The impact of high weight could increase further when high birth weight is also considered.
The purpose of this study was to evaluate the effect of high body weight on the development of asthma. This goal is complicated by the fact that weight is not one of the largest contributors to the development of asthma, and the effect of weight on asthma is likely to be relatively weak compared to other risk factors such as atopy, air pollution, and family history of asthma. It is further complicated by the facts that currently available cohort studies are diverse in both exposure measures and in outcome measures.
Several sources of bias should be considered in the interpretation of these results. First, misclassification of exposure is a potential source of bias in this systematic review, especially due to the acceptance of three different definitions of high body weight, the acceptance of high body weight at a variety of ages, and the possibility that BMI centile standards varied for different cohorts. However, significant misclassification of exposure would be expected to either bias findings towards the null result of no association between exposure and outcome or cause significant heterogeneity in the meta‐analysis model. Also, there is no indication that there is a threshold level for weight that sharply demarcates those at increased risk from those with average risk. The levels of homogeneity found in the significant results in the meta‐analysis suggest that the association found between high body weight and asthma is not due to misclassification of exposure.
Another potential source of bias is misclassification of outcome. This is a concern both for the meta‐analysis as a whole, which includes several different definitions of outcome and includes several different ages for the assessment of outcome, and within the individual studies, especially due to diagnostic bias. Regarding potential misclassification of outcome due to the acceptance of several different criteria for outcome in this meta‐analysis, it is important to note that the subset analysis examining the outcome of physician diagnosis of asthma shows results very similar to the other models. This argues against significant bias from the use of several different criteria for outcome. Misclassification of outcome from varying age at time of outcome remains a possible source of bias in this paper, although the relatively good homogeneity in the models would argue against a strong bias from this source.
What this study adds
This paper provides a systematic review and meta‐analysis of the studies on this topic and shows that both high birth weight and high BMI during childhood are predictive of future asthma
High birth weight is associated with a RR of 1.2 (95% CI 1.1 to 1.3) for future asthma, while high BMI during childhood is associated with a RR of 1.5 (95% CI 1.2 to 1.8) for future asthma. Over 100 000 children in the United States may suffer from asthma due to childhood overweight
Regarding misclassification of outcome within the individual studies, the results of the analysis of effect of birth weight argue against significant diagnostic bias. Parents (and doctors) of larger babies do not view them as more sickly than smaller babies, and so it is unlikely that they would report increased rates of either physician diagnosis of asthma or asthma treatment or hospitalisation due to high birth weight. Nevertheless, the possibility of diagnostic bias cannot be completely eliminated in the meta‐analysis and remains a potential limitation. An additional source of potential systematic error is publication bias, which is especially concerning because some of the included papers are based on findings from cohorts designed for other purposes. However, the Begg and Egger tests did not offer any evidence for publication bias, and a review of the grey literature did not reveal any additional research meeting inclusion criteria.
This study offers strong evidence that high body weight during middle childhood increases the odds of future asthma by approximately 1.5‐fold, and that high birth weight increases the odds of asthma 1.2‐fold. However, an important limitation of our analysis is the inability to adjust for individual confounding variables, such as other types of atopic disease, environmental tobacco exposure, family history of asthma, and gender. Although the individual studies adjusted for variables as they reached significance in their population, the meta‐analysis cannot examine how these might modify the relation between weight and asthma. Also, several studies did report results differently for boys and girls, but there were not enough of these to allow for subset analysis by gender in this paper. This is an important limitation, as some researchers have found association to differ by gender, and others have not.32,33,44
In order to examine the effect of other predictor variables on the relation between body weight and risk of subsequent asthma, the ideal future study would follow a very large cohort of children from birth through adolescence. Simple information such as weight and height would need to be collected at regular intervals, and asthma status would be assessed annually. Such a study could also collect data on types of dietary intake, age of pubertal changes, and gastric and atopic symptoms, and might even be able to perform forced expiratory volume (FEV1) testing to reduce diagnostic bias. This type of research could examine the effects of additional predictor variables both singly and in combination on the relation between high body weight and risk of asthma, and might result in new knowledge about the causes of the disease. Improved knowledge about the causal pathways leading to an association between high body weight and asthma could result in improved understanding of the pathophysiology involved in the dramatic increase in prevalence of asthma, which could potentially lead to important knowledge about methods for prevention of this common childhood disease.
Footnotes
Competing interests: none declared
References
- 1.Mannino D M, Homa D M, Akinbami L J.et al Surveillance for asthma—United States, 1980–1999. MMWR Surveill Summ 2002511–13. [PubMed] [Google Scholar]
- 2.Ball T M, Castro‐Rodriguez J A, Griffith K A.et al Siblings, day‐care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med 2000343538–543. [DOI] [PubMed] [Google Scholar]
- 3.Platts‐Mills T A, Rakes G, Heymann P W. The relevance of allergen exposure to the development of asthma in childhood. J Allergy Clin Immunol 2000105(2 pt 2)S503–S508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood R A. Pediatric asthma. JAMA 2002288745–747. [DOI] [PubMed] [Google Scholar]
- 5.Dietz W H. Overweight in childhood and adolescence. N Engl J Med 2004350855–857. [DOI] [PubMed] [Google Scholar]
- 6.Celedon J C, Palmer L J, Litonjua A A.et al Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med 2001164(10 pt 1)1835–1840. [DOI] [PubMed] [Google Scholar]
- 7.Arif A A, Delclos G L, Lee E S.et al Prevalence and risk factors of asthma and wheezing among US adults: an analysis of the NHANES III data. Eur Respir J 200321827–833. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis D, Chinn S, Potts J.et al Association of body mass index with respiratory symptoms and atopy: results from the European Community Respiratory Health Survey. Clin Exp Allergy 200232831–837. [DOI] [PubMed] [Google Scholar]
- 9.Young S Y, Gunzenhauser J D, Malone K E.et al Body mass index and asthma in the military population of the northwestern United States. Arch Intern Med 20011611605–1611. [DOI] [PubMed] [Google Scholar]
- 10.Chinn S. Obesity and asthma: evidence for and against a causal relation. J Asthma 2003401–16. [DOI] [PubMed] [Google Scholar]
- 11.Brenner J S, Kelly C S, Wenger A D.et al Asthma and obesity in adolescents: is there an association? J Asthma 200138509–515. [DOI] [PubMed] [Google Scholar]
- 12.von Mutius E, Schwartz J, Neas L M.et al Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax 200156835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein L H, Wu Y W, Paluch R A.et al Asthma and maternal body mass index are related to pediatric body mass index and obesity: results from the Third National Health and Nutrition Examination Survey. Obes Res 20008575–581. [DOI] [PubMed] [Google Scholar]
- 14.Redd S C. Asthma in the United States: burden and current theories. Environ Health Perspect 2002110(suppl 4)557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black P N, Sharpe S. Dietary fat and asthma: is there a connection? Eur Respir J 1997106–12. [DOI] [PubMed] [Google Scholar]
- 16.Woods R K, Walters E H, Raven J M.et al Food and nutrient intakes and asthma risk in young adults. Am J Clin Nutr 200378414–421. [DOI] [PubMed] [Google Scholar]
- 17.Hodge L, Salome C M, Peat J K.et al Consumption of oily fish and childhood asthma risk. Med J Aust 1996164137–140. [DOI] [PubMed] [Google Scholar]
- 18.Haby M M, Peat J K, Marks G B.et al Asthma in preschool children: prevalence and risk factors. Thorax 200156589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilliland F D, Berhane K T, Li Y F.et al Children's lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol 2003158576–584. [DOI] [PubMed] [Google Scholar]
- 20.Thomas E J, Kumar R, Dasan J B.et al Gastroesophageal reflux in asthmatic children not responding to asthma medication: a scintigraphic study in 126 patients with correlation between scintigraphic and clinical findings of reflux. Clin Imaging 200327333–336. [DOI] [PubMed] [Google Scholar]
- 21.Theodoropoulos D S, Pecoraro D L, Efstratiadis S E. The association of gastroesophageal reflux disease with asthma and chronic cough in the adult. Am J Respir Med 20021133–146. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Dales R, Tang M.et al Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol 2002155191–197. [DOI] [PubMed] [Google Scholar]
- 23.Seidell J C, de Groot L C, van Sonsbeek J L.et al Associations of moderate and severe overweight with self‐reported illness and medical care in Dutch adults. Am J Public Health 198676264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Pekkanen J, Laitinen J.et al Body build from birth to adulthood and risk of asthma. Eur J Public Health 200212166–170. [DOI] [PubMed] [Google Scholar]
- 25.Huang S L, Shiao G, Chou P. Association between body mass index and allergy in teenage girls in Taiwan. Clin Exp Allergy 199929323–329. [DOI] [PubMed] [Google Scholar]
- 26.Lau J, Ioannidis J P, Schmid C H. Quantitative synthesis in systematic reviews. Ann Intern Med 1997127820–826. [DOI] [PubMed] [Google Scholar]
- 27.Begg C B, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994501088–1101. [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M.et al Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997315629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau J, Antman E M, Jimenez‐Silva J.et al Cumulative meta‐analysis of therapeutic trials for myocardial infarction. N Engl J Med 1992327248–254. [DOI] [PubMed] [Google Scholar]
- 30.Wells G A, Shea B, O'Connell D.et alThe Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 28 March 2005)
- 31.Gilliland F D, Berhane K, Islam T.et al Obesity and the risk of newly diagnosed asthma in school‐age children. Am J Epidemiol 2003158406–415. [DOI] [PubMed] [Google Scholar]
- 32.Chinn S, Rona R J. Can the increase in body mass index explain the rising trend in asthma in children? Thorax 200156845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castro‐Rodriguez J A, Holberg C J, Morgan W J.et al Increased incidence of asthma‐like symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med 20011631344–1349. [DOI] [PubMed] [Google Scholar]
- 34.Rothman K J.Modern epidemiology. Oxford Press 2002
- 35.Bolte G, Schmidt M, Maziak W.et al The relation of markers of fetal growth with asthma, allergies and serum immunoglobulin E levels in children at age 5–7 years. Clin Exp Allergy 200434381–388. [DOI] [PubMed] [Google Scholar]
- 36.Sin D D, Spier S, Svenson L W.et al The relationship between birth weight and childhood asthma: a population‐based cohort study. Arch Pediatr Adolesc Med 200415860–64. [DOI] [PubMed] [Google Scholar]
- 37.Yuan W, Basso O, Sorensen H T.et al Fetal growth and hospitalization with asthma during early childhood: a follow‐up study in Denmark. Int J Epidemiol 2002311240–1245. [DOI] [PubMed] [Google Scholar]
- 38.Rasanen M, Kaprio J, Laitinen T.et al Perinatal risk factors for asthma in Finnish adolescent twins. Thorax 20005525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leadbitter P, Pearce N, Cheng S.et al Relationship between fetal growth and the development of asthma and atopy in childhood. Thorax 199954905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory A, Doull I, Pearce N.et al The relationship between anthropometric measurements at birth: asthma and atopy in childhood. Clin Exp Allergy 199929330–333. [DOI] [PubMed] [Google Scholar]
- 41.Fergusson D M, Crane J, Beasley R.et al Perinatal factors and atopic disease in childhood. Clin Exp Allergy 1997271394–1401. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz J, Gold D, Dockery D W.et al Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis 1990142555–562. [DOI] [PubMed] [Google Scholar]
- 43.Ogden C L, Flegal K M, Carroll M D.et al Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 20022881728–1732. [DOI] [PubMed] [Google Scholar]
- 44.Ford E S. The epidemiology of obesity and asthma. J Allergy Clin Immunol 2005115897–909. [DOI] [PubMed] [Google Scholar]
- 45.Laerum B N, Svanes C, Gulsvik A.et al Is birth weight related to lung function and asthma symptoms in Nordic‐Baltic adults? Respir Med 200498611–618. [DOI] [PubMed] [Google Scholar]
- 46.Gold D R, Damokosh A I, Dockery D W.et al Body‐mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol 200336514–521. [DOI] [PubMed] [Google Scholar]
- 47.Katz K A, Pocock S J, Strachan D P. Neonatal head circumference, neonatal weight, and risk of hayfever, asthma and eczema in a large cohort of adolescents from Sheffield, England. Clin Exp Allergy 200333737–745. [DOI] [PubMed] [Google Scholar]
- 48.Shaheen S O, Sterne J A, Montgomery S M.et al Birth weight, body mass index and asthma in young adults. Thorax 199954396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]