Abstract
Background and Aims
Clinical adrenal insufficiency has been reported with doses of inhaled fluticasone proprionate (FP) >400 μg/day, the maximum dose licensed for use in children with asthma. Following two cases of serious adrenal insufficiency (one fatal) attributed to FP, adrenal function was evaluated in children receiving FP outwith the licensed dose.
Methods
Children recorded as prescribed FP ⩾500 μg/day were invited to attend for assessment. Adrenal function was measured using the low dose Synacthen test (500 ng/1.73 m2 intravenously) and was categorised as: biochemically normal (peak cortisol response >500 nmol/l); impaired (peak cortisol ⩽500 nmol/l); or flat (peak cortisol ⩽500 nmol/l with increment of <200 nmol/l and basal morning cortisol <200 nmol/l).
Results
A total of 422 children had been receiving FP alone or in combination with salmeterol; 202 were not investigated (137 FP within license; 24 FP discontinued); 220 attended and 217 (age 2.6–19.3 years) were successfully tested. Of 194 receiving FP ⩾500 μg/day, six had flat responses, 82 impaired responses, 104 were normal, and in 2 the LDST was unsuccessful. Apart from the index child, the other five with flat responses were asymptomatic; a further child with impairment (peak cortisol 296 nmol/l) had encephalopathic symptoms with borderline hypoglycaemia during an intercurrent illness. The six with flat responses and the symptomatic child were all receiving FP doses of ⩾ 1000 μg/day.
Conclusion
Overall, flat adrenal responses in association with FP occurred in 2.8% of children tested, all receiving ⩾1000 μg/day, while impaired responses were seen in 39.6%. Children on above licence FP doses should have adrenal function monitoring as well as a written plan for emergency steroid replacement.
Keywords: asthma, adrenal suppression, high dose inhaled corticosteroids, fluticasone propionate
Inhaled corticosteroids (ICS) are the most effective treatment for controlling asthma in adults and children.1 In children, randomised controlled trials have repeatedly confirmed their effectiveness compared with placebo in the control of chronic persistent asthma.2
However, ICS, particularly at high doses, are associated with systemic side effects3,4 which can be divided into those attributable to excess, such as Cushingoid features and growth suppression,5 and effects related to hypothalamic‐pituitary axis suppression6 which can result in adrenocortical atrophy and glucocorticoid insufficiency. Patients are at risk of acute adrenal insufficiency if the adrenal cortex cannot generate an adequate endogenous corticosteroid response to stress, especially if corticosteroid treatment is interrupted abruptly.7,8
In 2001, a 5 year old girl presented to a hospital in the West of Scotland with a day's history of vomiting, impaired consciousness, and visual disturbance. She developed progressive unconsciousness and seizures and died within nine hours. Postmortem examination showed cerebral oedema and small adrenal glands. Four weeks later, her 7 year old brother was admitted with almost identical symptoms. He had hyponatraemia and cerebral oedema but responded to intensive care. After discharge, his initial plasma cortisol was noted to be inappropriately low (225 nmol/l). Subsequent adrenal testing showed a severely impaired cortisol response (<30 nmol/l) to a low dose Synacthen test (LDST). Both siblings had been receiving fluticasone proprionate (FP) for asthma for a number of years in doses of up to 2000 μg/day.
We attributed these events to acute adrenal insufficiency induced by high dose FP. This led us to screen adrenal function in all children with asthma attending the respiratory clinics at the Royal Hospital for Sick Children, Glasgow who were recorded as receiving FP outwith the manufacturer's licensed dose (>400 μg/day). Our primary objective was to identify any child with evidence of adrenal insufficiency, and ensure they had adequate adrenal replacement therapy and a plan for emergency management of symptomatic acute adrenal insufficiency.
There are few published studies of adrenal function in large numbers of children with asthma using high dose ICS.9,10,11 Here we report the results of adrenal function testing in the children screened.
Methods
Patient identification
Electronic versions of clinic letters of children attending the specialist respiratory clinics were searched for the words “fluticasone”, “flixotide”, or “seretide” and then scrutinised to identify every child prescribed inhaled FP >400 μg/day between January 2000 and April 2002. Parents and GPs of these children were sent an explanatory letter with an appointment for adrenal function testing.
At testing, the nurse inquired about the current inhaled FP dose, any dosage changes in the last six months, the inhaler device used, and the number of oral corticosteroid rescue courses in the last year. We did not attempt to assess compliance with inhaled FP.
All children who attended were tested, even if more detailed inquiry at the time indicated that their FP dose was now within license (⩽400 μg/day).
Endocrine assessment
Children were asked to fast from midnight and to omit any morning oral, but not inhaled, medications. The endocrine nurse explained the testing and the reasons for it. A brief enquiry was made for symptoms of chronic adrenal insufficiency. Height was measured using a calibrated stadiometer and weight measured using electronic scales. Where both parents attended, their heights were measured and a mid‐parental height calculated.
Following application of local anaesthetic cream, the children were cannulated between 08 00 and 10 00 hours. Adrenal function was assessed by a synthetic ACTH (Synacthen) test using a modification of the low dose protocol of Crowley and colleagues.12 Briefly, the Synacthen dose was calculated on the basis of body surface area, giving 500 ng/1.73 m2, and prepared using serial dilution of 250 μg Synacthen in 10 ml saline. Venous blood samples were taken at time zero, and at 15, 20, 25, 30, and 35 minutes thereafter. Serum cortisol was measured by immunoassay (Immulite 2000, Diagnostic Products Corporation, Los Angeles, CA, USA).
We interpreted the cortisol results as follows:13,14
Normal: peak >500 nmol/l
Impaired: peak ⩽500 nmol/l
Flat: peak ⩽500 nmol/l with increment <200 nmol/l and basal morning cortisol <200 nmol/l.
Statistics
Data were summarised using standard descriptive statistics. Relationships between variables were investigated using Pearson's correlation. All analyses were done using Minitab (Version 14) with a significance level of 5%.
Ethical aspects
Because of the serious adverse events encountered locally, it was considered clinically essential to check whether other children receiving high dose FP had evidence of adrenal insufficiency. The investigation plan was agreed with the Medical Director. In the circumstances, we did not consider it appropriate to obtain permission for testing from the local ethics committee. All parents were given a written and verbal explanation of the reasons for testing their child.
Results
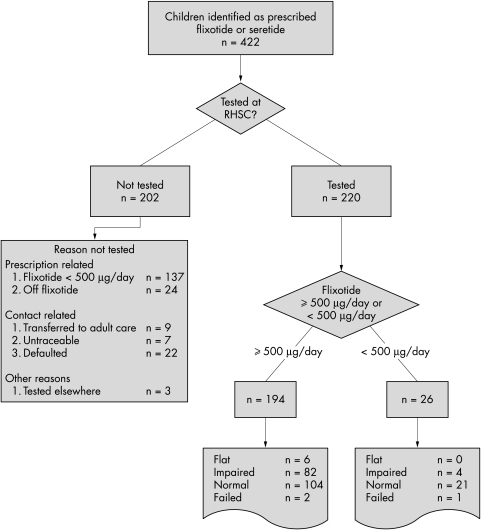
Between January 2000 and March 2002, 422 children were identified as prescribed FP, either alone or in combination with salmeterol (seretide). A flow diagram shows the number of children identified at each stage (fig 1). Two hundred and two children were not tested for reasons listed in fig 1.
Figure 1 Consort diagram of children studied.
Table 1 presents demographic details of the 220 children in whom testing was attempted. Children received FP from a metered dose inhaler with spacer (137/ 220; 62%), or as a dry powder. In 192 children, we had details of the duration of FP prescription (median 4 years; range 0–9).
Table 1 Details of 220 children tested by low dose Synacthen testing.
| Children studied | All tested | Normal response | Impaired response | Flat response |
|---|---|---|---|---|
| Number | 220 (217 successful; 3 failed) | 125 | 86 | 6 |
| Average FP dose (μg/day); mean/median (range) | 873/1000 (50–8000) | 743/500 (50–2000) | 1052/1000 (250–8000) | 1083/1000 (1000–1500) |
| Age (y); median (range) | 10.5 (2.6–19.3) | 10.5/10.5 (2.6–19.3) | 11/10.6 (4.5–17.4) | 8.3/8.1 (6.1–11.66) |
| M:F ratio | 1.1:1 (116:104) | 1.08:1 (65:60) | 1.25:1 (48:38) | 0.2:1 (1:5) |
| Height SDS; median (range) | −0.32 (−4 to 1.8) n = 193 | −0.18 (−2.9 to 1.8) n = 108 | −0.34 (−4.1 to 1.8) n = 75 | −0.55 (−2.3 to 0.90) n = 6 |
| Mid‐parental height SDS; median (range) | −0.57 (−3.5 to 1.4) n = 53 | −0.78 (−3.5 to 1.4) n = 26 | −0.49 (−2.7 to 0.9) n = 23 | −0.50 (−1.0 to 1.1) n = 3 |
| Weight SDS; median (range) | 0.27 (−3.8 to 3.9) n = 202 | 0.40 (−2.8 to 3.9) n = 116 | 0.18 (−3.8 to 2.2) n = 76 | 1.12 (−2.2 to 1.8) n = 6 |
| No. prescribed FP | ||||
| <500 μg/day | 26 (1 failed) | 21 | 4 | 0 |
| 500–1000 μg/day | 166 (1 failed) | 93 | 67 | 5 |
| >1000 μg/day | 28 (1 failed) | 11 | 15 | 1 |
| No. with basal cortisol <100 nmol/l | 16 | 4 | 8 | 4 |
Auxology
Height and weight standard deviation scores for the 220 children are in table 1, for all subjects and after sub‐division according to FP dose. There was no correlation between total daily FP dose and either height (r = 0.037, p = 0.612) or weight (r = 0.099, p = 0.161). In those children where mid‐parental height SD scores (SDS) were available, there was no significant difference between the child's height SDS and the mid‐parental height SDS (paired t test, p = 0.563, 95% CI −0.219 to 0.398).
Low dose Synacthen test results
The adrenal responses classified on the peak cortisol result and the FP doses at the time of testing are shown in table 1. Testing failed in three subjects. Of the 192 receiving FP ⩾500 μg/day successfully tested, 6 (3%) had flat, 82 impaired (43%), and 104 normal (54%) responses. Of the 25 receiving FP <500 μg/day at the time of testing successfully tested, none was flat (fig 1) and four were impaired (16%).
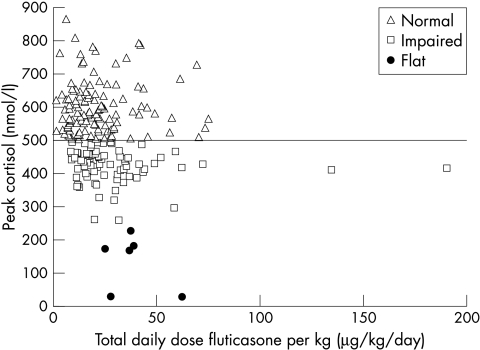
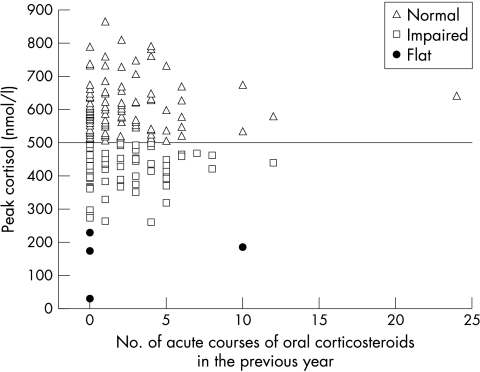
There was a weak but significant inverse correlation between peak cortisol and total daily dose (r = −0.213, p = 0.002). Correcting the total daily FP dose for subject's weight did not improve the correlation (r = −0.193, p = 0.006; fig 2). There was no relationship between peak cortisol level and age (r = −0.018, p = 0.794; table 2) or number of acute courses of prednisolone reported as given in the previous 12 months (r = 0.046, p = 0.526; fig 3).
Figure 2 Dose‐response curve showing relationship between peak stimulated cortisol (nmol/l) on LDST and total daily FP dose (μg/day) corrected for weight.
Table 2 Responses to low dose Synacthen in relation to age and dose; total number (<500 μg/day, 500–1000 μg/day, >1000 μg/day).
| Age (y) | n | Failed testing | Normal response | Impaired response | Flat response |
|---|---|---|---|---|---|
| <5 | 13 (2, 9, 1) | 1 (1, 0, 0) | 11 (2, 8, 1) | 1 (0, 1, 0) | 0 (0, 0, 0) |
| 5–10 | 86 (15, 63, 8) | 1 (0, 1, 0) | 48 (12, 32, 4) | 32 (3, 26, 3) | 5 (0, 4, 1) |
| >10 | 121 (8, 94, 19) | 1 (0, 0, 1) | 66 (7, 53, 6) | 53 (1, 40, 12) | 1 (0, 1, 0) |
Figure 3 Relation between peak stimulated cortisol on LDST and number of courses of oral corticosteroid (nmol/l) reported as taken in the last year.
Basal morning cortisol levels
There was no correlation between prescribed FP dose and the baseline morning serum cortisol levels (r = −0.115, p = 0.092). There was also no correlation between the number of children with baseline cortisol <100 nmol/l and FP dose (r = −0.152, p = 0.573) (table 1).
Clinical correlates of impaired adrenal responses
Brief details of the children who had flat responses or symptomatic impairment are given in table 3. Of the six with flat responses, none had symptoms of chronic adrenal insufficiency.
Table 3 Clinical details of children who had flat adrenal response (cases 1–6) or symptomatic adrenal insufficiency (case 7).
| Case | Age at test | Sex | Dose of FP (μg/d) | On oral prednisolone at time of testing? | Basal/peak cortisol (nmol/l) | Success‐fully weaned from ICS | Current status |
|---|---|---|---|---|---|---|---|
| 1 | 7.6 | M | 2000 | N | <25/<25 | Y | Well and off all treatment |
| 2 | 8.6 | F | 1000 | Y (4 mg daily) | 157/172 | N | Remains on prednisolone 5 mg daily and FP 500 μg/day |
| 3 | 9.3 | F | 1000 | N (but 7 short courses in previous 12 mth) | <25/<25 | N | Now on 400 μg/day mometasone; weaning off hydrocortisone replacement |
| 4 | 6.6 | F | 1000 | N | 167/138 | N | FP now reduced to 250 μg/day; oral prednisolone stopped at 7.5 years |
| 5 | 11.7 | F | 1000 | N | 98/227 | N | On 5 mg daily of prednisolone and 500 μg/day FP |
| 6 | 6.2 | F | 1000 | N (but >8 short courses in previous year) | <28/181 | N | Unsuccessful attempts to reduce FP; now on maintenance dose of prednisolone |
| 7 | 6.1 | M | 1000 | N | 118/269 | Y | Well and on treatment with Symbicort |
Two children had episodes of acute adrenal insufficiency: our index case (case 1, table 3); and one other (peak cortisol 289 nmol/l) who developed mild encephalopathy and hypoglycaemia with a tonsillitis who was the only one with symptoms of chronic adrenal insufficiency (tiredness, poor weight gain with dramatic improvement after adrenocortical replacement therapy) (case 7, table 3).
No child with peak LDST cortisol levels of >300 nmol/l test presented with either acute or chronic adrenal insufficiency.
Follow up and procedure adopted for steroid cover/replacement
Families and GPs were sent the results and a recommended plan including advice for managing any episodes of acute adrenal insufficiency (available from corresponding author on e‐mail request).
For all children, a focused effort was made at review to reduce the prescribed FP to a dose just sufficient to control the child's symptoms.
The identification and recall of all who had been prescribed FP >400 μg/day proved a major logistic exercise. While the majority were tested between May and December 2001, it was only by the end of 2004 (three years after our index cases) that we were confident that all at‐risk patients had been contacted and given appropriate advice.
Discussion
Because of its potency and availability in high dose forms, FP is commonly used when high dose ICS treatment is prescribed for children with moderate/severe asthma.15 While its use is well established,16 studies have shown that the dose‐response curve for FP appears to plateau at 100–200 μg/day. Doses >400 μg/day may occasionally confer extra benefit in some children with severe asthma, but at this level there may be evidence of adrenal suppression.17 Previous reports have highlighted the dangers of symptomatic adrenal insufficiency in children receiving high dose ICS, particularly FP.7,18,19,20
Our experience of two serious adverse events and published reports led us to test adrenal function in children prescribed FP >400 μg/day. Overall, we found evidence of adrenal suppression in 42.4% of the children tested (2.8% flat; 39.6% impaired). There has been no previous large survey using LDST in children prescribed above licence doses of inhaled FP for prolonged periods. Previous reports have generally either been case series21 or studies of adrenal function during short term trials of high dose FP.9,10 One exception is a cross‐sectional study of adrenal function in 50 children and adolescents receiving FP ⩾1000 μg/day for ⩾6 months who were thought to be adherent with therapy.11 Using a standard Synacthen test, biochemical adrenal insufficiency was found in only 12% of those tested. The greater numbers of children with impaired responses in this present report likely reflect the greater sensitivity of the LDST.14
Other estimates of the prevalence of biochemical adrenal suppression in children on high dose ICS vary. Our finding of 42% should be compared to the estimates of: 25% in the study of Kannisto and colleagues22 and 35% in the report of Broide et al in children and young adults,14 both using the LDST; 50% prevalence using insulin tolerance testing in the small study of Mahachoklertwattana and colleagues;10 and to the report of Fitzgerald et al where FP 750 μg/day was compared with beclomethasone dipropionate at 1500 μg/day in a 12 week randomised controlled crossover study—67% and 70% of children respectively had impaired adrenal function on LDST.9 These different prevalence estimates presumably reflect differences in the populations studied and methods used to characterise adrenal function. Nevertheless, they suggest that between one quarter and two thirds of children of children on high dose ICS will show biochemical adrenal suppression on sensitive testing.
In agreement with others, we confirmed that serum morning basal cortisol alone is not a reliable indicator of adrenocortical function in children with asthma on high dose ICS.11,13,14
We did not find other features of corticosteroid excess. Only one child, who was also receiving daily oral corticosteroids, had Cushingoid features. We found no evidence of a significant dose dependent effect of FP on height.23 In children with mid‐parental height measurements, there was no significant difference between the child's height SDS and the mid‐parental height SDS in keeping with a lack of significant growth suppression despite high FP doses. Dunlop et al also recently reported potentially severe adrenal suppression in some children who were growing at a normal rate despite taking high dose inhaled corticosteroids.23 Monitoring growth is, therefore, not an adequate screening test to identify children with adrenal suppression.
We were able to wean our index case from ICS treatment completely, while the child with symptoms of chronic adrenal insufficiency is now on a modest dose of an alternative ICS, suggesting that both did not have severe asthma. Systemic FP effects may have been more pronounced due to greater pulmonary absorption from relatively normal lungs.24,25 The attempts to reduce FP in the other children with flat responses resulted in unacceptable worsening of asthma symptoms, reflecting the value of high dose FP in children with more severe asthma.4,17
We made no attempt to evaluate compliance with inhaled FP treatment. Non‐compliance is well recognised in children receiving inhaled medications,26 has been shown to increase with duration of treatment,27 and is a potentially important confounder of any dose dependent systemic effects of inhaled corticosteroids. We found a very small although significant negative correlation between the daily FP dose and the peak cortisol level on LDST, similar to other studies. Sim et al reported a small but significant negative correlation (r = −0.42) between FP dose and peak cortisol on a short Synacthen test.11 The relevant graph in their paper (figure 111) shows a wide distribution of cortisol values at each FP dose level very similar to our own results (fig 2). Broide and colleagues14 found no relation between peak cortisol on LDST and daily dose of ICS. We also noted no relation between the number of reported rescue oral steroid courses in the last year and peak cortisol, although in some cases the reported number of courses used was improbably high. We speculate that the lack of a more definite relation between FP dose and biochemical adrenal impairment may reflect the effect of variable compliance.
While we found only a weak negative correlation between FP dose and peak cortisol level, it is reassuring that a flat response was only found in children prescribed FP ⩾1000 μg/day.
The question arises as to the daily ICS level that justifies adrenal testing.4,28 Some of our children were taking FP<500 μg/day when tested, but showed evidence of biochemical adrenal impairment. It is likely that some others of the 137 children on FP doses within license would also have had impaired responses. The decision not to investigate these children was a practical one based on the logistic difficulties of testing large numbers of children as well as the fact that the literature suggests, and our data confirms, that the risk of clinically significant adrenal side effects is related to FP doses well above license.4,17
We conclude that families and clinicians should be aware of the possibility of clinical adrenal suppression in children with asthma on inhaled FP doses above license (>400 μg/day). Since adrenal insufficiency is easily treated if recognised, families with children on high doses of FP (and other high dose inhaled corticosteroids) should be aware of the possibility of acute adrenal insufficiency and the potential need for emergency treatment. We would recommend they carry a steroid card, and have specific written advice about steroid replacement in the event of severe intercurrent illness.
Finally, these results support advice in current asthma guidelines to ensure that the dose of inhaled corticosteroids is only sufficient to control the disease and that doses be stepped down when control is achieved.29 In children with a poor response to treatment, ICS dose should not be increased without careful review.
What is already known on this topic
While inhaled corticosteroids are the most effective treatment for childhood asthma, high doses are associated with systemic side effects
Clinical adrenal insufficiency has been particularly associated with above license doses (>400 μg/day) of inhaled fluticasone propionate (FP)
What this study adds
Of children on above licensed doses of FP, 42% had biochemical adrenal insufficiency on sensitive testing
A flat adrenal response was only found in children receiving ⩾1000 μg/day FP
Acknowledgements
We gratefully acknowledge the calm forbearance of families and children prescribed off license doses of FP to adrenal function testing, the help of our consultant colleagues (Drs Cochrane, Gibson, and Devenny) in planning the testing protocol, and the assistance of all the nurses who performed the testing. We are especially grateful to Anne Wilson and Christine Kerr for their painstaking tabulation of all the data.
Abbreviations
FP - fluticasone proprionate
ICS - inhaled corticosteroids
LDST - low dose Synacthen test
SDS - standard deviation score
Footnotes
Competing interests: JYP has received financial support for clinical trials, attending conferences and postgraduate meetings, and from companies who make inhaled steroids, including GlaxoSmithKline, AstraZeneca, 3M Healthcare, and Novartis. His spouse has shares in GlaxoSmithKline, which makes fluticasone.
References
- 1.Anon British guideline on the management of asthma. Thorax 200358(suppl 1)i1–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calpin C, Macarthur C, Stephens D.et al Effectiveness of prophylactic inhaled steroids in childhood asthma: a systematic review of the literature. J Allergy Clin Immunol 1997100452–457. [DOI] [PubMed] [Google Scholar]
- 3.Lipworth B J. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta‐analysis. Arch Intern Med 1999159941–955. [DOI] [PubMed] [Google Scholar]
- 4.Russell G. The use of inhaled corticosteroids during childhood: plus ca change. Arch Dis Child 200489893–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price J, Hindmarsh P, Hughes S.et al Evaluating the effects of asthma therapy on childhood growth: what can be learnt from the published literature? Eur Respir J 2002191179–1193. [DOI] [PubMed] [Google Scholar]
- 6.Martin R J, Szefler S J, Chinchilli V M.et al Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med 20021651377–1383. [DOI] [PubMed] [Google Scholar]
- 7.Todd G R, Acerini C L, Ross‐Russell R.et al Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child 200287457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel L, Clayton P E. Adrenal insufficiency after treatment with fluticasone. Lowest possible dose of inhaled glucocorticoids should be given. BMJ 2002325836. [PubMed] [Google Scholar]
- 9.Fitzgerald D, Van A P, Mellis C.et al Fluticasone propionate 750 micrograms/day versus beclomethasone dipropionate 1500 micrograms/day: comparison of efficacy and adrenal function in paediatric asthma. Thorax 199853656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahachoklertwattana P, Sudkronrayudh K, Direkwattanachai C.et al Decreased cortisol response to insulin induced hypoglycaemia in asthmatics treated with inhaled fluticasone propionate. Arch Dis Child 2004891055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sim D, Griffiths A, Armstrong D.et al Adrenal suppression from high‐dose inhaled fluticasone propionate in children with asthma. Eur Respir J 200321633–636. [DOI] [PubMed] [Google Scholar]
- 12.Crowley S, Hindmarsh P C, Holownia P.et al The use of low doses of ACTH in the investigation of adrenal function in man. J Endocrinol 1991130475–479. [DOI] [PubMed] [Google Scholar]
- 13.Agwu J C, Spoudeas H, Hindmarsh P C.et al Tests of adrenal insufficiency. Arch Dis Child 199980330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broide J, Soferman R, Kivity S.et al Low‐dose adrenocorticotropin test reveals impaired adrenal function in patients taking inhaled corticosteroids. J Clin Endocrinol Metab 1995801243–1246. [DOI] [PubMed] [Google Scholar]
- 15.Devoy M. Use of inhaled corticosteroids in children. Arch Dis Child 200388461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams N P, Bestall J C, Lasserson T J.et al Inhaled fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev 2005;CD003135 [DOI] [PubMed]
- 17.Masoli M, Weatherall M, Holt S.et al Systematic review of the dose‐response relation of inhaled fluticasone propionate. Arch Dis Child 200489902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel L, Wales J K, Kibirige M S.et al Symptomatic adrenal insufficiency during inhaled corticosteroid treatment. Arch Dis Child 200185330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todd G, Dunlop K, McNaboe J.et al Growth and adrenal suppression in asthmatic children treated with high‐dose fluticasone propionate. Lancet 199634827–29. [DOI] [PubMed] [Google Scholar]
- 20.Drake A J, Howells R J, Shield J P.et al Symptomatic adrenal insufficiency presenting with hypoglycaemia in children with asthma receiving high dose inhaled fluticasone propionate. BMJ 20023241081–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todd G R, Acerini C L, Buck J J.et al Acute adrenal crisis in asthmatics treated with high‐dose fluticasone propionate. Eur Respir J 2002191207–1209. [DOI] [PubMed] [Google Scholar]
- 22.Kannisto S, Korppi M, Remes K.et al Adrenal suppression, evaluated by a low dose adrenocorticotropin test, and growth in asthmatic children treated with inhaled steroids. J Clin Endocrinol Metab 200085652–657. [DOI] [PubMed] [Google Scholar]
- 23.Dunlop K A, Carson D J, Steen H J.et al Monitoring growth in asthmatic children treated with high dose inhaled glucocorticoids does not predict adrenal suppression. Arch Dis Child 200489713–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brutsche M H, Brutsche I C, Munawar M.et al Comparison of pharmacokinetics and systemic effects of inhaled fluticasone propionate in patients with asthma and healthy volunteers: a randomised crossover study. Lancet 2000356556–561. [DOI] [PubMed] [Google Scholar]
- 25.Harrison T W, Wisniewski A, Honour J.et al Comparison of the systemic effects of fluticasone propionate and budesonide given by dry powder inhaler in healthy and asthmatic subjects. Thorax 200156186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson N A, Ferguson A E, Aitchison T C.et al Compliance with inhaled asthma medication in preschool children. Thorax 1995501274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonasson G, Carlsen K H, Mowinckel P. Asthma drug adherence in a long term clinical trial. Arch Dis Child 200083330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell G. Inhaled corticosteroids and adrenal insufficiency. Arch Dis Child 200287455–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins G, McMahon A D, Twaddle S.et al Stepping down inhaled corticosteroids in asthma: randomised controlled trial. BMJ 20033261115. [DOI] [PMC free article] [PubMed] [Google Scholar]