Abstract
Kawasaki disease is an acute inflammatory syndrome that takes the form of systemic vasculitis, and predominantly affects children. Important complications of this disease are coronary artery dilation and aneurysm formation. Recent studies indicate that Kawasaki disease patients have elevated expression, activity, or protein levels of matrix metalloproteinases (MMPs), and suggest that imbalances in MMPs or MMP/tissue inhibitor of MMP (TIMP) play important pathophysiological roles in the development of coronary artery lesions in this disease. However, it remains unclear whether MMP activities at the site of coronary artery lesions are indeed increased. Further studies on the effects of MMP inhibition on coronary outcome are needed to define the roles of MMPs and TIMPs in the formation of coronary artery lesions in Kawasaki disease; findings of such studies may support the use of MMP inhibitors for the prevention of coronary artery complications in patients with this disease.
Keywords: Kawasaki disease, coronary artery aneurism, matrix metalloproteinases, pathophysiology
The physiological condition of the extracellular matrix is maintained by a rigorously controlled balance between the synthesis and breakdown of component proteins. Matrix metalloproteinases (MMPs) and their endogenous inhibitors, tissue inhibitors of MMP (TIMPs), play central roles in this process.1 Accelerated matrix breakdown caused by increased activity of MMPs or quantitative imbalances between MMPs and TIMPs may thus result in several pathological conditions, including rheumatoid arthritis,2 tumour metastasis,3 and heart failure.4,5 Increased levels of MMPs have also been detected in aortic aneurysms in adult humans, and the development of aortic aneurysms is thought to result from the degradation of structural proteins in the arterial wall by proteolytic enzymes derived mainly from inflammatory cells infiltrating the media and adventitia.6,7,8 MMPs have been implicated in this process, and are thought to play a dominant role in the formation of aortic aneurysms.
Kawasaki disease is an acute inflammatory syndrome that takes the form of systemic vasculitis, and predominantly affects children. Important complications of this disease include coronary artery dilation and aneurysm formation, occurring in 10–15% of patients during the acute stage.9,10 Histopathological findings of coronary artery lesions (CALs) in Kawasaki disease indicate destruction of coronary artery wall with diffuse vasculitis,11 raising the possibility that MMPs are also involved in coronary arterial wall destruction and the formation of coronary aneurysms in Kawasaki disease (fig 1). This review discusses the possible roles of MMPs in the development of CALs in Kawasaki disease.
Figure 1 Histological sample showing panarteritis in the coronary artery of a patient who died 23 days after the onset of Kawasaki disease. Fragmentation of elastic fibres stained black is visible in the internal elastic lamina (arrow) and external elastic lamina (arrow head). Media (bracket) is almost completely destroyed, with severe inflammatory cell infiltration (Elastic van Gieson stain, ×20).
MMPs in Kawasaki disease
While the idea that MMP may be involved in the pathogenesis of coronary artery vasculitis in Kawasaki disease is relatively new, an increasing number of studies have indicated elevated expression, activity, or protein levels of MMPs in Kawasaki disease. A study conducted at this institute indicates that plasma levels of MMP‐1, ‐2, ‐3, and ‐9, as measured by enzyme linked immunoassay (ELISA), are significantly elevated in the acute stage of Kawasaki disease before treatment with intravenous gammaglobulin (IVGG), compared with levels in age matched controls.12 Levels of TIMP‐1 and ‐2 were also significantly higher in Kawasaki disease than in controls. Importantly, the data showed that levels of MMP‐9 before treatment were significantly higher in Kawasaki disease patients with CALs than in Kawasaki disease patients without CALs. Serial changes in MMPs and TIMPs after treatment with IVGG were also examined. Levels of MMP‐9 decreased after IVGG, regardless of the development of CALs. In contrast, MMP‐3 levels of patients with CAL remained elevated after IVGG, and were significantly higher than those of patients without CAL. The absence of differences in TIMP levels between patients with CAL and without CAL is worth noting, given the difference in MMP levels, suggesting that an imbalance between MMPs and TIMPs contributes to CAL formation. The pre‐IVGG MMP‐9/TIMP‐2 ratio and post‐IVGG MMP‐3/TIMP‐1 ratio were both significantly higher in patients who developed CALs than in those without CAL. These results suggest a close association between increased MMP levels with MMP/TIMP imbalance and formation of CALs in Kawasaki disease.
Elevated levels of MMPs or MMP/TIMP imbalances in Kawasaki disease have also been reported by several other investigators. A study by Matsuyama indicates that before treatment, serum levels of MMP‐3 and TIMP‐1 measured by ELISA are significantly higher in Kawasaki disease patients than in healthy children.13 The levels of MMP‐3 they found in Kawasaki disease patients were similar to levels found in a study at this institute. Takeshita et al14 also observed increased serum levels of MMP‐9 during the acute phase of Kawasaki disease, decreasing significantly from the subacute (after IVGG) through the convalescent phase. They also showed that expression of MMP‐9 mRNA as measured by RT‐PCR is increased in circulating leucocytes of Kawasaki disease patients, and that pathophysiological changes during the course of Kawasaki disease parallel changes in serum levels of MMP‐9. In addition, serum levels of MMP‐9 in that study significantly positively correlated with circulating leucocyte counts, and leucocytes were thus considered as a possible source of MMP‐9 secreted into the circulation.
Chua et al15 examined enzyme activity of MMP‐9 and TIMP‐1, in addition to protein levels, using gelatin zymography and ELISA. They showed that both enzyme activity and protein levels of these proteases in plasma are significantly elevated in the acute stage of Kawasaki disease, compared to afebrile patients diagnosed with gastroenteritis and dehydration. Both enzyme activity and protein levels of MMP‐9 and TIMP‐1 were significantly decreased in the convalescent stage. They also demonstrated that MMP‐9 activity and protein levels in patients who did not completely fulfil the diagnostic criteria for Kawasaki disease, but who were suspected of having the disease (so‐called incomplete form of Kawasaki disease), were increased to a degree similar to that of patients with the complete form of the disease. This is interesting because some patients with the incomplete form of Kawasaki disease have been found to develop CALs, despite having generally less severe clinical symptoms than patients with the complete form of Kawasaki disease.
In addition to reports indicating increased expression, activity, and protein levels of circulating MMPs in the acute phase of Kawasaki disease, a recent study by Gavin et al16 indicates that MMPs and TIMPs are also expressed in coronary artery during the acute phase of Kawasaki disease. In their study, MMP‐2 and ‐9 and TIMP‐1 and ‐2 were immunolocalised in the coronary arteries of autopsy specimens from children with fatal Kawasaki disease and controls who died of causes other than Kawasaki disease. MMP‐2 was prominent in the thickened neointima of coronary aneurysms and in the endothelial cells of new capillaries in areas of angiogenesis, suggesting that MMP‐2 participates in remodelling of the coronary arterial wall in acute Kawasaki disease, particularly in the process of neointimal proliferation and angiogenesis. In contrast, MMP‐9 was absent in control coronary arteries, but was expressed in coronary artery aneurysms and non‐aneurysmal coronary arteries. Interestingly, this change was not accompanied by increased expression of TIMP‐1, suggesting an imbalance between MMP‐9 and TIMP‐1 and thus an important role for MMP‐9 in the formation of CALs.
The available evidence seems to consistently indicate that circulating levels of MMPs, particularly MMP‐9, are significantly increased during the acute phase of Kawasaki disease. MMP‐9 may also be overproduced in the coronary arteries during acute Kawasaki disease. However, because MMPs exhibit proteolytic activity in local lesions,1 it remains unclear whether MMP activities at the site of CALs are indeed increased and thereby cause arterial wall destruction and subsequent CAL formation.
PATHOLOGICAL SIGNIFICANCE OF MMPs IN THE FORMATION OF CALs IN KAWASAKI DISEASE
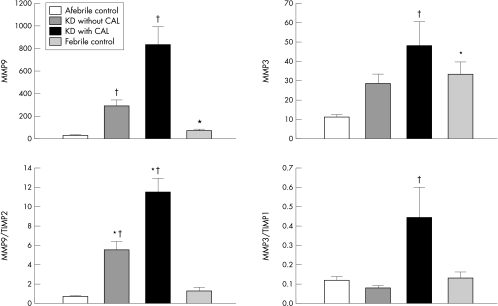
The most important issue regarding the increased MMP levels observed in Kawasaki disease is whether they play any pathophysiologically causative role in the development of CALs. MMPs are inflammatory markers, and thus elevated concentrations of circulating MMPs may merely be the result of the inflammatory process in the coronary arteries, rather than representing a cause of the process. For example, increased levels of circulating MMP are also observed in other inflammatory disorders, such as pneumonia12 or sepsis,14 which do not cause coronary aneurysm. This question could be directly resolved if studies could determine whether interventions that inhibit MMP production or activities prevent the formation of CALs. Unfortunately, such data are not presently available. This is partly because appropriate animal models of Kawasaki disease remain unavailable due to the unexplored aetiology of this disease. Nonetheless, some indirect data suggest that MMPs play a causative role in the development of coronary arterial wall destruction and resultant aneurysm formation. Several studies have compared MMP levels in Kawasaki disease to levels in other febrile (inflammatory) diseases, including bronchitis, pneumonia, sepsis, gastroenteritis, and encephalitis.12,14,15 The results have consistently shown that MMP levels, particularly MMP‐9, are much higher (about 4‐ to 8‐fold) in Kawasaki disease than in other inflammatory diseases, despite similar levels of inflammation as indicated by C reactive protein (CRP). Moreover, while MMP‐9 levels positively correlate with CRP levels in pneumonia, no such correlation is apparent in Kawasaki disease.12 Because MMP‐9 levels in Kawasaki disease are significantly higher in patients with CALs than in those without CALs, these data suggest that the increased MMP levels observed in Kawasaki disease are not merely the result of inflammation. In a study conducted at this institute, levels of MMPs and TIMPs in febrile non‐Kawasaki disease patients were significantly higher than those in afebrile healthy controls, but there was no significant difference in the MMP/TIMP ratio between the febrile patients and afebrile controls, indicating that the normal MMP/TIMP ratio is maintained in inflammatory diseases that do not cause coronary aneurysm. Only Kawasaki disease patients show imbalances between MMPs and TIMPs, with the balance tipped in favour of MMP activation (fig 2).12
Figure 2 Comparison of MMP levels and MMP/TIMP ratios between Kawasaki disease (KD) patients with or without coronary artery lesions (CALs), afebrile healthy controls, and febrile disease controls. *Statistical significance v afebrile controls; †statistical significance v febrile controls. Data for MMP‐9 are pre‐gammaglobulin therapy; data for MMP‐3 are post‐gammaglobulin therapy.
MMP and other factors in CAL development
In a study conducted at this institute, Kawasaki disease patients with CALs had significantly higher MMP levels and MMP/TIMP ratios than Kawasaki disease patients without CALs, but there was some overlap between the two groups. Interaction between MMPs or between MMPs and TIMPs may affect their activity and modulate the pathophysiological process of arterial wall destruction.1 Studies indicate that in addition to MMPs and TIMPs, serine proteases (including urokinase and tissue plasminogen activators) play important roles in the development of aneurysms in adults.17,18 Many inflammatory cytokines that have been shown to be elevated in Kawasaki disease19,20,21 can cause endothelial damage and may modulate MMP activities.1,22 It thus appears likely that complex interplay among these systems is involved in the process of arterial extracellular matrix degradation and resultant aneurysm formation. A related study conducted at this institute indicates that neutrophil elastase levels are significantly increased in the acute phase of Kawasaki disease, and that levels are significantly higher in patients with CALs than in patients without CALs.12 Neutrophil elastase has been shown to activate MMPs,1,23 and may also suppress TIMP activity.24 Neutrophil elastase may thus accelerate coronary arterial wall destruction by tipping the balance in favour of MMP activation in the early stages of Kawasaki disease. Plasminogen activators and plasmin (a serine protease) have also been shown to act as triggers for activation of the MMP pathway.25,26 A recent study of aortic aneurysm in a rat model indicates that blocking plasminogen activator activity via local overexpression of plasminogen activator inhibitor‐1 (PAI‐1) prevents formation of arterial aneurysms by inhibiting MMP activation, suggesting that plasminogen activator mediated MMP activation plays a critical role in arterial wall destruction and subsequent aneurysm formation.18,27 A study conducted at this institute indicates that circulating levels of PAI‐1 are markedly elevated in Kawasaki disease, and that patients with higher PAI‐1 levels are susceptible to CALs,28 suggesting that this fibrinolytic system is also involved in the pathogenesis of CALs in Kawasaki disease.
Genetic factors may also influence the formation of aneurysms in Kawasaki disease; the available evidence suggests that genetic factors contribute to aneurysm formation in adults.29,30,31 A polymorphism in the MMP‐9 gene promoter has been found in patients with intracranial aneurysm or myocardial infarction; variations of this polymorphism result in variations at the level of transcription.30 Polymorphisms in the MMP‐3 promoter and the gene encoding TIMP‐1 are reportedly associated with abdominal aortic aneurysm.31 Many factors other than MMPs and TIMPs may thus be involved in aneurysm formation in Kawasaki disease. Future studies of the roles of MMPs and TIMPs in the development of CALs in Kawasaki disease should take into account interactions among the various factors mentioned above.
Clinical implications of elevated MMP levels in Kawasaki disease
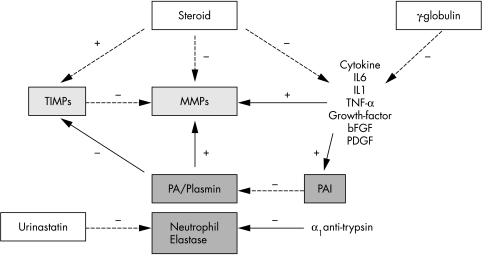
Study results indicating that MMP levels are increased in the acute phase of Kawasaki disease may have important therapeutic implications for preventing CALs in patients with this disease (fig 3). High dose IVGG combined with oral aspirin, to reduce inflammation in the coronary arterial wall, has become the standard of care during acute phase Kawasaki disease.32 Although IVGG reportedly decreases cytokine levels,33,34 the downstream mechanisms of action for IVGG remain unknown. A broad range of cytokines have been shown to stimulate MMP production, release, and activation.22 The efficacy of IVGG in preventing CALs may therefore be related to protection against arterial wall destruction via reduction of cytokine induced MMP stimulation. However, some Kawasaki disease patients that have been administered high dose IVGG continue to suffer from CAL.35 Recently, use of corticosteroids36 or urinastatine37,38 has been proposed as an effective treatment for Kawasaki disease. Although an early study indicated that Kawasaki disease patients receiving corticosteroid have a high frequency of CALs,39 several recent studies suggest that corticosteroid therapy can be effective against Kawasaki disease.36,40,41 The potent anti‐inflammatory effects of steroids are well established, including suppression of inflammatory cytokines.42 Furthermore, an experimental study has shown that steroids have a direct inhibitory effect on MMP activation.43 The favourable effects of steroid therapy reported in recent studies thus appear to be consistent with their indirect or direct inhibitory effects on MMPs. Urinastatine, an inhibitor of neutrophil elastase, has also been shown to reduce fever duration, and may have beneficial effects on coronary outcomes in Kawasaki disease.37,38 The markedly elevated levels of neutrophil elastase observed in Kawasaki disease suggest a mechanism for such beneficial effects of urinastatine therapy in Kawasaki disease patients. The time course of changes in MMP and neutrophil elastase levels may also be important for the timing of therapy designed to reduce MMP and neutrophil elastase levels. Steroids and urinastatine are now primarily used to treat Kawasaki disease patients who are unresponsive to IVGG therapy.38,41 However, marked activation of MMPs and neutrophil elastase may occur in the very early stages of Kawasaki disease, and it may therefore be beneficial to start steroid/urinastatine therapy as soon as possible together with IVGG. Finally, numerous synthetic MMP inhibitors are now undergoing clinical trials for the treatment of disorders such as cancer and rheumatoid arthritis.44,45,46,47 MMPs may represent a viable direct target for therapeutic intervention in Kawasaki disease specifically aimed at preventing CALs.
Figure 3 Therapeutic implications of MMPs, based on interaction between MMPs and their regulators. + indicates stimulating effects; − indicates inhibitory effects.
Summary
MMPs and MMP/TIMP imbalances may play important roles in the development of CALs in Kawasaki disease. However, there is still a lack of direct evidence indicating that activated MMPs in the coronary arteries of patients with Kawasaki disease cause arterial wall destruction and subsequent coronary artery aneurysm. Further studies of the effects of MMP inhibition on coronary outcomes are needed to define the roles of MMPs and TIMPs in CAL formation in Kawasaki disease. The results of such studies may support the use of MMP inhibitors for the prevention of coronary artery complications in patients with Kawasaki disease.
Acknowledgements
The author deeply appreciates the assistance of Dr Kei Takahashi (Department of Pathology, School of Medicine, Toho University), who generously provided histology pictures for this paper.
Abbreviations
CAL - coronary artery lesion
IVGG - intravenous gammaglobulin
MMP - metalloproteinase
TIMP - tissue inhibitor of MMP
Footnotes
Funding: supported by a grant from Kawano Memorial Foundation: No. 10‐3 (HS)
Competing interests: none declared
References
- 1.Parks W C, Mecham R P. eds. Matrix metalloproteinases. San Diego, CA: Academic Press, 19981–21.
- 2.Woessner J F. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 199152145–2154. [PubMed] [Google Scholar]
- 3.Strtler‐Stevenson W G. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol 19961481345–1350. [PMC free article] [PubMed] [Google Scholar]
- 4.Senzaki H, Gluzband Y A, Pak P H.et al Synergistic exacerbation of diastolic stiffness from short‐term tachycardia‐induced cardiodepression and angiotensin II. Circ Res 199882503–512. [DOI] [PubMed] [Google Scholar]
- 5.Senzaki H, Paolocci N, Gluzband Y A.et al β‐adrenergic blockade prevents MMP activation and diastolic dysfunction in short‐term tachycardia‐induced cardiodepression with angiotensin II. Circ Res 200086807–815. [DOI] [PubMed] [Google Scholar]
- 6.Vine N, Powell J T. Metalloproteinases in degenerative aortic disease. Clin Sci 1991816233–239. [DOI] [PubMed] [Google Scholar]
- 7.Thompson R W, Holmes D R, Mertens R A.et al Production and localization of 92‐KD gelatinase in abdominal aortic aneurysms: an elastolytic metalloproteinase expressed by aneurysm‐infiltrating macrophages. J Clin Invest 199596318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies M J. Aortic aneurysm formation. Lessons from human studies and experimental models. Circulation 199898193–195. [DOI] [PubMed] [Google Scholar]
- 9.Dajani A S, Taubert K A, Gerber M A.et al Diagnosis and therapy of Kawasaki disease. Circulation 1993871776–1780. [DOI] [PubMed] [Google Scholar]
- 10.Senzaki H, Chen C H, Ishido H.et al Arterial hemodynamics in patients after Kawasaki disease. Circulation 20051112119–2125. [DOI] [PubMed] [Google Scholar]
- 11.Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: I. Pathology and morphogenesis of the vascular changes. Jpn Circ J 197943633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senzaki H, Masutani S, Kobayashi J.et al Circulating matrix metalloproteinases and their inhibitors in patients with Kawasaki disease. Circulation 2001104860–863. [DOI] [PubMed] [Google Scholar]
- 13.Matsuyama T. Tissue inhibitor of metalloproteinases‐1 and matrix metalloproteinase‐3 in Japanese healthy children and in Kawasaki disease and their clinical usefulness in juvenile rheumatoid arthritis. Pediatr Int 199941239–245. [DOI] [PubMed] [Google Scholar]
- 14.Takeshita S, Tokutomi T, Kawase H.et al Elevated serum levels of matrix metalloproteinase‐9 (MMP‐9) in Kawasaki disease. Clin Exp Immunol 2001125340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chua P K, Melish M E, Yu Q.et al Elevated levels of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 during the acute phase of Kawasaki disease. Clin Diagn Lab Immunol 200310308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavin P J, Crawford S E, Shulman S T.et al Systemic arterial expression of matrix metalloproteinases 2 and 9 in acute Kawasaki disease. Arterioscler Thromb Vasc Biol 200323576–581. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P, Moons L, Lijnen R.et al Urokinase‐generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet 199717439–444. [DOI] [PubMed] [Google Scholar]
- 18.Allaire E, Hasenstab D, Kenagy R D.et al Prevention of aneurysm development and rupture by local overexpression of plasminogen activator inhibitor‐1. Circulation 199898249–255. [DOI] [PubMed] [Google Scholar]
- 19.Lang B A, Silverman E D, Laxer R M.et al Spontaneous tumor necrosis factor production in Kawasaki disease. J Pediatr 1989115939–943. [DOI] [PubMed] [Google Scholar]
- 20.Leung D Y, Cotran R S, Kurt‐Jones E.et al Endothelial cell activation and high interleukin‐1 secretion in the pathogenesis of acute Kawasaki disease. Lancet 198921298–1302. [DOI] [PubMed] [Google Scholar]
- 21.Barron K. Immune abnormalities in Kawasaki disease: prognostic implications and insight into pathogenesis. Cardiol Young 19911206–211. [Google Scholar]
- 22.Bond M, Fabunmi R P, Baker A H.et al Synergistic upregulation of metalloproteinase‐9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF‐κB. FEBS Lett 199843529–34. [DOI] [PubMed] [Google Scholar]
- 23.Nagase H, Suzuki K, Cawston T E.et al Involvement of a region near valine‐69 of tissue inhibitor of metalloproteinases (TIMP)‐1 in the interaction with matrix metalloproteinase 3 (stromelysin 1). Biochem J 1997325163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada Y, Watanabe S, Nakanishi I.et al Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteases. FEBS Lett 1988229157–160. [DOI] [PubMed] [Google Scholar]
- 25.Baramova E N, Bajou K, Remacle J M.et al Involvement of PA/plasmin system in the processing of pro‐MMP‐9 and in the second step of pro‐MMP‐2 activation. FEBS Lett 1997405157–162. [DOI] [PubMed] [Google Scholar]
- 26.Mazzieri R, Masiero L, Zanetta L.et al Control of type IV collagenase activity by components of the urokinase‐plasmin system: a regulatory mechanism with cell‐bound reactants. EMBO J 1997162319–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamarina N A, McMillan W D, Shively V P. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery 19971222264–271. [DOI] [PubMed] [Google Scholar]
- 28.Senzaki H, Kobayashi T, Nagasaka H.et al Plasminogen activator inhibitor‐1 in patients with Kawasaki disease: diagnostic value for the prediction of coronary artery lesion and implication for a new mode of therapy. Pediatr Res 200353983–988. [DOI] [PubMed] [Google Scholar]
- 29.Powell J T, Bashir A, Dawson S.et al Genetic variation on chromosome 16 is associated with abdominal aortic aneurysm. Clin Sci 19907813–16. [DOI] [PubMed] [Google Scholar]
- 30.Peters D G, Kassam A, St Jean P L.et al Functional polymorphism in the matrix metalloproteinase‐9 promoter as a potential risk factor for intracranial aneurysm. Stroke 1999302612–2616. [DOI] [PubMed] [Google Scholar]
- 31.Yoon S, Tromp G, Vongpunsawad S.et al Genetic analysis of MMP3, MMP9, and PAI‐1 in Finnish patients with abdominal aortic or intracranial aneurysms. Biochem Biophys Res Commun 1999265563–568. [DOI] [PubMed] [Google Scholar]
- 32.Harada K. Intravenous γ‐globulin treatment in Kawasaki disease. Acta Pediatr Jan 199133805–810. [DOI] [PubMed] [Google Scholar]
- 33.Amran D, Renz H, Lack G.et al Suppression of cytokine‐dependent human T‐cell proliferation by intravenous immunoglobulin. Clin Immunol Immunopathol 199473180–186. [DOI] [PubMed] [Google Scholar]
- 34.Leung D W M, Schlievert P M, Meissner H C. The immunopathogenesis and management of Kawasaki syndrome. Arthritis Rheum 1998411538–1547. [DOI] [PubMed] [Google Scholar]
- 35.Wallace C A, French J W, Kahn S J.et al Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics 2000105e78. [DOI] [PubMed] [Google Scholar]
- 36.Shinohara M, Sone K, Tomomasa T.et al Corticosteroids in the treatment of the acute phase of Kawasaki disease. J Pediatr 1999135465–469. [DOI] [PubMed] [Google Scholar]
- 37.Nakano M, Kamijo Y, Toyaoda M. Preventive effects of urinastatin for coronary artery aneurysm formation in Kawasaki disease. Proceedings of the 5th International Kawasaki Disease Symposium 1995364–371.
- 38.Nakano M, Ueda E, Kamijo Y. Preventive effects of urinastatin therapy for coronary artery complications in Kawasaki disease. Prog Med 20002085–92. [Google Scholar]
- 39.Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary involvement. Pediatrics 197963175–179. [PubMed] [Google Scholar]
- 40.Onouchi Z, Kawasaki T. Overview of pharmacological treatment of Kawasaki disease. Drugs 199958813–822. [DOI] [PubMed] [Google Scholar]
- 41.Wright D A, Newburger J W, Baker A.et al Treatment of immune‐globulin‐resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr 1996128146–149. [DOI] [PubMed] [Google Scholar]
- 42.Jonat C, Rahmsdorf H J, Park K K.et al Antitumor promotion and antiinflammation down‐regulation of AP‐1 (Fos/Jun) activity by glucocorticoid hormone. Cell 1990621189–1204. [DOI] [PubMed] [Google Scholar]
- 43.Wilheim S M. Human skin fibroblast stromelysin: structure, glycosilation, substarte specificity, and differential expression in normal and tomorgenic cells. Proc Natl Acad Sci U S A 1987846725–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowinsky E K, Humphrey R, Hammond L A.et al Phase I and pharmacologic study of the specific matrix metalloproteinase inhibitor BAY 12–9566 on a protracted oral daily dosing schedule in patients with solid malignancies. J Clin Oncol 200018178–186. [DOI] [PubMed] [Google Scholar]
- 45.Crul M, Beerepoot L V, Stokvis E.et al Clinical pharmacokinetics, pharmacodynamics and metabolism of the novel matrix metalloproteinase inhibitor ABT‐518. Cancer Chemother Pharmacol 200250473–478. [DOI] [PubMed] [Google Scholar]
- 46.Rudek M A, Figg W D, Dyer V.et al Phase I clinical trial of oral COL‐3, a matrix metalloproteinase inhibitor, in patients with refractory metastatic cancer. J Clin Oncol 200115584–592. [DOI] [PubMed] [Google Scholar]
- 47.Levitt N C, Eskens F A, O'Byrne K J.et al Phase I and pharmacological study of the oral matrix metalloproteinase inhibitor, MMI270 (CGS27023A), in patients with advanced solid cancer. Clin Cancer Res 200171912–1922. [PubMed] [Google Scholar]