Abstract
Background
Oligosaccharides may alter postnatal immune development by influencing the constitution of gastrointestinal bacterial flora.
Aims
To investigate the effect of a prebiotic mixture of galacto‐ and long chain fructo‐oligosaccharides on the incidence of atopic dermatitis (AD) during the first six months of life in formula fed infants at high risk of atopy.
Methods
Prospective, double‐blind, randomised, placebo controlled trial; 259 infants at risk for atopy were enrolled. A total of 102 infants in the prebiotic group and 104 infants in the placebo group completed the study. If bottle feeding was started, the infant was randomly assigned to one of two hydrolysed protein formula groups (0.8 g/100 ml prebiotics or maltodextrine as placebo). All infants were examined for clinical evidence of atopic dermatitis. In a subgroup of 98 infants, faecal flora was analysed.
Results
Ten infants (9.8%; 95 CI 5.4–17.1%) in the intervention group and 24 infants (23.1%; 95 CI 16.0–32.1%) in the control group developed AD. The severity of the dermatitis was not affected by diet. Prebiotic supplements were associated with a significantly higher number of faecal bifidobacteria compared with controls but there was no significant difference in lactobacilli counts.
Conclusion
Results show for the first time a beneficial effect of prebiotics on the development of atopic dermatitis in a high risk population of infants. Although the mechanism of this effect requires further investigation, it appears likely that oligosaccharides modulate postnatal immune development by altering bowel flora and have a potential role in primary allergy prevention during infancy.
Keywords: infant nutrition, prebiotics, atopic dermatitis
The prevalence of atopic diseases has steadily increased during the last decade in developed countries.1,2,3 The composition of the intestinal flora plays a key role in postnatal development of the immune system.4 In human milk, neutral oligosaccharides are an important factor that promotes an intestinal flora dominated by bifidobacteria and lactobacilli.5,6 Based on the analysis of human milk oligosaccharides, a prebiotic mixture of 90% short chain galacto‐oligosaccharides (GOS) and 10% long chain fructo‐oligosaccharides (FOS) has been developed.7,8 Studies in preterm9 and term 10,11,12 infants have shown that feed supplementation with GOS/FOS produces an intestinal flora similar to that found in breast fed infants. Atopic dermatitis (AD) is usually the first manifestation of allergy during early infancy. It has been reported that AD is associated with delayed maturation of TH1 immune responses during early infancy with raised total IgE and specific IgE to dietary antigens in the serum.13 Infants with early onset allergic disease are also at risk of other manifestations of allergic disease, a phenomenon described as the allergic march.14 The intestinal flora is part of a complex ecosystem and many of its constituent bacteria remain unidentified.15 However, there is strong evidence that the intestinal flora influences the postnatal development of the immune system.16 Stimulation of the entire intestinal flora by prebiotics might be a more effective method of altering immune development than by adding a single bacterial species to the intestinal ecosystem. However, positive results with probiotics17 have been reported. The present study was designed to investigate the effect of supplementing infant formula feed with 0.8 g/100 ml of a mixture of GOS/FOS on the incidence of AD during the first six months of life. We hypothesised that the prebiotic formula would significantly reduce the cumulative incidence of AD in high risk infants at 6 months of age compared with an unsupplemented control group fed an identical formula.
Study population and methods
The study was a double blind, randomised, placebo controlled trial using a parallel group design. Written parental informed consent was obtained from each participating family and the study protocol was approved by the Ethical Committee of the Macedonio Melloni Maternity Hospital, Milan, Italy. Term infants born between 1 April 2003 and 31 March 2005 at the Macedonio Melloni Maternity Hospital, Milan and with a parental history of atopic eczema, allergic rhinitis, or asthma in either mother or father were eligible for the study. In all cases the parental diagnosis was based on a documented physician's certification.
According to the hospital's policy, breast feeding was recommended to all mothers.
The parents were informed about the study at discharge from the maternity unit and were asked to contact the hospital if they started formula feeding. Inclusion criteria were: gestational age between 37 and 42 weeks, birth weight appropriate for gestational age, and start of formula feeding within the first two weeks of life. When the mother contacted the hospital, the infants were randomly assigned to one of two formula feeds. The recipe of both formulae was based on a hypoallergenic formula with extensively hydrolysed cows' milk whey protein and was supplemented either with 0.8 g GOS/FOS per 100 ml or a 0.8 g maltodextrin/100 ml as placebo. Randomisation was by means of a random numbers table and blinding was maintained by coding the two trial formulae with the suffix “N” or “O” to the product name.
The study formulae were fed ad libitum.
Mixed breast and bottle feeding was accepted until the sixth week of life. When the mother started formula feeding according to the inclusion criteria but continued breast feeding for more than six weeks, the infant was excluded from the study.
Study infants were seen on a monthly basis. The parents were interviewed with the aid of a diary. The results obtained before starting test formula feeding (examination day 1) and at the age of 3 and 6 months (examination days 2 and 3, respectively) were used for this analysis.
At each visit the infant's skin was examined for AD according to the diagnostic criteria described by Harrigan and Rabinowitz17 and Muraro et al.18 The diagnosis of AD was confirmed if the following features were detected: pruritus, involvement of the face, skull facial and/or extensor part of the extremities, and a minimal duration of the symptoms of four weeks.
The severity of the skin alterations was scored by the SCORAD index based on extension, intensity of the skin symptoms, as well as on the subjective symptoms of pruritus and sleep loss as recommended by the European Task Force on AD.19,20 The extent of AD was determined by using the SCORAD figure for infants under 2 years. Subjective symptoms and information concerning stool frequency and consistency were obtained during the interviews of the parents based on their diary record. All examinations and interviews were performed exclusively by two of the authors (GM and SA). Anthropometric measurements and recording of crying, regurgitation, vomiting, and stool characteristics were used as secondary parameters to describe safety as well as acceptance and tolerance. For all infants, growth parameters were measured at each study visit. Body weight was measured using a scale with an accuracy of ±5 g. The crown–heel length was measured using a special board for newborn and infants with an accuracy of ±1 mm and the head circumference was measured using a metal tape with accuracy ±1 mm.
The incidence of crying (score 1–3: 1 = practically not crying; 2 = crying in connection with feeding; 3 = crying independently from the meals), regurgitation (score 1–3: 1 = 0; 2 = 1–2; 3 = >2 regurgitations per day), and vomiting (score 1–3: 1 = 0; 2 = 1; 3 = >1 vomiting episodes per day) was recorded on the basis of the parent's interview.
Stool characteristics were recorded with respect to consistency (score 1–5: 1 = watery; 2 = soft; 3 = seedy; 4 = formed; 5 = hard) and frequency. Stool consistency was evaluated on the basis of the appearance of the fresh sample used for the analysis, the questionnaire, and the report of the parents during the interview. The consistency of each stool sample was recorded on the day before the study day; the mean of the scores obtained was used to characterise the stool consistency of that day.
In a subgroup of 98 infants, the parents agreed to collect stool samples for microbiological analysis. The stool samples were obtained at all three examination visits. A volume of 0.2 g of a fresh faecal sample was homogenised in a cryo‐protective glycerol transport medium (glycerol 10 ml, oxoid 0.1 g, water to 100 ml) and immediately frozen at −80°C. The samples were transported on dry ice. For the identification of bifidobacteria and lactobacilli, commercially available selective media were used (bifidobacteria: Heipha No 20580e, Heidelberg, Germany; lactobacilli: modified Rogosaagar Heipha 2068e, Heidelberg, Germany) as described previously.21 The numbers are presented as colony forming units (CFU)/g stool.
Statistics
The results were analysed on a per protocol basis.
Time balanced randomisation was performed with the software RANCODE (IDV Gauting, Germany; seed numbers randomised by reaction time) with a random permuted block size of four. Interval data (e.g. anthropometrics) were compared between the groups by two sided t test. Bacterial counts in the stool samples were compared by Mann‐Whitney U test due to non‐parametric data distribution.
Scores with ordinal data (e.g. stool consistency) were compared between the groups by Mann‐Whitney U test.
Scores with nominal data (e.g. crying, vomiting, regurgitation) and other nominal data were compared by table analysis of the coded raw data (χ2 test). In the case of 2×2 tables, Fisher's exact test was used.
For analyses of the primary outcomes, 95% confidence intervals were calculated using the Wilson score method.22
Sample size was calculated based on analysis of the previous years' incidence of AD in the hospital and assuming an effect size similar to that reported for probiotics16 at that time. Based on this assumption, 108 subjects per group completing the protocol were calculated to provide a power of 80%. The study was completed after a full two year enrolment period to exclude seasonal effects. The drop out rate was slightly higher than assumed (20% versus 15%). Thus, the statistical analysis was performed on the basis of 206 instead of 216 completed cases as planned. A p value <0.05 was considered to be statistically significant.
Results
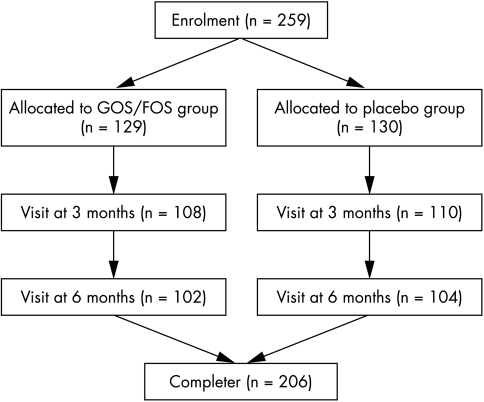
A total of 259 infants were enrolled in the study (fig 1). The most relevant clinical data of the study population are summarised in table 1. Fifty three infants left the trial before completing the study. The main reason for drop out was the continuation or reestablishment of breast feeding. The characteristics at birth and the reasons for drop out were not significantly different between the two groups (table 2).
Figure 1 Disposition of all subjects enrolled in the study.
Table 1 Most relevant clinical data of the study population.
| GOS/FOS formula | Placebo formula | p value | |
|---|---|---|---|
| Enrolled (n) (m/f) | 129 (65/64) | 130 (63/67) | ns |
| Completed (n) (m/f) | 102 (52/50) | 104 (49/55) | ns |
| Age of mother (years)* | 30.0±5.7 | 29.0±5.3 | ns |
| Number of births* | 1.53±0.71 | 1.57±0.77 | ns |
| Vaginal delivery (n(%)) | 72 (70.6) | 69 (66.3) | ns |
| Infants with parental history of allergy: only mother (n) | 69 | 71 | ns |
| Infants with parental history of allergy: only father (n) | 28 | 27 | ns |
| Infants with bi‐parental history of allergy (n) | 5 | 6 | ns |
| Birth weight (g)* | 3344±456 | 3376±482 | ns |
| Birth length (cm)* | 49.2±1.5 | 49.4±1.9 | ns |
| Birth head circumference (cm)* | 34.4±1.2 | 34.6±1.2 | ns |
| Age at first bottle feeding (days)* | 11.2±7.0 | 11.8±9.2 | ns |
| Age at full bottle feeding (days)* | 21.2±10.1 | 21.5±9.2 | ns |
| Weight gain 0–6 months (g/day)* | 27.4±4.1 | 26.4±3.7 | ns |
| Length gain 0–6 months (cm/week)* | 0.75±0.14 | 0.74±0.10 | ns |
| Head circumference gain 0–6 months (cm/week)* | 0.33±0.04 | 0.34±0.05 | ns |
*Data are presented as mean±SD.
Table 2 Drop out characteristics.
| GOS/FOS formula | Placebo formula | p value | |
|---|---|---|---|
| n (%) | 27 (20.9%) | 26 (20.0%) | ns |
| Male/female | 13/14 | 14/12 | ns |
| Birth weight (g)* | 3350±450 | 3380±460 | ns |
| Birth length (cm)* | 49.2±1.7 | 49.2±1.8 | ns |
| Reasons for dropping out | |||
| Continuation/reestablishment breast feeding | 22 | 21 | ns |
| Hardness of stool | 0 | 2 | ns |
| Flatulence | 1 | 1 | ns |
| Move to other city | 1 | 0 | ns |
| No information | 3 | 2 | ns |
*Data are presented as mean±SD.
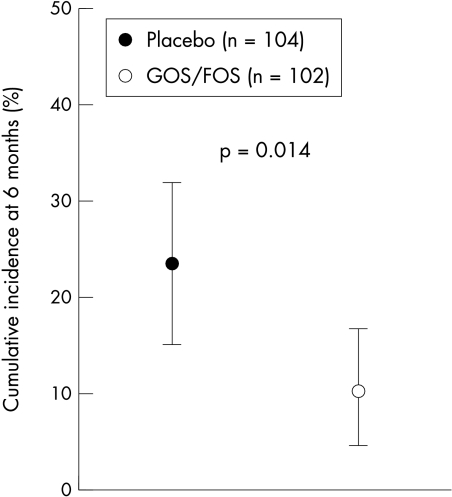
During the six month study period, 10 infants (9.8%; 95% CI 5.4–17.1%) in the GOS/FOS group and 24 infants (23.1%; 95% CI 16.0–32.1%) in the placebo group developed AD (fig 2).
Figure 2 Cumulative incidence of AD at 6 months of age in the group fed a formula supplemented with GOS and FOS or maltodextrins as placebo. Data are expressed as mean (95% CI).
In the group of infants with AD, the SCORAD scores were not significantly different between the group fed the GOS/FOS supplemented formula and the group fed the placebo formula (medians (interquartile range) at 3 months of age: 9.8 (4.8) versus 9.8 (7.5), p = 0.86; and at 6 months of age: 8.9 (12.4) versus 12.8 (5.5), p = 0.18).
In the subgroup of infants with a complete set of stool samples, supplementation with GOS/FOS resulted in a significant increase in the number of bifidobacteria when compared with the placebo group. There was no significant influence on lactobacilli counts (table 3).
Table 3 Bifidobacteria and lactobacilli counts as colony forming units (CFU) per gram fresh stool on three consecutive examination days.
| GOS/FOS formula | Placebo formula | p value | |
|---|---|---|---|
| n (m/f) | 50 | 44 | |
| At start of the study | |||
| Bifidobacteria (CFU/g stool)* | 8.17 (2.3) | 8.33 (2.4) | ns |
| Lactobacilli (CFU/g stool)* | 4.70 (0.0) | 4.70 (0.6) | 0.040 |
| At 3 months of age | |||
| Bifidobacteria (CFU/g stool)* | 9.56 (0.9) | 8.30 (1.1) | <0.0001 |
| Lactobacilli (CFU/g stool)* | 6.04 (1.5) | 6.12 (2.1) | ns |
| At 6 months of age | |||
| Bifidobacteria (CFU/g stool)* | 10.28 (0.7) | 8.65 (1.2) | <0.0001 |
| Lactobacilli (CFU/g stool)* | 5.99 (3.6) | 5.90 (2.0) | ns |
Data are presented as median (interquartile range).
Stool characteristics (stool frequency and stool consistency) were significantly influenced by the diet (table 4).
Table 4 Stool characteristics at 3 and 6 months of age.
| GOS/FOS formula | Placebo formula | p value | |
|---|---|---|---|
| n (m/f) | 102 (52/50) | 104 (49/55) | |
| At start of formula feeding | |||
| Frequency* (n/day) | 2.59±0.9 | 2.39±1.2 | ns |
| Consistency (score)* | 2.3±0.5 | 2.2±0.5 | ns |
| Infants with score < 2 (n) | 20 | 16 | |
| Infants with score > 4 (n) | 0 | 0 | |
| At 3 months of age | |||
| Frequency* (n/day) | 2.34±0.8 | 1.55±0.6 | <0.0001† |
| Consistency (score)* | 2.08±0.5 | 2.82±0.8 | <0.0001‡ |
| Infants with score < 2 (n) | 28 | 0 | |
| Infants with score > 4 (n) | 0 | 0 | |
| At 6 months of age | |||
| Frequency* (n/day) | 1.75±0.6 | 1.50±0.6 | 0.0059† |
| Consistency (score)* | 2.44±0.7 | 3.22±0.9 | <0.0001‡ |
| Infants with score < 2 (n) | 8 | 0 | |
| Infants with score > 4 (n) | 0 | 9 |
*Data are presented as mean±SD.
†t test.
‡U test.
Regarding acceptance and tolerance of the formulae, there were significantly lower reports of regurgitation and crying in the group fed the GOS/FOS supplemented formula, whereas there was no difference in the reported incidence of vomiting between the two groups (table 4).
No adverse effects were observed during the entire study based on the diary record given by the parents and the results of the monthly examinations.
Discussion
In the present study, the cumulative incidence of AD during the first six months of life was significantly reduced by supplementation of a hypoallergenic formula (extensively hydrolysed cows' milk whey protein) with 0.8 g GOS/FOS per 100 ml. To our knowledge this is the first report showing that prebiotic oligosaccharides can influence the incidence of AD.
The majority of the infants investigated in this study had a maternal history of atopy which is strongly related to the risk of AD at 6 months of life.23 It is well known that the incidence of AD peaks at 6 months,24 but the reported absolute incidence varies substantially between different studies. However, the incidence in infants with comparable risk as in the present study is reported to be approximately 30%.23,25
In the present study, a hydrolysed whey protein was used as the basis for both study formulae in accord with recent recommendations for the nutritional management of infants at risk,26 which might explain the fact that only mild forms of AD occurred.27
Table 5 Regurgitation, vomiting, and crying at 3 and 6 months of age.
| GOS/FOS formula | Placebo formula | p value | |
|---|---|---|---|
| n (m/f) | 102 (52/50) | 104 (49/55) | |
| At 3 months of age | |||
| Regurgitation (n) | 0.0192* | ||
| 0 episodes/day | 75 | 62 | |
| 1 episode/day | 23 | 27 | |
| 2 or more episodes/day | 4 | 15 | |
| Vomiting (n) | ns* | ||
| 0 episodes/day | 99 | 92 | |
| 1 episode/day | 5 | 10 | |
| 2 episodes/day | 0 | 0 | |
| Crying (n) | 0.0175* | ||
| No | 66 | 48 | |
| In connection with feeding | 33 | 54 | |
| Independent from feeding | 3 | 2 | |
| At 6 months of age | |||
| Regurgitation (n) | 0.0027* | ||
| 0 episodes/day | 81 | 69 | |
| 1 episode/day | 19 | 19 | |
| 2 or more episodes/day | 2 | 16 | |
| Vomiting (n) | ns* | ||
| 0 episodes/day | 95 | 100 | |
| 1 episode/day | 7 | 4 | |
| 2 episodes/day | 0 | 0 | |
| Crying (n) | 0.0057* | ||
| No | 76 | 57 | |
| In connection with feeding | 26 | 44 | |
| Independent from feeding | 0 | 3 |
*χ2 test.
The primary hypothesis of this study was that dietary prebiotics could modulate the postnatal development of the immune system by altering intestinal flora. Although it was not possible to obtain stool samples from all infants, data from the subgroup providing stool samples showed a significant effect of GOS/FOS supplementation on the bifidobacteria counts, similar to a study previously performed in the same hospital10 using identical microbiological methods.21 As in the previous studies, the effect of GOS/FOS supplementation on lactobacilli counts was less pronounced. However, in studies using molecular techniques for microbial identification, the effect of GOS/FOS on lactobacilli counts has also been clearly shown.28
There is accumulating evidence that human milk oligosaccharides (HMOS) can influence the immune system not only via the intestinal flora but also by direct interaction with immune cells. HMOS have been shown to affect the cytokine production and activation of human cord blood derived T cells in vitro.29 Furthermore, HMOS can affect the formation of platelet–neutrophil complexes and activation of associated neutrophils in blood of healthy volunteers. Formation of these complexes requires selectins and could be influenced by HMOS.30 Thus, HMOS could serve as anti‐inflammatory components of human milk and contribute to the lower incidence of inflammatory diseases such as necrotising enterocolitis in breast fed versus formula fed infants.31
Recently, some effects of dietary carbohydrates such as fructans (inulin and oligofructose) on immune modulation were also investigated. In animal studies, the first evidence of immune modulation was shown primarily on activated immune cells in Peyer's patches, including IL‐10 production and natural killer cell cytotoxicity, the concentration of secretory IgA in ileum and caecum, and splenocyte cytokine production. It was speculated that part of the observed effects could be due to interaction of prebiotics with carbohydrate receptors on immune cells.32 In a murine type I allergy model, the allergic reaction following sensitisation with ovalbumin was attenuated in animals fed with dietary short chain galacto‐ and long chain fructo‐oligosaccharides.33
Misikangas et al34 described an association with dietary inulin and adenoma growth in a mouse model with multiple intestinal neoplasia. In contrast to this single observation, Pool‐Zobel in a review of 12 studies (animal and human studies) concluded that inulin‐type fructans reduce the risk of colon cancer.35 The Scientific Committee on Nutrition considered the studied GOS/FOS mixture as safe for infant nutrition up to a concentration of 0.8 g/100 ml.36
The results of the present study do not allow conclusions concerning the mechanism of the effect that was observed on AD. The complexity of the structures of HMOS indicates that breast feeding is providing further support besides stimulation of the intestinal flora. In comparison, the studied prebiotic mixture is mainly active via its influence on intestinal flora. In summary, the present data show a significant effect of a dietary mixture of GOS/FOS on the incidence of AD at 6 months of age. Although further studies are needed to understand completely the mechanism behind the immune modulating effect of the studied prebiotics, the data support the potential role of prebiotics as dietary manipulation for primary allergy prevention during infancy.
What is already known on this topic
The intestinal flora plays an important role in postnatal development of the immune system
Dietary prebiotics (mixture of galacto‐oligosaccharides and long‐chain fructo‐oligosaccharides) stimulate an intestinal flora dominated by bifidobacteria
What this study adds
The studied prebiotic mixture reduces the incidence of AD in infants at risk, which proves the concept of the immune modulating capacity of prebiotics
Supplementary Material
Acknowledgements
The authors thank Dr Kock, Laborzentrum Pohlheim, Germany for microbiological analysis, Dr Carlo Gelmetti for dermatological support, and Mr Stanley Norman for editorial advice.
Abbreviations
AD - atopic dermatitis
FOS - fructo‐oligosaccharides
GOS - galacto‐oligosaccharides
HMOS - human milk oligosaccharides
Footnotes
Funding: the study was partially supported by a grant from Numico Research Friedrichsdorf, Germany and the EARNEST program (Food‐CT‐00678)
Competing interests: none declared
References
- 1.Holgate S T. The epidemic of allergy and asthma. Nature 1999402(6760 suppl)B2–B4. [DOI] [PubMed] [Google Scholar]
- 2.Ahberg N, Hesselmar B, Aberg B.et al Increase of asthma, allergic rhinitis and eczema in Swedish schoolchildren between 1979 and 1991. Clin Exp Allergy 199525815–819. [DOI] [PubMed] [Google Scholar]
- 3.Kemp A, Björksen B. Immune deviation and the hygiene hypothesis: a review of the epidemiological evidence. Pediatr Allergy Immunol 20031474–80. [DOI] [PubMed] [Google Scholar]
- 4.Björkstén B, Sepp E, Julge K.et al Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol 2001108516–520. [DOI] [PubMed] [Google Scholar]
- 5.Kunz C, Rudloff S. Baier W, et al. Oligosaccharides in human milk: structural, functional, and metabolic aspects, Ann Rev Nutr 200020699–722. [DOI] [PubMed] [Google Scholar]
- 6.Boehm G, Stahl B. Oligosaccharides. In: Mattila‐Sandholm T, ed. Functional dairy products. Cambridge: Woodhead, 2003203–243.
- 7.Boehm G, Fanaro S, Jelinek J.et al Prebiotic concept for infant nutrition. Acta Paediatr 200392(suppl 441)64–67. [DOI] [PubMed] [Google Scholar]
- 8.Boehm G, Jelinek J, Stahl B.et al Prebiotics in infant formulas. J Clin Gastroenterol 200438S76–S79. [DOI] [PubMed] [Google Scholar]
- 9.Boehm G, Lidestri M, Casetta P.et al Supplementation of an oligosaccharide mixture to a bovine milk formula increases counts of faecal bifidobacteria in preterm infants. Arch Dis Child Fetal Neonatal Ed 200286F178–F181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moro G, Minoli I, Mosca M.et al Dosage related bifidogenic effects of galacto‐ and fructo‐oligosaccharides in formula fed term infants. J Pediatr Gastroenterol Nutr 200234291–295. [DOI] [PubMed] [Google Scholar]
- 11.Schmelze H, Wirth S, Skopnik H.et al Randomised double‐blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta palmitic acid level, and nondigestible oligosaccharides. J Pediatr Gastroenterol Nutr 200336343–351. [DOI] [PubMed] [Google Scholar]
- 12.Knol J, Scholtens B, Kafka C.et al Colon microflora in infant fed formula with galacto‐ and fructo‐oligosaccharides: more like breast fed infants. J Pediatr Gastroenterol Nutr 20054036–42. [DOI] [PubMed] [Google Scholar]
- 13.Prescott S L. Development of allergen‐specific T‐cell memory in atopic and normal children. Lancet 1999353186–200. [DOI] [PubMed] [Google Scholar]
- 14.Illi S, von Mutius E, Lau S.et al The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol 2004113925–931. [DOI] [PubMed] [Google Scholar]
- 15.Köhler H, McCormick B A, Walker W A. Bacterial‐enterocyte crosstalk: cellular mechanisms in health and disease. J Pediatr Gastroenterol Nutr 200336175–185. [DOI] [PubMed] [Google Scholar]
- 16.Kalliomäki M, Salminen S, Arvilommi H.et al Probiotics in primary prevention of atopic diseases: a randomized placebo‐controlled trial. Lancet 20013571076–1079. [DOI] [PubMed] [Google Scholar]
- 17.Harrigan E, Rabinowitz L G. Atopic dermatitis. Pediatr Allergy Immunol 199919383–389. [Google Scholar]
- 18.Muraro A, Dreborg S, Halken S.et al Dietary prevention of allergic diseases in infants and small children. Part II: Evaluation of methods in allergy prevention studies and sensitization markers. Definitions and diagnostic criteria for allergic diseases. Pediatr Allergy Immunol 200414196–205. [DOI] [PubMed] [Google Scholar]
- 19.Kunz B, Oranje A P, Labreze L.et al Clinical validation and guidelines for the SCORAD index: Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 199719510–19. [DOI] [PubMed] [Google Scholar]
- 20.Anon Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 199318623–31. [DOI] [PubMed] [Google Scholar]
- 21.Fanaro S, Vigi V, Chierici R.et al Fecal flora measurements of breast fed infants using an integrated transport and culturing system. Acta Paediatr 200392634–635. [DOI] [PubMed] [Google Scholar]
- 22.Newcombe R G. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 199817857–872. [DOI] [PubMed] [Google Scholar]
- 23.Moore M M, Rifas‐Shiman S L, Rich‐Edwards J W.et al Perinatal predictors of atopic dermatitis occurring in the first six months of life. Pediatrics 2004113468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruno G, Milita O, Ferrara M.et al Prevention of atopic diseases in high risk babies (long‐term follow‐up). Allergy Proc 199314181–186. [DOI] [PubMed] [Google Scholar]
- 25.Laitinen K, Kalliomäki M, Poussa T.et al Evaluation of diet and growth in children with and without atopic eczema: follow‐up study from birth to 4 years. Br J Nutr 200594565–574. [DOI] [PubMed] [Google Scholar]
- 26.Host A, Koletzko B, Dreborg S.et al Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child 19998180–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg von A, Koletzko S, Grübl A.et al The effect of hydrolyzed cow's milk formula for allergy prevention in the first year of life: The German Infant Nutritional Intervention Study, a randomized double‐blind trial. J Allery Clin Immunol 2003111533–540. [DOI] [PubMed] [Google Scholar]
- 28.Bakker‐Zierikzee A M, Alles M, Knol J.et al Effects of infant formula containing a mixture of galacto‐ and fructo‐oligosaccharides on viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr 200594783–790. [DOI] [PubMed] [Google Scholar]
- 29.Eiwegger T, Stahl B, Schmitt J.et al Human milk‐derived oligosaccharides and plant‐derived oligosaccharides stimulate cytokine production of cord blood T‐cells in vitro. Pediatr Res 200456536–540. [DOI] [PubMed] [Google Scholar]
- 30.Bode L, Rudloff S, Kunz C.et al Human milk oligosaccharides reduce platelet‐neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J Leukoc Biol 200476820. [DOI] [PubMed] [Google Scholar]
- 31.van den Berg A, van Elburg R M, Teerlink T.et al A randomized controlled trial of enteral glutamine supplementation in very low birth weight infants: plasma amino acid concentrations. J Pediatr Gastroenterol Nutr 20054166–71. [DOI] [PubMed] [Google Scholar]
- 32.Watzl B, Girrbach S, Roller M. Inulin, oligofructose and immunomodulation. Br J Nutr 200593(suppl 1)S49–S55. [DOI] [PubMed] [Google Scholar]
- 33.Garssen J, Vos P, M'Rabet L.et al Oral exposure to a mixture of galacto‐oligosaccharides and long chain fructo‐oligosaccharides as a new concept for allergy prevention. Allergy Clin Immunol Int 2005(suppl 1)572
- 34.Misikangas M, Pajari A M, Päivärinta E.et al Promotion of adenoma growth by dietary inulin is associated with increase in cyclin D1 and decrease in adhesion proteins in Min/+ mice mucosa. J Nutr Biochem 200516402–409. [DOI] [PubMed] [Google Scholar]
- 35.Pool‐Zobel B. Inulin‐type fructans and reduction in colon cancer risk: review of experimental and human data. B J Nutr 200593(suppl 1)S73–S90. [DOI] [PubMed] [Google Scholar]
- 36.Scientific Committee on Food Additional statement on the use of resistant short chain carbohydrates (oligofructosyl‐saccharose and oligo‐galactosyl‐lactose) in infant formulae and follow‐on formulae. SCF/CS/NUT/IF/47 Final. 14 December 2001
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.