Abstract
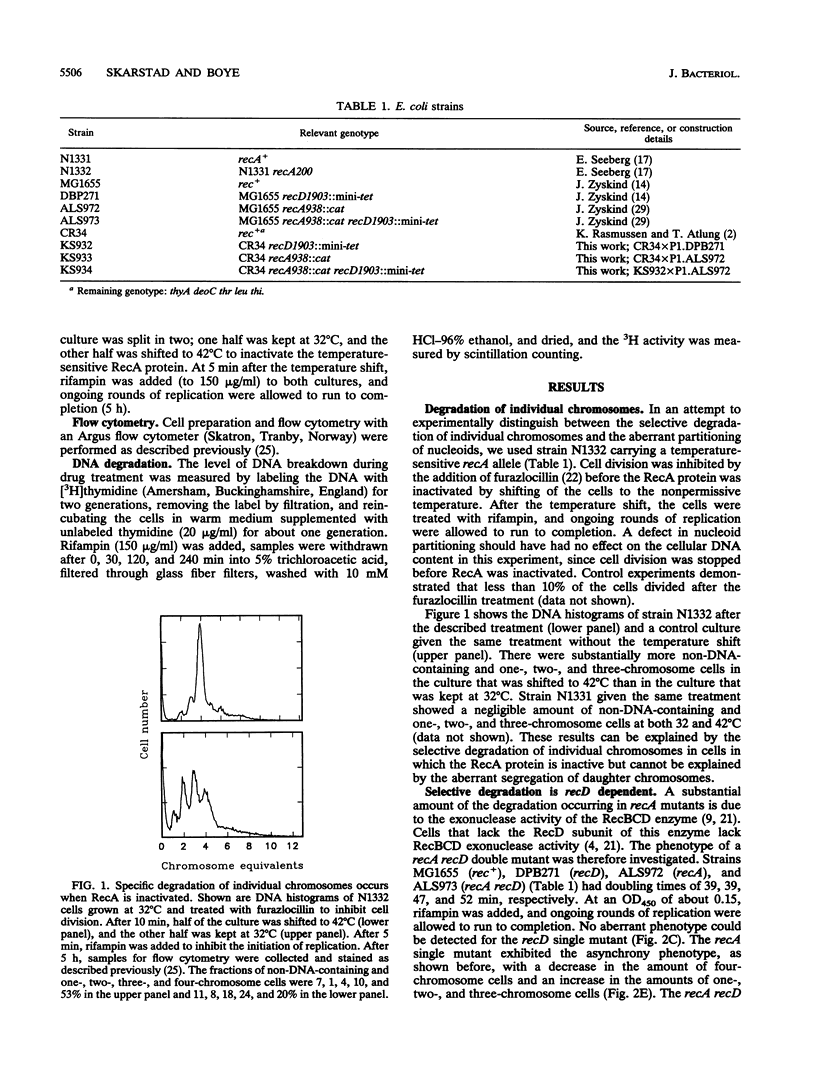
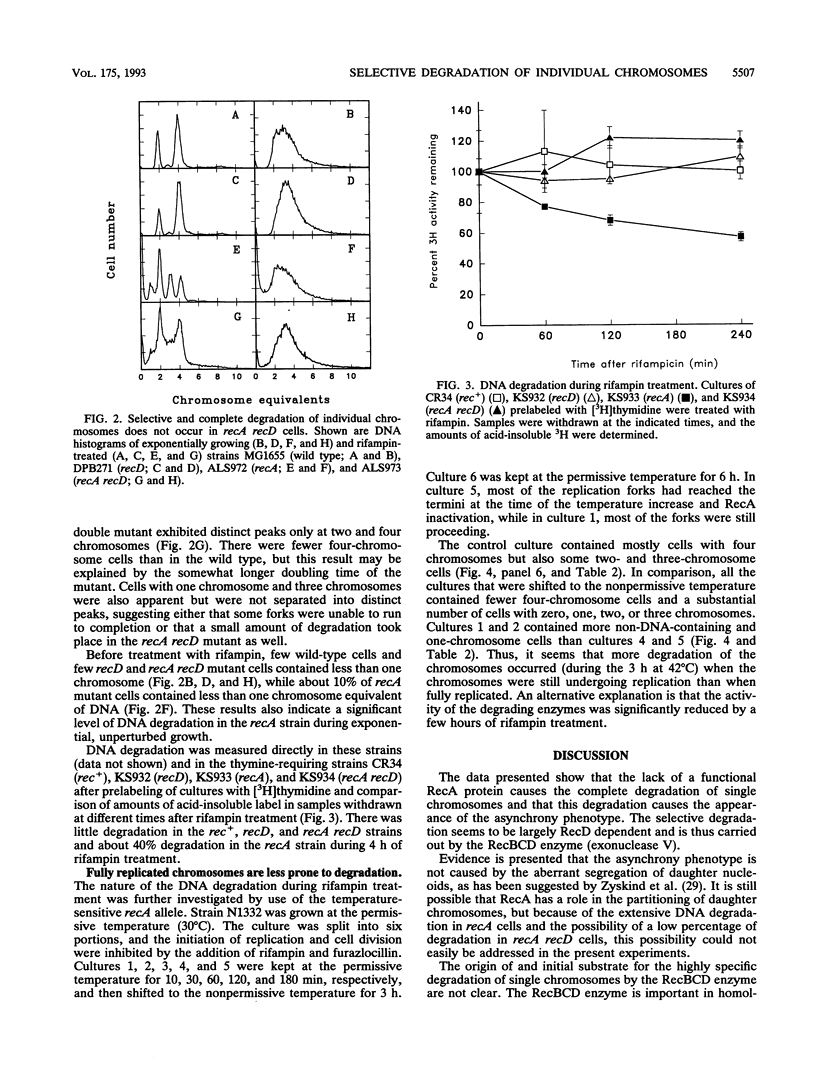
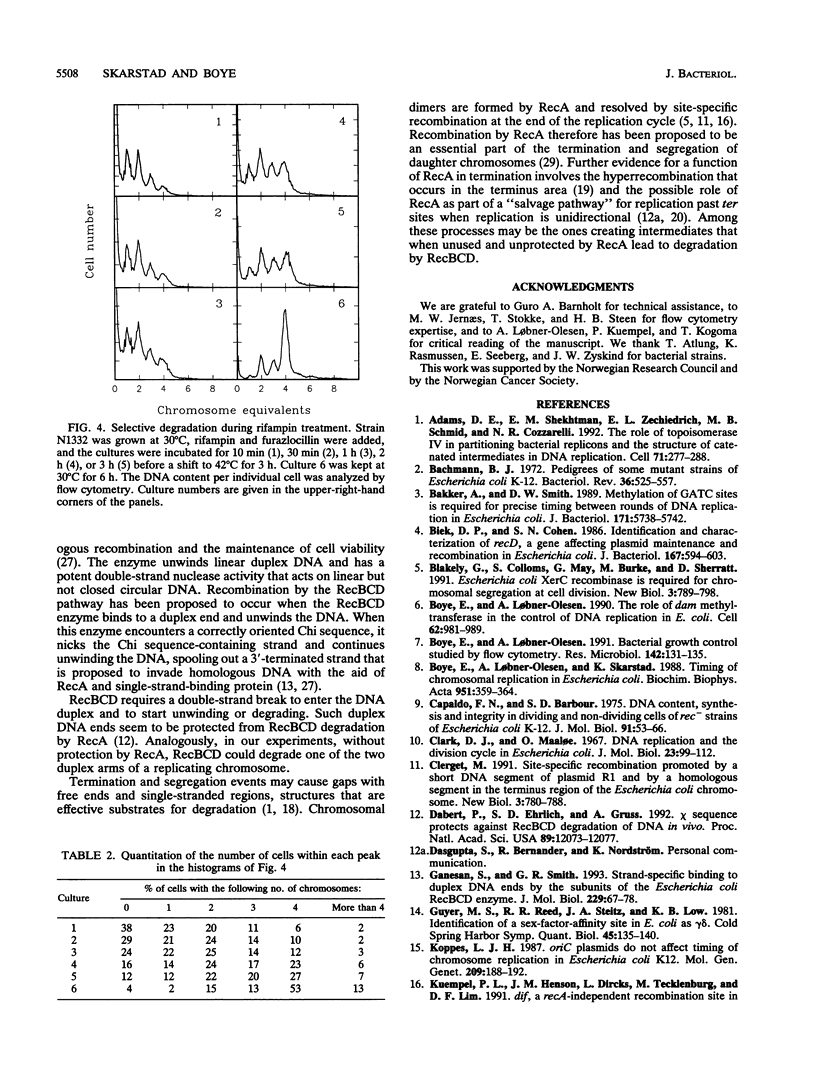
Rapidly growing wild-type Escherichia coli cells contain two, four, or eight fully replicated chromosomes after treatment with rifampin, reflecting that all replication origins are initiated simultaneously. Cells with defects in the timing of the initiation of replication may contain three, five, six, or seven fully replicated chromosomes after such treatment. This phenotype, termed the asynchrony phenotype, is also seen in recombination-deficient recA mutants. It is shown here that for recA strains, the phenotype can be explained by a selective and complete degradation of individual chromosomes. The selective degradation is largely recD dependent and is thus carried out by the RecBCD exonuclease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. E., Shekhtman E. M., Zechiedrich E. L., Schmid M. B., Cozzarelli N. R. The role of topoisomerase IV in partitioning bacterial replicons and the structure of catenated intermediates in DNA replication. Cell. 1992 Oct 16;71(2):277–288. doi: 10.1016/0092-8674(92)90356-h. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A., Smith D. W. Methylation of GATC sites is required for precise timing between rounds of DNA replication in Escherichia coli. J Bacteriol. 1989 Oct;171(10):5738–5742. doi: 10.1128/jb.171.10.5738-5742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek D. P., Cohen S. N. Identification and characterization of recD, a gene affecting plasmid maintenance and recombination in Escherichia coli. J Bacteriol. 1986 Aug;167(2):594–603. doi: 10.1128/jb.167.2.594-603.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely G., Colloms S., May G., Burke M., Sherratt D. Escherichia coli XerC recombinase is required for chromosomal segregation at cell division. New Biol. 1991 Aug;3(8):789–798. [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A. Bacterial growth control studied by flow cytometry. Res Microbiol. 1991 Feb-Apr;142(2-3):131–135. doi: 10.1016/0923-2508(91)90020-b. [DOI] [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A., Skarstad K. Timing of chromosomal replication in Escherichia coli. Biochim Biophys Acta. 1988 Dec 20;951(2-3):359–364. doi: 10.1016/0167-4781(88)90107-8. [DOI] [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A. The role of dam methyltransferase in the control of DNA replication in E. coli. Cell. 1990 Sep 7;62(5):981–989. doi: 10.1016/0092-8674(90)90272-g. [DOI] [PubMed] [Google Scholar]
- Capaldo F. N., Barbour S. D. DNA content, synthesis and integrity in dividing and non-dividing cells of rec- strains of Escherichia coli K12. J Mol Biol. 1975 Jan 5;91(1):53–66. doi: 10.1016/0022-2836(75)90371-x. [DOI] [PubMed] [Google Scholar]
- Clerget M. Site-specific recombination promoted by a short DNA segment of plasmid R1 and by a homologous segment in the terminus region of the Escherichia coli chromosome. New Biol. 1991 Aug;3(8):780–788. [PubMed] [Google Scholar]
- Dabert P., Ehrlich S. D., Gruss A. Chi sequence protects against RecBCD degradation of DNA in vivo. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12073–12077. doi: 10.1073/pnas.89.24.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S., Smith G. R. Strand-specific binding to duplex DNA ends by the subunits of the Escherichia coli RecBCD enzyme. J Mol Biol. 1993 Jan 5;229(1):67–78. doi: 10.1006/jmbi.1993.1008. [DOI] [PubMed] [Google Scholar]
- Guyer M. S., Reed R. R., Steitz J. A., Low K. B. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- Koppes L. J. OriC plasmids do not affect timing of chromosome replication in Escherichia coli K12. Mol Gen Genet. 1987 Aug;209(1):188–192. doi: 10.1007/BF00329857. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Low B., Godson G. N., Birge E. A. Isolation and characterization of an Escherichia coli K-12 mutant with a temperature-sensitive recA- phenotype. J Bacteriol. 1974 Oct;120(1):407–415. doi: 10.1128/jb.120.1.407-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J. M., Louarn J., François V., Patte J. Analysis and possible role of hyperrecombination in the termination region of the Escherichia coli chromosome. J Bacteriol. 1991 Aug;173(16):5097–5104. doi: 10.1128/jb.173.16.5097-5104.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løbner-Olesen A., Kuempel P. L. Chromosome partitioning in Escherichia coli. J Bacteriol. 1992 Dec;174(24):7883–7889. doi: 10.1128/jb.174.24.7883-7889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y. M., Shi Q., Li Q. G., Sheng Z. J. recA gene dependence of replication of the Escherichia coli chromosome initiated by plasmid pUC13 integrated at predetermined sites. Mol Gen Genet. 1991 Feb;225(2):234–240. doi: 10.1007/BF00269854. [DOI] [PubMed] [Google Scholar]
- Rinken R., Thomas B., Wackernagel W. Evidence that recBC-dependent degradation of duplex DNA in Escherichia coli recD mutants involves DNA unwinding. J Bacteriol. 1992 Aug;174(16):5424–5429. doi: 10.1128/jb.174.16.5424-5429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L. S., Botta G., Park J. T. Effects of furazlocillin, a beta-lactam antibiotic which binds selectively to penicillin-binding protein 3, on Escherichia coli mutants deficient in other penicillin-binding proteins. J Bacteriol. 1981 Jan;145(1):632–637. doi: 10.1128/jb.145.1.632-637.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Boye E. Perturbed chromosomal replication in recA mutants of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2549–2554. doi: 10.1128/jb.170.6.2549-2554.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Boye E., Steen H. B. Timing of initiation of chromosome replication in individual Escherichia coli cells. EMBO J. 1986 Jul;5(7):1711–1717. doi: 10.1002/j.1460-2075.1986.tb04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Steen H. B., Boye E. Escherichia coli DNA distributions measured by flow cytometry and compared with theoretical computer simulations. J Bacteriol. 1985 Aug;163(2):661–668. doi: 10.1128/jb.163.2.661-668.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., von Meyenburg K., Hansen F. G., Boye E. Coordination of chromosome replication initiation in Escherichia coli: effects of different dnaA alleles. J Bacteriol. 1988 Feb;170(2):852–858. doi: 10.1128/jb.170.2.852-858.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in procaryotes. Microbiol Rev. 1988 Mar;52(1):1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippe-Schindler R., Zahn G., Messer W. Control of the initiation of DNA replication in Escherichia coli. I. Negative control of initiation. Mol Gen Genet. 1979 Jan 10;168(2):185–195. doi: 10.1007/BF00431444. [DOI] [PubMed] [Google Scholar]
- Zyskind J. W., Svitil A. L., Stine W. B., Biery M. C., Smith D. W. RecA protein of Escherichia coli and chromosome partitioning. Mol Microbiol. 1992 Sep;6(17):2525–2537. doi: 10.1111/j.1365-2958.1992.tb01429.x. [DOI] [PubMed] [Google Scholar]



