Abstract
Objectives
Vitamin D deficiency (VDD) is common in immigrant children with increased skin pigmentation living in higher latitudes. We assessed the pattern of and risk factors for VDD in immigrant East African children living in Melbourne (latitude 37°49′ South).
Study design
A prospective survey of 232 East African children attending a clinic in Melbourne. Data were collected by questionnaire, medical assessment and laboratory tests.
Results
Low 25‐hydroxyvitamin D (25‐OHD) levels (<50 nmol/l) occurred in 87% of children, and VDD (25‐OHD <25 nmol/l) in 44%. Risk factors included age <5 years, female gender, increased time in Australia, decreased daylight exposure and winter/spring season. Anaemia (20%), vitamin A deficiency (20%) and iron deficiency (19%) were also identified.
Conclusions
Asymptomatic VDD is common in East African immigrant children residing at a temperate latitude. Risk factors for VDD limit endogenous vitamin D production. Screening of immigrant children with increased skin pigmentation for VDD, anaemia, iron and vitamin A deficiency is appropriate. VDD in adolescent females identifies an increased risk of future infants with VDD.
Severe vitamin D deficiency (VDD) causes rickets in infants and children, and osteomalacia in adolescents and adults due to decreased bone mineralisation.1 VDD in pregnancy is associated with restricted fetal and infant growth,2,3 and predisposes to neonatal VDD and hypocalcaemia.4 Vitamin D status in childhood and adolescence may play a role in the prevention of osteoporosis.5 Adequate status may reduce the adult risk of diabetes, ischaemic heart disease, hypertension and tuberculosis.6
In Melbourne, nutritional rickets was documented during the 1960s; 70% of the affected children were migrants of Mediterranean origin.7 More recently, VDD has been documented in veiled or dark skinned pregnant women,8 and in immigrant infants from different backgrounds presenting with rickets.9
In the absence of supplementation, skin pigmentation and exposure to solar ultraviolet B (UVB) irradiation determine serum levels of 25‐hydroxyvitamin D (25‐OHD) through endogenous production.1 Adults and adolescents living in climates with reduced UVB exposure are at increased risk of VDD,10,11 particularly those individuals with dark skin,12 with reduced sun exposure13 or wearing covering clothing for socio‐cultural reasons.14 Knowledge of the risk factors in specific populations is important in preventing VDD in pregnant women and infants,8 and may also contribute to the prevention of osteoporosis.15
The increased rates of VDD in adult East African immigrants living in Melbourne, Australia16 suggested that their immigrant offspring are also at risk of VDD. We aimed to prospectively assess the prevalence, severity, pattern of and risk factors for VDD in these children. Malnutrition, iron and vitamin A deficiency are prevalent in African children,17 and VDD is associated with underweight18 and with iron deficiency anaemia.19 We aimed to determine if VDD was part of a broader nutritional problem in these children.
Methods
Subjects and sampling
Consecutive immigrant children aged 0–17 years attending an immigrant health clinic at the Royal Children's Hospital (RCH) were enrolled prospectively. The clinic was established in consultation with community representatives after a number of health issues including VDD16 were studied in a community based project involving adult members of the East African community in Melbourne. Potential participants were referred to the clinic by East African community health workers. It was not possible to collect data on non‐attendance. All families attending the clinic were offered enrolment in the study, and it is our impression that enrolment rates were close to 100%. All enrolled children were indigenous to countries in East Africa (Somalia, Sudan, Ethiopia, Kenya, Egypt, Eritrea or Djibouti). Subjects were enrolled between December 2000 and November 2002. Informed parental or carer's consent was obtained using interpreters trained in the languages of the region. The study was approved by the RCH Human Research Ethics Committee.
Data collection
Data relating to demographics, country of origin and residence prior to migration (including refugee camps), feeding practices, immunisation status, medical history, sun exposure, carer's education, carer's concerns about the child's health, and utilisation of health services in Australia were collected by administered questionnaire using trained interpreters.
Subjects were assessed by a paediatrician for evidence of illness including VDD. Full blood examination and serum levels of ferritin, 25‐OHD and retinol were routinely tested. Serum levels of parathyroid hormone (PTH), alkaline phosphatase (ALP) and calcium (Ca2+) were measured at the paediatrician's discretion, and wrist radiographs were performed in some cases. PTH and ALP results performed more than 60 days after the vitamin D test, or subsequent to treatment, were excluded from the analysis.
Definitions
The definitions of clinical entities (eg, VDD, iron deficiency) used in the study for the different laboratory tests are detailed in table 1. Data for daily effective ultraviolet radiation (UVR) levels for Melbourne, measured in “standard erythemal dose” (SED) units, were obtained from the Australian Radiation Protection and Nuclear Safety Agency.
Table 1 Definitions of deficiency used in this study for laboratory test results.
| Clinical entity | Test | Level | |||
|---|---|---|---|---|---|
| Vitamin D deficiency28 | Serum 25‐OHD* | All ages | |||
| <25 nmol/l | |||||
| Vitamin D insufficiency28 | Serum 25‐OHD | All ages | |||
| ⩾25 nmol/l and <50 nmol/l | |||||
| Elevated PTH46 | Serum PTH | All ages | |||
| >6.8 pmol/l | |||||
| Elevated ALP | Serum ALP | Aged <2 years | Aged 2–9 years | Aged 10–15 years | Aged ⩾16 years |
| >350 μ/l | >300 μ/l | >350 μ/l | >120 μ/l | ||
| Hypocalcaemia | Serum calcium | All ages | |||
| <2.1 mmol/l | |||||
| Anaemia47 | Haemoglobin | Aged <2 years | Aged 2–5 years | Aged 6–11 | Aged ⩾12 years |
| <105 g/l | <110 g/l | <115 g/l | <120 g/l | ||
| Microcytosis | MCV | Aged <2 years | Aged 2–5 years | 6–11 years | Aged ⩾12 years |
| <70 fl | <75 fl | <77 fl | <78 fl | ||
| Iron deficiency | Serum ferritin | Aged <6 years | Aged 6–9 years | Aged ⩾10 years | |
| <6 μg/l | <10 μg/l | Males <23 μg/l | |||
| Females <6 μg/l | |||||
| Underweight† | Weight‐for‐age | z score <−2 | |||
| Overweight† | BMI | z score >2 | |||
| Stunting† | Height‐for‐age | z score <−2 | |||
*Quantitative liquid phase radioimmunoassay (Gamma‐B 25‐hydroxy vitamin D kit; IDS, Boldon, UK).
†Age and gender specific z scores (standard deviation from the reference mean) derived from the revised NCHS growth charts.48
ALP, alkaline phosphatase; BMI, body mass index; 25‐OHD, 25‐hydroxyvitamin D; MCV, mean cell volume; PTH, parathyroid hormone.
Statistical analysis
All data were analysed using Stata 7.0 or 8.0. (Stata Statistical Software, College Station, TX). For univariate analysis, t tests were used to examine differences between continuous parametric variables, and the two‐sample Wilcoxon rank‐sum (Mann‐Whitney) test to examine differences between non‐parametric continuous variables. Relative risks were calculated as risk ratios. Correlations were assessed by Spearman rank correlation coefficients. Clothing covering the head was used as a clinically useful marker of covering clothing limiting sun exposure. The program used for the anthropometric calculations only allowed calculation of weight‐for‐height z scores for heights ranging from 45 to 121.5 cm (n = 77).
Multivariate logistic regression was performed to assess risk factors for VDD. The model included VDD as the dependent variable and gender, age, body mass index (BMI) z score, carer education, time in Australia, duration of breast feeding, iron deficiency, vitamin A deficiency, sun exposure and season of 25‐OHD test as independent variables. The model also included a variable to measure the potential interaction between season and the reported hours of sun exposure per week. An initial model, containing all the variables, was compared to a reduced model after exclusion of the variable with the largest p value. The latter was compared to the next model after the removal of the next variable. The likelihood ratio test was used to compare pairs of models. The final model was that for which removal of a variable resulted in a significant p value from the likelihood ratio test.
Results
Data were collected from 232 children during the study period. Descriptive demographic, univariate risk factor and nutritional data, and the associations with VDD, are listed in table 2.
Table 2 Descriptive demographic, univariate risk factor and nutritional data for 232 East African migrant children and adolescents, and the association with vitamin D deficiency.
| Parameter (number (%) unless stated) | All children, n = 232 | 25‐OHD <25 nm/l, n = 103 (44%) | 25‐OHD ⩾25 nm/l n = 129 (56%) | Risk ratio* (95% CI) |
|---|---|---|---|---|
| Demographic data | ||||
| Mean (SD), age (years) | 8.9 (4.4) | 9.9 (4.5) | 8.2 (4.2) | Diff 1.7 (0.6 to 2.8)† |
| Female | 107 (46%) | 58 (56%) | 49 (38%) | 1.5 (1.1 to 2.0) |
| Univariate risk factors for VDD | ||||
| Median (IQR) months in Australia | 7.9 (3.2–35) | 10.7 (5–37) | 5.0 (2.3–25) | p = 0.0002‡ |
| Resident in Australia >6 months | 126 (54%) | 73 (71%) | 53 (41%) | 2.0 (1.5 to 2.9) |
| Exposed ⩽14 h daylight/week. | 95 (41%) | 57 (55%) | 38 (29%) | 1.8 (1.3 to 2.4) |
| With head cover | 62 (27%) | 48 (47%) | 14 (11%) | 2.4 (1.9 to 3.1) |
| Tested winter/spring | 103 (44%) | 66 (64%) | 37 (29%) | 2.2 (1.6 to 3.0) |
| Nutritional data | ||||
| Underweight for age | 15 (6%) | 7 (7%) | 8 (6%) | 1.1 (0.4 to 2.9) |
| Stunting (height for age) | 12 (5%) | 7 (7%) | 5 (4%) | 1.8 (0.6 to 5.4) |
| Overweight (BMI) | 11 (5%) | 5 (5%) | 6 (5%) | 1.0 (0.3 to 3.3) |
| Vitamin A deficiency | 46 (20%) | 30 (29%) | 16 (12%) | 1.7 (1.2 to 2.2) |
| Anaemia | 46 (20%) | 22 (21%) | 24 (19%) | 1.1 (0.8 to 1.5) |
| Iron deficiency | 45 (19%) | 17 (17%) | 28 (22%) | 0.8 (0.5 to 1.2) |
| Iron deficiency anaemia§ | 23 (10%) | 9 (9%) | 14 (11%) | 0.9 (0.5 to 1.5) |
*25‐OHD <25 nmol/l compared to ⩾25 nmol/l; †difference in mean (95% CI); ‡two‐sample Wilcoxon rank‐sum (Mann‐Whitney) test; §anaemia with hypoferritinaemia.
BMI, body mass index; 95% CI, 95% confidence interval; IQR, interquartile range; 25‐OHD, 25‐hydroxyvitamin D; SD, standard deviation; VDD, vitamin D deficiency.
The countries of origin ranged from latitude 30°N to latitude 5°S. The majority of children were native to Somalia (43%) or the Sudan (25%). All had darkly pigmented skin. A total of 96 children (41%) had spent a median of 2.7 years (interquartile range (IQR) 1.5–4.3) in refugee camps prior to migrating to Australia. The median duration of stay in Melbourne (latitude 37°49′ South) was 8 months (IQR 3–35, range 0.4–88). Of the carers interviewed, 70% were parents (fathers 15%, mothers 55%) and 89% had formal education (33% primary, 39% secondary, 17% tertiary). Since arriving in Australia 63% of subjects had visited a general practitioner or a child health nurse.
VDD was documented in 103 children (44%), and 202 children (87%) had VDD or vitamin D insufficiency (VDI). None of the children had clinical signs of rickets. By univariate analysis, children with VDD were more likely to be older, female, to have spent longer in Australia, to have decreased daylight exposure, to wear covering clothing, to have had 25‐OHD tested in winter or spring, and to have low serum vitamin A (table 2). There were 47 females aged 10 years or more. Compared to the other subjects, 66% (vs 39%) were vitamin D deficient (risk ratio (RR) 1.7; 95% confidence interval (95% CI) 1.29 to 2.23), 72% (vs 15%) wore covering clothing (RR 4.8; 95% CI 3.25 to 7.02) and 57% (vs 37%) reported decreased daylight exposure (RR 1.6; 95% CI 1.15 to 2.13). VDD was not associated with carer's education, or with growth, iron deficiency or anaemia variables (table 2). Independent risk factors associated with VDD by multivariate analysis were age (<5 years), female gender, longer duration of time in Australia (>6 months), decreased daylight exposure (⩽14 h/week) and 25‐OHD tested in winter or spring (table 3).
Table 3 Risk factors for vitamin D deficiency by multivariate analysis.
| Variable | Odds ratio (95% CI) |
|---|---|
| Age <5 years | 1.5 (1.0 to 2.2) |
| Female* | 2.1 (1.1 to 4.1) |
| Time >6 months in Australia | 3.4 (1.8 to 6.7) |
| Sun exposure ⩽14 h/week | 3.3 (1.3 to 8.5) |
| Vitamin D tested winter/spring | 9.0 (3.7 to 22.0) |
*Because of the strong co‐linearity between gender and head covering (98% of those who were covered were female), only gender was included in the logistic regression model.
95% CI, 95% confidence interval.
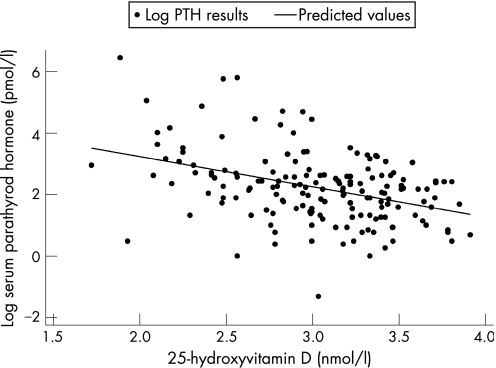
In 115 children tested, PTH levels were elevated in 22 of 71 children (31%) with VDD and six of 43 children (14%) with VDI (RR 2.2; 95% CI 0.98 to 5.04). The highest 25‐OHD level with an elevated serum PTH was 35.8 nmol/l. Serum 25‐OHD and serum PTH were inversely correlated (r = −0.37; 95% CI −0.22 to −0.49; p<0.0001) (fig 1). In 103 children tested, ALP levels were elevated in 16 of 60 children (27%) with VDD and 13 of 43 (30%) with VDI. Serum calcium was low in three of 124 (2%) children tested, two of whom were vitamin D deficient and one vitamin D insufficient. When performed, wrist radiographs were abnormal in 15 of 36 (42%) children with VDD and one of 13 children with VDI. Abnormal radiographs included one with active rickets, 11 with healed rickets and four with osteopaenia.
Figure 1 Correlation between 25‐hydroxyvitamin D levels and parathyroid hormone (PTH) levels in 115 East African immigrant children and adolescents.
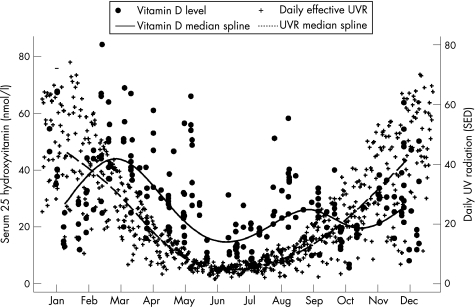
UVR measurements were correlated with vitamin D levels collected over the study period (fig 2). Vitamin D levels lagged behind relative UVR levels but followed a curve parallel to the UVR curve, with the nadir occurring during the winter month of June.
Figure 2 Association between daily UV radiation levels (Melbourne) and 25‐hydroxyvitamin D levels of 232 East African immigrant children and adolescents with date of measurement. SED, standard erythemal dose; UV, ultraviolet.
Most children had normal growth and none were malnourished (table 2). The median (range) z scores were: weight‐for‐age −0.1 (−4.1 to 4.4); height‐for‐age 0.2 (−4.7 to 5.7); weight‐for‐height −0.3 (−2.6 to 3.8) (n = 77); and BMI −0.3 (−3.0 to 4.8). Underweight was associated with an increased risk of vitamin A deficiency (RR 2.2; 95% CI 1.1 to 4.3). Neither underweight nor stunting was associated with anaemia, iron deficiency, VDD or exposure to a refugee camp. Overweight was associated with carer's education greater than primary school level (RR 8.0; 95% CI 1.1 to 64.0) but was not significantly associated with living in Australia for >6 months (RR 3.8; 95% CI 0.8 to 17.0). At the time of enrolment, 220 children (96%) had been or were being breastfed, for a median duration of 12 months (range 1–44). In addition, 103 children (44%) had received infant formula, for a median duration of 12 months (range 2–48). In those who were aged 4 years or younger at enrolment, having received milk formula as an infant was associated with a reduced risk of VDD (RR 0.4; 95% CI 0.1 to 0.97; n = 37).
The proportion of children with anaemia, iron deficiency and iron deficiency anaemia is detailed in table 2. Time spent in a refugee camp did not significantly alter the risk of anaemia (RR 1.5; 95% CI 0.9 to 2.6), iron deficiency (RR 1.2; 95% CI 0.7 to 2.0) or iron deficiency anaemia (RR 1.3; 95% CI 0.6 to 2.8). Of the 51 children aged <5 years, 20 (39%) were anaemic (RR 2.7; 95% CI 1.7 to 4.5) and 18 (35%) iron deficient (RR 2.4; 95% CI 1.4 to 3.9). Children breastfed for more than 12 months were more likely to be iron deficient (RR 2.5; 95% CI 1.1 to 5.6) compared with children breastfed for shorter periods in the group aged 4 years and less. There is evidence in adult indigenous African women that lower reference ranges for haemoglobin may be appropriate.20,21 When the haemoglobin cut‐off for anaemia was reduced by 10 g/l, 17 children (7%) were anaemic and 14 (6%) had iron deficiency anaemia.
A total of 46 children (20%), and seven children under 5 years (13%) of age, were vitamin A deficient. None of the children had xerophthalmia. Vitamin A deficiency was associated with VDD (RR 2.7; 95% CI 1.1 to 6.3) but not with having spent time in a refugee camp prior to migrating to Australia (RR 0.7; 95% CI 0.4 to 1.2). Vitamin A deficiency was not associated with the other factors analysed, including health‐care visits, carer's education or growth.
Discussion
The majority (87%) of East African immigrant children and adolescents in this study were vitamin D deficient or insufficient. The associated pattern of risk factors for VDD (younger age, female gender/covering clothing, residence in Australia for a longer time, decreased daylight exposure, and vitamin D level tested in winter or spring) indicates a reduction in exposure to UVR in affected individuals.10,11,12,13,18,22,23,24,25,26,27,28,29 The city of Melbourne is at significantly higher latitude than the countries of origin of this population. In Boston (latitude 42° North), Caucasian adults had minimal pre‐vitamin D production during winter.10 This study documents the compounding effect of risk factors for VDD on the seasonal variation in vitamin D production in a population of dark skinned children in Melbourne, suggesting that inadequate endogenous production of vitamin D is the main cause of VDD in this group. These risk factors lead to worsening VDD with time spent in Australia. Public health campaigns designed to prevent solar skin damage and cancer through reduced exposure to UVR need to consider the public health implications of their intervention for population subgroups at risk of VDD.6
Although VDD was asymptomatic in our subjects, there is evidence of biological consequences in some children. One quarter of children tested had elevated PTH levels with VDD or VDI, and half of the children with VDD who had radiographs, had abnormal results. In Finnish adolescents, VDD was associated with increased PTH levels and decreased bone mineral accumulation over time.28,29 Peak bone mass, an important factor in osteoporosis fracture risk, is mostly attained by late adolescence.30 Suboptimal vitamin D status in children and adolescents may affect the attainment of peak bone mass and increase the risk of osteoporosis and fractures later in life.
Significant levels of VDD have been documented in dark skinned pregnant women in Melbourne and veiled pregnant women living in Melbourne and in the Middle East.8,14,31 We observed that 65% of females aged 10 years or more had VDD. Cultural factors such as covering clothing limit sun exposure in young women from this background. Our findings of an increased rate of covering clothing and limitation of sun exposure in older females indicates that these risk factors for VDD are likely to persist throughout adolescence into the child‐bearing years. VDD in adolescent females, therefore, increases the risk of VDD, hypocalcaemia and rickets in future offspring.32 Our finding that a younger age (less than 5 years) is a risk factor for VDD may indicate decreased vitamin D stores in this age group, possibly as a result of maternal VDD, prolonged breastfeeding and/or increased vitamin D requirements at a time of increased skeletal growth. Establishing an awareness of VDD and a cultural tolerance of vitamin D supplementation in children and adolescents at risk of VDD is an important ongoing public health intervention in the prevention of VDD in current and future generations.
The serum level of 25‐OHD required for appropriate skeletal growth in childhood is debated.33 Seasonal vitamin D supplementation increases calcitriol levels and suppresses PTH levels in Spanish children with 25‐OHD levels <30 nmol/l,34 suggesting that 25‐OHD levels below 30 nmol/l are clinically important in children. We found a number of children with 25‐OHD levels between 25 and 36 nmol/l who had elevated PTH levels. This supports the proposal that the target 25‐OHD level should be higher than 25 nmol/l.34,35 Although we found no associated biochemical or radiological abnormalities in children with 25‐OHD levels above 36 nmol/l, we will continue to consider 25‐OHD levels less than 50 nmol/l (20 ng/ml) to be abnormally low, until there are data to prove that lower levels are safe.
We found a high prevalence of anaemia (20%) and iron deficiency (19%) in this population, and higher rates of anaemia (39%) and iron deficiency (35%) in children aged less than 5 years, comparable with another group of African refugee children.36 Using WHO criteria, the level of anaemia in this population is a “moderate” public health problem.21 In contrast, the prevalence of anaemia (1.1%) in young Australian children of mixed origin37 is not a public health problem, while the prevalence in Tanzanian preschool children (84%) and school children (55–67%)38,39 is considered a severe public health problem by WHO criteria. In data published elsewhere, we have not been able to demonstrate an association between anaemia and intestinal parasite infestation in children from this study.40 Although malaria was not suspected in any subject, asymptomatic malarial parasitaemia may explain some cases of anaemia in children from this background living in Australia41 and we have subsequently added screening to our clinic protocol. Routine assessment of immigrant children from developing countries should, therefore, include screening for anaemia, iron deficiency and malarial parasitaemia, especially when these conditions are prevalent in the country of origin.
Our finding that 20% of children were vitamin A deficient is concerning, given the morbidity and mortality risks associated with mild vitamin A deficiency.42,43 We are surprised that vitamin A deficiency is prevalent in children who spent time in refugee camps, as vitamin A supplementation is routine in many camps. A treatment strategy would be to provide routine vitamin A supplements to immigrant children from countries where vitamin A deficiency is prevalent. We can not explain the association of vitamin A deficiency with VDD.
Overall, 5% of children were overweight and the risk of being overweight was associated with better educational levels in the child's carer. We did not collect other data describing socio‐economic status. This group of immigrant children may be at risk of obesity given the increasing prevalence of obesity in Australian children.44
We recommend that all dark skinned children and adolescents who migrate to higher latitudes, or who wear covering clothing, have their vitamin D status assessed. We are concentrating on a cheap, high‐dose intermittent cholecalciferol liquid supplement in an olive oil base as vitamin D supplementation for the treatment and prevention of VDD in these children. We have concerns about compliance with daily dose regimes in marginalised communities living in Australia.45
The high prevalence of VDD in this immigrant population has implications for a wider population of children, defined generically by having increased skin pigmentation and living at higher latitudes, or by wearing covering clothing. Better data are needed to document the prevalence of VDD in different population subgroups defined in this way.
What is already known on this topic
Endogenous production of 25‐hydroxyvitamin D is determined by exposure of human skin to UVB radiation and skin pigmentation.
Suboptimal vitamin D status in children and adolescents may affect the attainment of peak bone mass and increase the risk of osteoporosis later in life.
Known risk factors for vitamin D deficiency in children include winter season, immigrant or refugee status, skin pigmentation, atmospheric pollution and geographical latitude.
What this study adds
This prospective study identifies a high prevalence of vitamin D deficiency and a number of measurable risk factors in a large cohort of East African immigrant children and adolescents.
This study documents the interaction between seasonal variation in UVB radiation and socio‐cultural factors limiting exposure to sunlight in this population.
This will assist in the planning of public health interventions in populations at risk of vitamin D deficiency as defined by the known risk factors.
Acknowledgements
We wish to thank the families from the East African community in Melbourne for taking part in this study. We thank Nigisti Mulholland for her assistance in the planning and running of this study. We wish to thank the following services for their assistance: The Australian Radiation Protection and Nuclear Safety Agency; Interpreter Services, Royal Children's Hospital, Melbourne; and Biochemical Laboratory Services, Royal Children's Hospital, Melbourne.
Abbreviations
ALP - alkaline phosphatase
BMI - body mass index
95% CI - 95% confidence interval
IQR - interquartile range
25‐OHD - 25‐hydroxyvitamin D
PTH - parathyroid hormone
RCH - Royal Children's Hospital
RR - risk ratio
SD - standard deviation
SED - standard erythemal dose
UVB - ultraviolet B
UVR - ultraviolet radiation
VDD - vitamin D deficiency
VDI - vitamin D insufficiency
Footnotes
Competing interests: None.
The study was conducted utilising the facilities and the material support of the Clinical Epidemiology and Biostatistics Unit, Murdoch Children's Research Institute, the Centre for International Child Health, University of Melbourne Department of Paediatrics, and the Royal Children's Hospital. No other funding was required.
References
- 1.Chesney R W. Metabolic bone disease. In: Behrman RE, Kliegman R, Jenson HB, eds. Nelson textbook of pediatrics. 16th ed. Philadelphia: WB Saunders, 20002132–2138.
- 2.Namgung R, Tsang R C. Factors affecting newborn bone mineral content: in utero effects on newborn bone mineralization. Proc Nutr Soc 200059(1)55–63. [DOI] [PubMed] [Google Scholar]
- 3.Brunvand L, Quigstad E, Urdal P.et al Vitamin D deficiency and fetal growth. Early Hum Dev 199645(1–2)27–33. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed I, Atiq M, Iqbal J.et al Vitamin D deficiency rickets in breast‐fed infants presenting with hypocalcaemic seizures. Acta Paediatr 199584(8)941–942. [DOI] [PubMed] [Google Scholar]
- 5.Brown J P, Josse R G. Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ 2002167(90100)1S–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Fuller K E, Casparian J M. Vitamin D: balancing cutaneous and systemic considerations. South Med J 200194(1)58–64. [PubMed] [Google Scholar]
- 7.Mayne V, McCredie D. Rickets in Melbourne. Med J Aust 19722(16)873–875. [PubMed] [Google Scholar]
- 8.Grover S R, Morley R. Vitamin D deficiency in veiled or dark‐skinned pregnant women. Med J Aust 2001175(5)251–252. [DOI] [PubMed] [Google Scholar]
- 9.Nozza J M, Rodda C P. Vitamin D deficiency in mothers of infants with rickets. Med J Aust 2001175(5)253–255. [DOI] [PubMed] [Google Scholar]
- 10.Webb A R, Kline L, Holick M F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 198867(2)373–378. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal K S, Mughal M Z, Upadhyay P.et al The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child 200287(2)111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris S, Dawson‐Hughes B. Seasonal changes in plasma 25‐hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr 199867(6)1232–1236. [DOI] [PubMed] [Google Scholar]
- 13.Das G, Crocombe S, McGrath M.et al Hypovitaminosis D among healthy adolescent girls attending an inner city school. Arch Dis Child 200691(7)569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gannage‐Yared M H, Chemali R, Yaacoub N.et al Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res 200015(9)1856–1862. [DOI] [PubMed] [Google Scholar]
- 15.Pasco J A, Henry M J, Nicholson G C.et al Vitamin D status of women in the Geelong Osteoporosis Study: association with diet and casual exposure to sunlight. Med J Aust 2001175(8)401–405. [DOI] [PubMed] [Google Scholar]
- 16.Skull S A, Ngeow J Y, Biggs B A.et al Vitamin D deficiency is common and unrecognized among recently arrived adult immigrants from The Horn of Africa. Intern Med J 200333(1–2)47–51. [DOI] [PubMed] [Google Scholar]
- 17.UNICEF The State of the World's Children Report 2003 ‐ child participation. New York: UNICEF, 2002
- 18.Lulseged S, Fitwi G. Vitamin D deficiency rickets: socio‐demographic and clinical risk factors in children seen at a referral hospital in Addis Ababa. East Afr Med J 199976(8)457–461. [PubMed] [Google Scholar]
- 19.Grindulis H, Scott P H, Belton N R.et al Combined deficiency of iron and vitamin D in Asian toddlers. Arch Dis Child 198661(9)843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson‐Spear M A, Yip R. Hemoglobin difference between black and white women with comparable iron status: justification for race‐specific anemia criteria. Am J Clin Nutr 199460(1)117–121. [DOI] [PubMed] [Google Scholar]
- 21.WHO Iron deficiency anaemia: assessment, prevention, and control. A guide for programme managers. Geneva: World Health Organization, 2001
- 22.Du X, Greenfield H, Fraser D R.et al Vitamin D deficiency and associated factors in adolescent girls in Beijing. Am J Clin Nutr 200174(4)494–500. [DOI] [PubMed] [Google Scholar]
- 23.Fuleihan G E ‐ H, Nabulsi M, Choucair M.et al Hypovitaminosis D in healthy schoolchildren. Pediatrics 2001107(4)e53. [DOI] [PubMed] [Google Scholar]
- 24.Guillemant J, Cabrol S, Allemandou A.et al Vitamin D‐dependent seasonal variation of PTH in growing male adolescents. Bone 199517(6)513–516. [DOI] [PubMed] [Google Scholar]
- 25.Lehtonen‐Veromaa M, Mottonen T, Irjala K.et al Vitamin D intake is low and hypovitaminosis D common in healthy 9‐ to 15‐year‐old Finnish girls. Eur J Clin Nutr 199953(9)746–751. [DOI] [PubMed] [Google Scholar]
- 26.Meulmeester J F, van den Berg H, Wedel M.et al Vitamin D status, parathyroid hormone and sunlight in Turkish, Moroccan and Caucasian children in The Netherlands. Eur J Clin Nutr 199044(6)461–470. [PubMed] [Google Scholar]
- 27.Oliveri M B, Ladizesky M, Mautalen C A.et al Seasonal variations of 25 hydroxyvitamin D and parathyroid hormone in Ushuaia (Argentina), the southernmost city of the world. Bone Miner 199320(1)99–108. [DOI] [PubMed] [Google Scholar]
- 28.Outila T A, Karkkainen M U, Lamberg‐Allardt C J. Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr 200174(2)206–210. [DOI] [PubMed] [Google Scholar]
- 29.Lehtonen‐Veromaa M K, Mottonen T T, Nuotio I O.et al Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3‐y prospective study. Am J Clin Nutr 200276(6)1446–1453. [DOI] [PubMed] [Google Scholar]
- 30.Matkovic V, Jelic T, Wardlaw G M.et al Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross‐sectional model. J Clin Invest 199493(2)799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishal A A. Effects of different dress styles on vitamin D levels in healthy young Jordanian women. Osteoporos Int 200112(11)931–935. [DOI] [PubMed] [Google Scholar]
- 32.Dijkstra S H, van Beek A, Janssen J W.et al High prevalence of vitamin D deficiency in newborns of high‐risk mothers. Arch Dis Child Fetal Neonatal Eds 200792750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pettifor J. What is the optimal 25(OH)D level for bone in children? In: Norman AW, Bouillion R, Thomasset M, eds. Vitamin D endocrine system: structural, biological, genetic and clinical aspects. Riverside, CA: University of California, Riverside, 2000903–907.
- 34.Docio S, Riancho J A, Perez A.et al Seasonal deficiency of vitamin D in children: a potential target for osteoporosis‐preventing strategies? J Bone Miner Res 199813(4)544–548. [DOI] [PubMed] [Google Scholar]
- 35.Norman A W. Sunlight, season, skin pigmentation, vitamin D, and 25‐hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr 199867(6)1108–1110. [DOI] [PubMed] [Google Scholar]
- 36.Geltman P L, Radin M, Zhang Z.et al Growth status and related medical conditions among refugee children in Massachusetts, 1995–1998. Am J Public Health 200191(11)1800–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karr M, Alperstein G, Causer J.et al Iron status and anaemia in preschool children in Sydney. Aust N Z J Public Health 199620(6)618–622. [DOI] [PubMed] [Google Scholar]
- 38.Stoltzfus R J, Chwaya H M, Tielsch J M.et al Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. Am J Clin Nutr 199765(1)153–159. [DOI] [PubMed] [Google Scholar]
- 39.Tatala S, Svanberg U, Mduma B. Low dietary iron availability is a major cause of anemia: a nutrition survey in the Lindi District of Tanzania. Am J Clin Nutr 199868(1)171–178. [DOI] [PubMed] [Google Scholar]
- 40.Rice J E, Skull S A, Pearce C.et al Screening for intestinal parasites in recently arrived children from East Africa. J Paediatr Child Health 200339(6)456–459. [DOI] [PubMed] [Google Scholar]
- 41.Cooley L A, Nott L, McGregor A R. Prevalence of Plasmodium falciparum infection in recently arrived African immigrants. Conference proceedings of the Australasian Society for Infectious Diseases Annual Scientific Meeting, Alice Springs, May 2004. Abstract 26, p56
- 42.Sommer A, Katz J, Tarwotjo I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am J Clin Nutr 198440(5)1090–1095. [DOI] [PubMed] [Google Scholar]
- 43.Fawzi W W, Herrera M G, Willett W C.et al Dietary vitamin A intake and the risk of mortality among children. Am J Clin Nutr 199459(2)401–408. [DOI] [PubMed] [Google Scholar]
- 44.Wake M A, McCallum Z. Secondary prevention of overweight in primary school children: what place for general practice? Med J Aust 2004181(2)82–84. [DOI] [PubMed] [Google Scholar]
- 45.Burns C. A pilot study on compliance in Aboriginal paediatric patients receiving oral antibiotic medication. Austr J Hospital Pharmacy 199222217–221. [Google Scholar]
- 46.Behrman R E, Kliegman R, Jenson H B. eds. Nelson textbook of pediatrics. Philadelphia: WB Saunders 2000
- 47.Nathan D O, Orkin S H, Look A T.et alNathan and Oski's hematology of infancy and childhood. 6th edn. Philadelphia: WB Saunders, 2003
- 48.Kuczmarski R J, Ogden C L, Grummer‐Strawn L M.et al CDC growth charts: United States. Adv Data 20003141–27. [PubMed] [Google Scholar]