Abstract
Non‐steroidal anti‐inflammatory drugs (NSAID) are increasingly popular in hospital medicine and general practice and are readily available over the counter. The vast majority of healthy children who ingest therapeutic doses of NSAID for a limited duration tolerate them without any significant adverse effects. However, the risk of renal toxicity is potentially increased in situations where there is stimulation of the renin‐angiotensin system such as with volume depletion or in pre‐existing chronic renal disease. We describe four cases which illustrate this complication occurring in a children's hospital. We have not proven cause and effect, but further research is needed to define the true risk of the potential renal complications of NSAID in patients at risk of dehydration.
Keywords: NSAID, renal complications, dehydration, intra‐vascular volume depletion, acute renal failure
Non‐steroidal anti‐inflammatory drugs (NSAID) are widely used because of their proven efficacy and excellent safety profile in a diverse range of clinical conditions. Ibuprofen is readily available as an over‐the‐counter NSAID. The risk of serious adverse events among children less than 2 years old receiving short‐term treatment with ibuprofen is very small.1 Approximately 1–5% of the general population exposed to NSAID develop a spectrum of nephrotoxic syndromes needing treatment, which include acute renal failure (ARF), interstitial nephritis, hypertension, hyperkalaemia, hyponatraemia and hypernatraemia.2,3 There have been reports in the literature that suggest that as many as 20% of patients who take NSAID are predisposed to the development of renal toxicity because of volume contracted states, low cardiac output or other conditions which compromise renal perfusion.4 It is well recognised that the use of NSAID in a volume depleted patient can lead to ARF via inhibition of prostaglandin synthesis.5,6 Recent case reports have highlighted this complication in volume depleted children receiving ibuprofen.7,8 There have been relatively few reports of other renal side effects in children resulting from the use of NSAID.9 Acute interstitial nephritis is relatively uncommon in children as a cause of ARF.10 We report the cases of four children who developed ARF resulting from the use of therapeutic doses of NSAID in a children's hospital. The aim of this case series is to illustrate the need to be aware of the effect of NSAID on volume depleted patients.
Patients
The four reported cases were diagnosed at a children's hospital. None of the children had pre‐existing renal disease and all four had documented normal renal function during the preceding 24–48 h. NSAID treatment in therapeutic doses preceded the ARF in each patient. The drug, highest serum urea and creatinine, and time to normalisation of the renal parameters are given in table 1. The patients were treated by stopping the relevant NSAID and intravenous rehydration which led to rapid normalisation of renal function in three of the four patients.
Table 1 Patient details.
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age/sex | 13, female | 7, male | 14, male | 13, male |
| Underlying pathology | Relapse of Crohn's disease | JIA, fasted for surgery | JIA with vomiting | Craniopharyngioma |
| Post‐operative vomiting | Diabetes insipidus | |||
| NSAID | Diclofenac sodium | Ibuprofen, indomethacin, diclofenac sodium | Diclofenac sodium | Diclofenac sodium |
| Highest urea (mmol/l) | 22 | 12.9 | 10.7 | 10.7 |
| Highest creatinine (μmol/l) | 629 | 146 | 376 | 226 |
| Normalisation (days) | Permanent impairment | 3 | 4 | 5 |
JIA, juvenile idiopathic arthritis.
Patient 1 was a 13 year old girl with Crohn's disease of 1 year's duration. She presented to her local hospital with a relapse of Crohn's disease. She was mildly dehydrated clinically. She was treated with intravenous hydrocortisone, mesalazine, cefotaxime and metronidazole. She received four doses of 50 mg diclofenac sodium per rectum for analgesia following which she became anuric. Within 36 h of presentation to her local hospital, her serum creatinine had risen from 87 to 360 μmol/l. Her serum potassium had risen to 5.6 mmol/l. Her haemoglobin was 10.2 g/dl and platelet count was 200×109/l. Her blood film was normal. Her serum urea was 22 mmol/l and creatinine rose further to 629 μmol/l over the next 24 h. An ultrasound scan of her kidneys was normal.
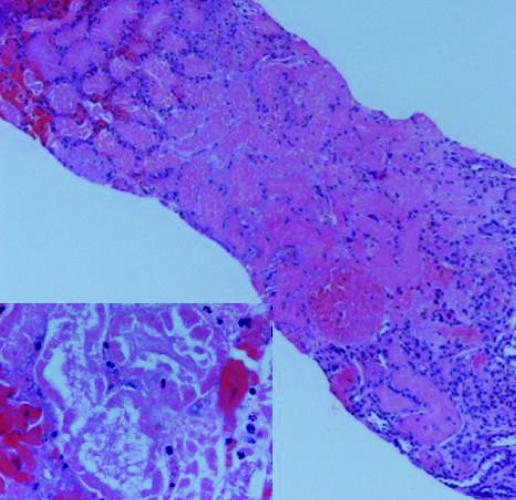
An autoimmune screen including ANA, dsDNA, p and c ANCA, anti Sm, anti GBM and anti‐mitochondrial antibodies was negative. Immunoglobulins were normal. Renal biopsy demonstrated acute cortical haemorrhagic infarction with medullary sparing and acute tubular necrosis. There were no histopathological features of interstitial nephritis (fig 1).
Figure 1 Extensive necrosis of the renal cortex of patient 1 (haematoxylin and eosin, original magnification ×40). Inset shows necrotic tubules (haematoxylin and eosin, original magnification ×400).
The patient was treated with haemodialysis for 26 days and her renal function remains mildly impaired 3 years later with a predicted GFR of 75 ml/min/1.73 m2 and persistent proteinuria with an albumin:creatinine ratio of 26.6 mg/mmol creatinine.
Patient 2 was a 7 year old boy with recently diagnosed systemic juvenile idiopathic arthritis (JIA). He had been symptomatic for 4 weeks and had fasted for the insertion of a Broviac line. He was on ibuprofen (10 mg/kg/dose 8 hourly) and indomethacin (25 mg 12 hourly) for 48 h preceding the surgery. He had never been treated with methotrexate. He received per rectal diclofenac sodium post‐operatively. He became clinically mildly dehydrated as a result of vomiting post‐operatively. He was treated with intravenous rehydration after which his serum urea and creatinine normalised over the next 3 days.
Patient 3 had undifferentiated JIA for 5 years and had been on methotrexate (15 mg/m2) for 6 months, prednisolone forte and diclofenac sodium (75 mg 12 hourly). He developed shingles with vomiting and was treated with intravenous acyclovir. Clinically, he was slightly dehydrated. He was treated with intravenous rehydration and his renal profile normalised over 4 days in spite of continuing treatment with intravenous acyclovir.
Patient 4 was a 13 year old boy who had a craniopharyngioma resected. He was given regular oral diclofenac sodium as post‐operative analgesia (50 mg 8 hourly). He developed diabetes insipidus 48 h after surgery. He was given 250 μg of intravenous desmopressin 12 hourly for 24 h prior to the onset of his renal impairment. The maximum negative balance while on diclofenac sodium was 570 ml. He was treated with intravenous rehydration after which his serum urea and creatinine normalised over the next 5 days.
Discussion
Under normal physiological circumstances, renal blood flow is not dependent on prostaglandin synthesis, except in certain states where there is activation of the renin‐angiotensin system such as following volume depletion induced either by haemorrhage, salt loss or hypo‐albuminaemia. In these conditions, circulating vasoconstrictors are released, maintaining vascular resistance and blood pressure at the potential expense of regional organ blood flow. To maintain renal blood flow, counter‐regulatory renal prostaglandins are released that counteract vasoconstrictors and normalise renal blood flow. It is also important to be aware of the importance of prostaglandins in maintaining glomerular filtration rate in patients with cardiovascular compromise, pre‐existing renal disease, volume contraction and diuretic use.6,11,12,13
NSAID taken under these circumstances blunt this counter‐regulatory response and intensify the renal vasoconstriction, leading to ARF.14 If the vasoconstriction is sufficiently intense and of extended duration, acute cortical necrosis may ensue. The histopathological appearance of acute cortical necrosis seen on the renal biopsy in patient 1 is compatible with this pathogenesis. Mesalazine and cefotaxime are known to cause interstitial nephritis. Although patients 2, 3 and 4 did not have renal biopsies, the ARF was related to the use of NSAID and the ARF resolved within a period of 3–5 days when the NSAID were discontinued and the patient rehydrated while continuing other potentially nephrotoxic drugs. Renal recovery was rapid and complete except for patient 1.
In cases of non‐anuric renal failure, rehydration usually restores renal function. However, caution should be exercised as volume overload can potentially be a problem in patients who are anuric or oliguric. Swift diagnosis of NSAID induced ARF with prompt discontinuation of the offending agent usually reverses the condition within 72–96 h.15 However, if it remains unrecognised, the condition may progress rapidly, needing dialysis.
It is probable that the number of episodes of renal dysfunction due to NSAID treatment is underestimated because the kidney dysfunction is often mild, asymptomatic, transient and non‐anuric.5 This complication may be under‐reported because many patients improve spontaneously after removal of the offending drug,
Virtually all NSAID have been implicated, although some subclasses may be less toxic because of renal conversion of active drug to an inactive metabolite. Indomethacin appears to be associated with the highest risk of this complication.15 Aspirin, although not used as an anti‐pyretic in children, is less potent at suppressing renal prostaglandin production. Naproxen, diclofenac, piroxicam and ibuprofen appear to have an effect between that of aspirin and indomethacin in their relative capacities to acutely compromise renal function. In situations of hypovolaemia, paracetamol should be the drug of choice for anti‐pyretic treatment and the use of NSAID should be carefully monitored and avoided if possible. Paracetamol does not affect Cox 1 or 2 activities and has no adverse renal effect in therapeutic doses.
The use of NSAID is known to be safe in children with a self‐limiting illness.1 The common factors in our patients in a paediatric hospital were that all the children had NSAID with complex medical problems and they all had an element of dehydration. Caution should be exercised in patients with underlying chronic medical conditions when prescribing NSAID or buying them over the counter.
Most cases of NSAID induced renal impairment are mild and self‐limiting. This report does not prove cause and effect, but in an era of widespread popularity of NSAID use in children, further research is needed to define the true risk of the potential renal complication of this group of drugs, especially in patients at risk of dehydration.
Abbreviations
ARF - acute renal failure
JIA - juvenile idiopathic arthritis
NSAID - non‐steroidal anti‐inflammatory drugs
Footnotes
Competing interests: None.
Parental/guardian informed consent was obtained for publication of the persons' details in this report.
References
- 1.Lesko S M, Mitchell A A. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics 1999104(4)e39. [DOI] [PubMed] [Google Scholar]
- 2.Whelton A, Watson J. Non‐steroidal anti‐inflammatory drugs: effects on kidney function. In: De Broe ME, Porter GA, Bennett WM, Verpooten GA, eds. Clinical nephrotoxins: renal injury from drugs and chemicals. Dordrecht, The Netherlands: Kluwer, 1997209–222.
- 3.Whelton A, Hamilton C W. Non‐steroidal anti‐inflammatory drugs: effects on kidney function. J Clin Pharmacol 199131588–598. [DOI] [PubMed] [Google Scholar]
- 4.Bush T M, Shlotzhauer T L, Imai K. Non‐steroidal anti‐inflammatory drugs: proposed guidelines for monitoring toxicity. West J Med 1991155(1)39–42. [PMC free article] [PubMed] [Google Scholar]
- 5.Ulinski T, Guigonis V, Dunan O.et al Acute renal failure after treatment with non‐steroidal anti‐inflammatory drugs. Eur J Pediatr 2004163(3)148–150. [DOI] [PubMed] [Google Scholar]
- 6.Moghal N E, Hulton S A, Milford D V. Care in the use of ibuprofen as an antipyretic in children. Clin Nephrol 199849(5)293–295. [PubMed] [Google Scholar]
- 7.Moghal N E, Hedge S, Eastham K M. Ibuprofen and acute renal failure in a toddler. Arch Dis Child 200489276–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong W, Coward R J, Morris M C. Ibuprofen induced acute renal failure in an infant. NZ Med J 2001114(1140)431. [PubMed] [Google Scholar]
- 9.Mann J F E, Goerig M, Brune K.et al Ibuprofen as an over‐the‐counter drug: is there a risk for renal injury? Clin Nephrol 199339(1)1–6. [PubMed] [Google Scholar]
- 10.McRae Dell K, Kaplan B S, Meyers C M. Tubulo‐interstitial nephritis. In: Barrat TM, Avner ED, Harmon WE, eds. Pediatric nephrology, 4th edn. Baltimore: Lippincott Williams & Wilkins, 1999823–834.
- 11.Patrono C, Dunn M J. The clinical significance of inhibition of renal prostaglandin synthesis. Kidney Int 1987321–12. [DOI] [PubMed] [Google Scholar]
- 12.Clive D M, Stoff J S. Renal syndromes associated with non‐steroidal anti‐Inflammatory drugs. N Engl J Med 1984310563–572. [DOI] [PubMed] [Google Scholar]
- 13.Becker‐Cohen R, Frishberg Y. Severe reversible renal failure due to naproxen‐associated acute interstitial nephritis. Eur J Pediatr 2001160(5)293–295. [DOI] [PubMed] [Google Scholar]
- 14.Bennett W M, Henrich W L, Stoff J S. The renal effects of non‐steroidal anti‐inflammatory drugs: summary and recommendations. Am J Kidney Dis 199628(No 1, Suppl 1)S56–S62. [DOI] [PubMed] [Google Scholar]
- 15.Whelton A. Nephrotoxicity of non‐steroidal anti‐inflammatory drugs: physiologic foundations and clinical implications. Am J Med 1999106(5B)13S–24S. [DOI] [PubMed] [Google Scholar]