Abstract
Objectives
To determine physical activity levels in paediatric patients who underwent the Fontan procedure, and their relationship to functional status and exercise capacity.
Study Design
We studied 147 patients (ages 7–18 years) at a median of 8.1 years after Fontan, as part of the Pediatric Heart Network cross‐sectional study of Fontan survivors. Assessment included medical history, self‐reported physical activity, parent‐completed Child Health Questionnaire (CHQ), cardiopulmonary exercise testing and physical activity level measured by accelerometry (MTI Actigraph).
Results
Measured time spent in moderate and vigorous activity was markedly below normal at all ages, particularly in females, and was not significantly related to self‐reported activity levels, or to maximum Vo2, Vo2 at anaerobic threshold or maximum work rate on exercise testing. Lower measured activity levels were significantly related to lower perceived general health but not to self‐esteem, physical functioning, social impact of physical limitations or overall physical or psychosocial health summary scores. Reduced exercise capacity was more strongly related than measured activity levels to lower scores in general health, self‐esteem and physical functioning.
Conclusions
Physical activity levels are reduced after Fontan, independent of exercise capacity, and are associated with lower perceived general health but not other aspects of functional status.
Keywords: congenital heart disease, cardiac surgery, functional status, exercise
Reduced exercise capacity has been noted for patients with a functional single ventricle who have had the Fontan procedure,1,2 and is related to both cardiac and pulmonary factors.3,4,5,6,7,8,9 While the maximal exercise capacity of children with congenital heart disease has been studied,10 little is known about the levels of habitual physical activity, especially after the Fontan procedure.11,12,13 Only one study12 objectively quantified habitual physical activity levels by accelerometry, and another case study used a heart rate monitor.14 Rehabilitation programs for Fontan patients have shown the positive benefits of physical activity and exercise training in increasing exercise capacity.15,16,17,18
Functional status is a concern after the Fontan procedure.19 Multiple terms have been previously used to express the concept of functional status, and include functional status, functional outcomes, quality of life and health‐related quality of life, with no standardised definitions. We define functional status as the patient's perception of their physical and psychosocial well‐being and functional capacity as framed within the context of health. Exercise capacity as objectively measured through standardised cardiopulmonary exercise testing is taken to be a specific aspect of functional status. The determinants of functional status are complex, with medical morbidity being only one of many potential factors. Physical activity levels may be both an indicator of and a contributor to physical health, and may reflect exercise capacity as well as self‐efficacy, or the individual's personal beliefs regarding their own abilities and competencies. The assessment and definition of physical activity levels in these patients may characterise an additional aspect of functional status which may be amenable to intervention.
The purpose of this study was to determine the habitual physical activity levels in children and adolescents after the Fontan procedure, and to examine the relationships among physical activity, patient and medical characteristics, attitudes towards exercise, exercise capacity and functional status. We hypothesised that functional status would be more strongly related to measured physical activity levels than to exercise capacity.
Methods
This study was ancillary to the Fontan Cross‐Sectional Study performed by the Pediatric Heart Network (PHN), which consists of seven paediatric cardiac centres in the United States and Canada, a Data Coordinating Center at the New England Research Institutes, and the PHN Chair. The PHN performs multi‐centre clinical studies with funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The primary aim of the Fontan Cross‐Sectional Study was to assess the correlation between measures of functional status, ventricular function and exercise performance in paediatric Fontan procedure survivors. Participation in ancillary studies is optional, and this ancillary study involved some but not all participating patients from three PHN centres (Toronto, New York and Salt Lake City). The ancillary study was approved by the institutional ethics review board of each institution, and participants and/or their parent or legal guardian gave written informed consent or assent.
Study subjects
Inclusion criteria for the Fontan Cross‐Sectional Study included age 6–18 years at enrolment and having had the Fontan procedure at least 6 months before initial study testing. Subjects agreed to have an echocardiogram, complete a parent report functional status questionnaire and blood testing within 3 months of enrolment. Exclusion criteria included the presence of a non‐cardiac medical or psychiatric disorder, current or planned participation in a competing research study, lack of reading fluency by the primary care giver in English or Spanish, and current or planned pregnancy. Only patients who also completed the cardiopulmonary exercise testing component of the Fontan Cross‐Sectional Study were approached for participation in this ancillary study.
Measurements
Medical record review and questionnaires
A detailed medical record review was performed to determine patient demographics, underlying cardiac anatomy, pre‐Fontan characteristics (procedures, complications, clinical status), Fontan procedure features and immediate clinical course, and outcomes during follow‐up. Parents completed the Child Health Questionnaire (CHQ) Parent Report form (CHQ‐PF50) as a generic measure of functional status.20 Scoring of the 12 subscales of the CHQ contributes to overall Psychosocial and Physical Functioning Summary Scores. Children also completed a questionnaire to assess self‐reported physical activity habits and attitudes. This questionnaire is not designed to be an objective measure of physical activity but to provide qualitative information, and was used in clinical practice at one of the study centres. Normal comparison data are not available.
Subjects between ages 10 and 18 years completed the Congenital Heart Adolescent or Teenage (CHAT) questionnaire, a congenital heart disease‐specific functional status instrument. Interviews were conducted with 37 adolescents with congenital heart disease, and themes were identified.21 Items were developed, the questionnaire was piloted and revised, and then applied to a population of 162 adolescents with congenital heart disease attending paediatric cardiology outpatient clinics at The Hospital for Sick Children. The rate of completion of all components was >97%. Principal component factor analysis was performed for item reduction, and scores were developed in three single item domains (general health, social life been affected, perceived condition seriousness) and five multi‐item domains (activity limitation score, emotional concerns score, friendship problems, career concerns, symptom discomfort). The items and domains showed excellent Cronbach's α and construct validity. Test–retest reliability was assessed for 21 subjects who completed the questionnaire twice, and showed excellent intra‐class correlations for each domain, ranging from 0.74 to 0.94.
Cardiopulmonary exercise testing
Age for the study was taken as age at enrolment in the ancillary study, which was usually at the time of exercise testing. Standardised height and weight measurements were taken at the time of exercise testing. Maximal exercise testing was performed using a ramp protocol on an electronically braked cycle ergometer. All subjects were encouraged to exercise to exhaustion. Expired gases were measured while the subjects were sitting on the ergometer during 3 min of quiet rest prior to beginning the study, and were then collected throughout the exercise protocol using commercially available metabolic carts. Oxygen consumption (Vo2) and carbon dioxide production (Vco2) were measured on a breath‐by‐breath basis. Peak Vo2 was defined as the highest Vo2 achieved by the subject during the test. Ventilatory anaerobic threshold (VAT) was measured using the V‐slope method in those subjects in whom it could be accurately determined. Values for Vo2 were indexed to body weight and also expressed as a percentage of predicted values for healthy age‐ and gender‐matched subjects as reported by Cooper et al,22 using a similar ergometer protocol. All exercise values were interpreted locally and reported to the PHN Data Coordinating Center.
Measurement of habitual physical activity levels
The Actigraph (Manufacturing Technology, Fort Walton Beach, FL, USA) uni‐axial accelerometer was used to objectively measure habitual physical activity. Previous studies have demonstrated that the Actigraph accelerometer is a reliable and valid measure of physical activity in children and adolescents during laboratory treadmill walking and running and free‐living activities.23,24 For this study, the unit was programmed to store data at 1 min epochs. The accelerometer (the size of a small pager) was attached to an elastic belt and worn at the mid‐axillary line during waking hours, except during bathing/showering or other water activities (eg, swimming). At bedtime, the accelerometer was removed. A log was kept by the patient to record the reasons and times when the accelerometer was removed. The failure to record activity occurring while the accelerometer is removed is an inherent limitation of this methodology, but review of log records has shown the impact to be minimal. Subjects were required to have at least three (preferably four) complete days of data which included one weekend day to be included in the analysis. The majority of subjects achieved the 4 days of monitoring (93%). Upon return of the accelerometer to the investigators, the activity counts per consecutive time block stored in the unit were downloaded to a computer for subsequent data reduction and analysis. Activity counts per time block were converted to level of physical activity intensity (metabolic equivalent tasks or METS) during that time block as moderate physical activity (3–5.9 METS) (MPA) and vigorous physical activity (⩾6 METS) (VPA) based on age‐specific cut‐points.25 Time blocks of activity were then summed over the total daily time the unit was worn to give the total number of minutes spent in both MPA and VPA.
Data analysis
Accelerometer data were grouped by subject age (7–9, 10–12, 13–15 and 16–18 years) and gender so that comparisons could be made with recent data obtained using identical methodology in normal, healthy youths.26 Individual values for MPA and VPA combined (MVPA), and VPA alone were plotted relative to the 50th percentiles reported by Pate et al.27 The percentage of those subjects meeting the current physical activity guideline of >60 min per day of MVPA28 was calculated. Since the distribution of time spent in MVPA was skewed, a normalising logarithmic transformation was performed, and the logMVPA was used in all correlation analyses. Multiple general linear regression analyses were used to relate logMVPA to patient and medical history characteristics and questions from the Physical Activity Questionnaire. Pearson correlation analyses were used to relate logMVPA to CHQ Domain and Summary Scores, CHAT scores and the results of cardiopulmonary testing. To determine if exercise variables from exercise testing were more related to CHQ and CHAT scores than logMVPA, separate Pearson correlation analyses were performed to relate each of three exercise variables to the scores. All analyses were performed using SAS software, Version 9.1 (SAS Institute, Cary, NC, USA) by the principal investigator (BWM).
Results
Patient characteristics
A total of 147 patients (62% males) were recruited at a median age of 11.6 years (range 7.0–18.4 years) and at a median interval from Fontan procedure of 8.1 years (range 1.5–16 years). Hypoplastic left heart syndrome was the underlying cardiac diagnosis in 10%, and the median number of palliative surgical procedures before the Fontan procedure was three (range 0–9). The median age at Fontan procedure was 2.9 years (range 1.1–14.0 years). At assessment, the patients tended to have higher body mass index (BMI) than the normal population, with a mean Z score of +0.35±1.22 (p = 0.004). No patient was receiving supplemental oxygen, and 81% were either previously or currently receiving an angiotensin‐converting enzyme (ACE) inhibitor. The characteristics of our study population are very similar to that of the larger cohort from which the patients were derived, as recently reported.29
Of the 147 patients, 99 completed the questionnaires, exercise testing and activity monitoring. Other patients did not complete certain parts of the measurements, or there were technical problems with the activity monitoring. In total, 144 patients completed the questionnaires, 133 had exercise testing and 108 had activity monitoring data available.
Physical Activity Questionnaire
In comparison with their friends, 33% of subjects felt they were less active and 80% of subjects participated in gym class at school. Self‐reported symptoms during exercise included fatigue in 38%, shortness of breath in 44% and pain in 21%. The mean self‐reported time spent being physically active was 7 h per week (range 0.5–47). The mean self‐reported time spent doing sedentary pursuits (television watching, playing videogames, recreational computer use) was 28 h per week (range 0–119).
Objectively‐measured physical activity levels
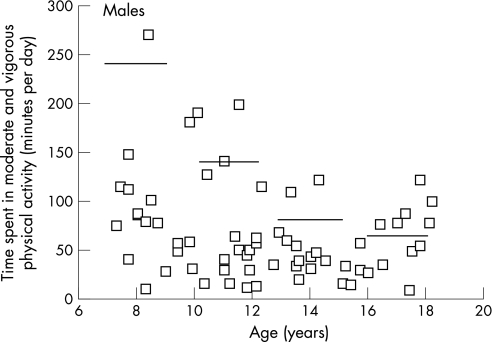
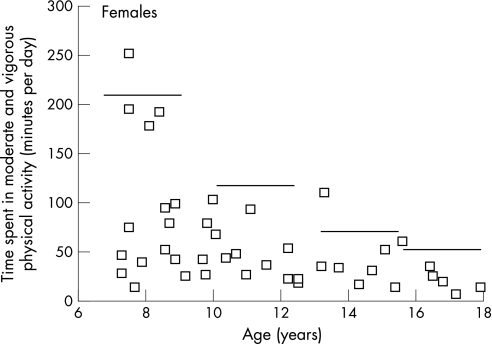
Of 124 subjects who wore the accelerometers, 13 were initially excluded due to non‐compliance (<3 days) or accelerometer malfunctioning and three outliers were excluded following exploratory data analysis. The final sample for the measured physical activity data included 108 patients (60 males, 48 females) and the results are shown in fig 1. The amount of time that the accelerometer was worn generally increased across age groups, which would be expected given the sleep–wake patterns of adolescents. For the total sample, mean counts per minute (p = 0.07), MPA (p = 0.18), VPA (p = 0.08) and MVPA (p = 0.15) were not significantly different for males compared with females. In general, there was a non‐significant trend for age‐related decline in counts per minute (p = 0.08), MPA (p = 0.10), VPA (p = 0.03) and MVPA (p = 0.07) except for the higher values in the 16–18 year old boys. Most values in the study subjects were less than the reference median for MVPA (even more dramatic for VPA, not shown) (figs 1 and 2), and the mean values for the study subjects actually fell below or approximate the 5th percentile of the reference values. Several cases of 0 min per day of VPA were recorded, and furthermore, 75% of male and female patients recorded ⩽2 min per day and ⩽3 min per day of VPA, respectively. Approximately 38% of the study subjects achieved the current physical activity recommendations for children and adolescents.27
Figure 1 Daily time spent in moderate and vigorous physical activity for each Fontan patient by patient age for males. Reference lines are the age group 50th percentiles for normal, healthy children.27
Figure 2 Daily time spent in moderate and vigorous physical activity for each Fontan patient by patient age for females. Reference lines are the age group 50th percentiles for normal, healthy children.27
Exercise capacity
From cardiopulmonary exercise testing completed in 133 subjects, the mean per cent of predicted for maximum Vo2 was 67%±15% (n = 128; p<0.001 vs normal), mean per cent of predicted for Vo2 at VAT was 96%±25% (n = 92; p = 0.14) and mean per cent of predicted for maximum work load was 58%±15% (n = 130; p<0.001).
Factors associated with objectively‐measured physical activity levels
Patient and medical history characteristics
Increasing minutes of MVPA (after logarithmic transformation; logMVPA) was significantly related to both younger patient age and male gender, as described above. Of the other patient and medical history variables, greater logMVPA was independently related in multivariable general linear regression analysis to an underlying cardiac diagnosis other than hypoplastic left heart syndrome (p = 0.03), fewer procedures performed before the Fontan procedure (p = 0.003) and lower family income (p<0.001), after adjusting for gender (p = 0.003) and age (p<0.001) (R2 = 0.39). After controlling for these variables, no other patient or medical history factor was significantly and independently associated with logMVPA, including care giver level of education, other cardiac morphologies, pre‐Fontan procedures including palliative procedures, other surgeries and catheter interventions, pre‐Fontan pulmonary arterial pressures, age at Fontan procedure, Fontan connection type, post‐Fontan procedures, history of ventricular dysfunction, arrhythmia, thrombosis, stroke, protein‐losing enteropathy, current echocardiographic evidence of ventricular dysfunction or other complications, prior or current use of ACE inhibition and BMI.
Self‐reported physical activity levels and attitudes
From analysis of the Physical Activity Questionnaire, greater logMVPA was independently related in general linear regression analysis to the presence of other family members who participated in competitive sports (p = 0.05), absence of complaints of dyspnoea with exercise (p = 0.03) and agreement that physical activity can prevent overweight (p = 0.02) (model R2 = 0.14). In Pearson correlation analysis, increased objectively measured logMVPA was not related to self‐reported time spent being physical active (r = –0.04; p = 0.68). Decreased logMVPA was significantly related to increasing total time spent in sedentary pursuits (r = –0.34; p<0.001), particularly television watching (r = –0.29; p = 0.002) and computer use (r = –034; p<0.001), but not time spent playing video games (r = –0.03; p = 0.77).
Functional status
In Pearson correlation analysis, logMVPA was not significantly related to any domains or summary scores from the CHQ Parent Report as shown in table 1, except for increasing physical activity being associated with greater general health perceptions. Physical activity levels were not significantly related to any of the domains of the disease‐specific CHAT questionnaire.
Table 1 Relationship between objectively‐measured physical activity levels† and health status.
| Variable | Pearson correlation coefficient | |||
|---|---|---|---|---|
| Physical activity level | Per cent predicted MaxVo2 | Per cent predicted Vo2 at VAT | Per cent predicted MaxWork | |
| R | R | r | r | |
| CHQ Parent Report | ||||
| Global health | 0.08 | 0.39* | 0.45* | 0.33* |
| Physical functioning limitations | 0.04 | 0.21* | 0.24* | 0.29* |
| Impact of emotional or | –0.04 | –0.08 | –0.11 | 0.02 |
| behavioural problems ‐ school or work | ||||
| Impact of physical limitations ‐ school or work | 0.11 | 0.21* | 0.24* | 0.29* |
| Bodily pain | 0.14 | 0.12 | 0.02 | 0.11 |
| General behaviour problems | –0.03 | –0.19* | –0.15 | –0.09 |
| Mental health | 0.05 | 0.03 | 0.14 | 0.05 |
| Self esteem | 0.08 | 0.17 | 0.28* | 0.07 |
| General health perceptions | 0.23* | 0.25* | 0.15 | 0.36* |
| Impact on parent of child's health ‐ parent worries/concerns | –0.03 | 0.14 | 0.22* | 0.17 |
| Impact on parent of child's health ‐ time restrictions | –0.05 | 0.02 | 0.05 | 0.15 |
| Family activities | 0.13 | –0.07 | –0.03 | 0.03 |
| Family cohesion | 0.14 | 0.11 | 0.26* | 0.02 |
| Overall physical functioning summary score | 0.14 | 0.29* | 0.26* | 0.38* |
| Overall psychosocial functioning summary score | –0.04 | –0.05 | 0.05 | 0.01 |
| CHAT Questionnaire | ||||
| General health | 0.04 | –0.19 | –0.34* | –0.11 |
| Social life | –0.01 | 0.03 | –0.19 | –0.08 |
| Seriousness of condition | –0.01 | 0.01 | 0.01 | –0.03 |
| Activity limitations | –0.22 | –0.32* | –0.34* | –0.32* |
| Emotional concerns | 0.02 | –0.04 | 0.01 | –0.18 |
| Friendship concerns | –0.21 | –0.17 | –0.12 | –0.25* |
| Career concerns | –0.04 | –0.12 | –0.16 | –0.22* |
| Symptom concerns | –0.02 | –0.25* | –0.22 | –0.21* |
*p<0.05.
†Reported as number of minutes per day spent in moderate or vigorous physical activity, after logarithmic transformation.
CHAT, Congenital Heart Adolescent and Teenage Questionnaire; CHQ, Child Health Questionnaire; MaxVo2, maximum oxygen consumption at peak exercise; MaxWork, maximum work rate; Vo2 at VAT, oxygen consumption at anaerobic threshold.
Exercise capacity
In Pearson correlation analysis, increasing logMVPA was not significantly related to per cent of predicted maximum Vo2 (r = –0.10; p = 0.34) or Vo2 at VAT (r = –0.11; p = 0.36), but there was a trend with increasing maximum work rate (r = 0.20; p = 0.052). There was no significant interaction between age at assessment and the exercise variables, indicating no influence of age on the lack of association between activity levels and exercise capacity.
Association between exercise capacity and functional status
In general, exercise test variables were more strongly and significantly related to functional status scores than objectively measured physical activity levels, as shown in table 1.
For the CHQ Parent Report, greater exercise capacity was related to higher scores for global health, physical functioning, impact of physical limitations, freedom from bodily pain, general health perceptions and physical functioning summary score. Regarding the disease‐specific functional status scores of the CHAT questionnaire, greater exercise capacity was associated with lower scores for general health perceptions, activity limitations and symptom concerns.
Discussion
A primary aim of this study was to describe and define the habitual physical activity levels of Fontan patients using an objective measurement device. The results show the well‐known age‐related decline and gender differences in physical activity levels of children and adolescents26,30; however, the 16–18 year old boys in this study showed higher values than the 13–15 year old boys, while the physical activity levels were only slightly higher in boys compared with girls. This probably reflects the overall poor physical activity levels at all ages and in both boys and girls who have had a Fontan procedure. Using the MTI accelerometer, Fredriksen et al12 found that boys with congenital heart disease had lower physical activity levels compared with healthy boys, while there were no differences between girls with congenital heart disease and healthy girls. In this same study, there were no differences in physical activity levels between younger and older children with congenital heart disease. Leitch et al31 found that total and activity energy expenditure assessed by doubly labelled water were not significantly different for 5 year old children following surgical repair of cyanotic congenital heart disease compared with age‐matched healthy control subjects. Australian adolescents with congenital heart disease completed an assessment questionnaire and were reported to be equally active in comparison to normal, healthy peers, but they differed qualitatively by participation in less VPA.11 However, there was a low response rate in that study (38%), and those with more severe congenital heart disease were found to be more likely to be non‐respondents.
Compared with the percentiles derived from normal, healthy children and adolescents participating in the Amherst Health and Activity Study26 (figs 1 and 2), the mean objectively measured physical activity levels of our Fontan patients are well below the 50th percentile, and actually fall below or approximate the 5th percentile for most patients. In addition, the mean physical activity values are lower than those reported for 9 and 15 year old European children.32 The percentage of our Fontan patients meeting a recommendation of 60 min per day of MVPA is quite low compared with other studies of normal, healthy children and adolescents assessed using accelerometry.26,32 The variability in the data does indicate that a few Fontan patients achieve adequate and even high levels of physical activity. However, although we screened the data for outliers, the amount of monitor tampering (ie, shaking) or the degree of non‐compliance are unknown. Overall, it can be concluded that the physical activity level of Fontan patients is substantially lower than normal healthy youths. This study shows the utility of using the MTI accelerometer in monitoring the habitual physical activity levels of Fontan patients, and may assist in establishing proper recommendations for physical activity for these patients.
The reasons for the lower physical activity of Fontan patients have not been fully explored, but possible explanations may include lower cardiorespiratory functional capacity, parental rearing style and imposed limitations, and/or psychosocial factors (eg, perceived competence or self‐efficacy). In a study of 100 adolescents (55 boys and 45 girls) aged 12–18 years with varying severity of congenital heart disease, it was found that the subjects' self‐efficacy influenced their degree of participation in physical activity and sports, and the recommendations of the cardiologist also played an important role in determining the attitudes of the mother and the self‐efficacy of the patient.13 However, self‐efficacy among an Australian sample was not significantly different from a normal, healthy reference group and these patients also received family support.11 We did not assess self‐efficacy as part of our study. However, the number of procedures prior to the Fontan procedure, the presence of underlying hypoplastic left heart syndrome and dyspnoea during exercise were related to reduced MVPA. Given the general relationships between more severe cardiac abnormalities and exercise capacity, it is perplexing that exercise capacity was not related to MVPA in our study. However, there was a trend for MVPA to be related to maximum work rate, which perhaps is a better indicator of ability to perform MVPA. Additional study is required to clarify these relationships.
The results showing that exercise capacity is more strongly related to functional status than MVPA does not support and contradicts or opposes our hypothesis. Several indicators of functional status (global health, physical functioning, impact of physical limitations, freedom from bodily pain, general health perceptions and physical functioning summary score) were significantly related to exercise capacity variables, whereas only general health perception was significantly associated with MVPA. Taken together, these results suggest that even though Fontan patients generally have a low level of physical activity, those with higher levels of exercise capacity show better functional status.
We have observed that while self‐reported time spent being physically active was not significantly correlated with objectively measured physical activity levels, there were significant associations with some of the attitude questions and with self‐reported time spent in sedentary pursuits. There has been little research in congenital heart disease regarding attitudes towards physical activity. Studies of overweight children have shown that they have similar attitudes towards physical activity as do normal weight children.33 Television viewing has received particular focus regarding the development of childhood obesity.34,35,36,37 To date, the only proven intervention that has been successful at preventing childhood obesity has been one aimed at reducing television viewing.38 There have been no previous studies of time spent in sedentary pursuits among children with congenital heart disease. A recent study in congenital heart disease patients has shown that activity restrictions were significantly associated with the subsequent development of greater adiposity.39 Our study population of Fontan patients has significantly greater Z score of BMI compared with normal values, although this was not associated with objectively measured physical activity levels. Obesity in Fontan patients must be prevented, given the potential to increase pulmonary vascular resistance as well as a host of other important morbidities. Increasing self‐efficacy for physical activity together with rehabilitation programs may both prevent obesity and improve functional status.40
A few studies have examined physical activity and exercise training for rehabilitation in Fontan patients. Minamisawa and colleagues studied 16 patients with an exercise program lasting 2–3 months, and noted significant improvements in exercise capacity.17 Opocher and colleagues studied 10 younger Fontan patients with an 8 month activity program, and noted significant improvements in maximum Vo2 and maximum oxygen pulse.16 Brassard and colleagues studied five adolescent Fontan patients with an 8 week exercise program, and showed significant improvements in skeletal muscle function.15 Rhodes and colleagues studied 19 patients with serious congenital heart disease, including 11 Fontan patients, with a 12 week exercise training program, and noted improvements in maximum Vo2, peak work rate, VAT and maximum oxygen pulse.18 A follow‐up study of these patients showed sustained benefits.41 While the results are promising, all of these studies have small patient numbers and lack a control group. No study to date has examined an intervention aimed at increasing self‐efficacy for physical activity, or assessed whether exercise training improves habitual physical activity levels.
The results of this study must be viewed in the light of potential limitations. Since there was no formal comparison of patient characteristics between patients enrolled and not‐enrolled in the main study, and enrolled and not‐enrolled in the current study, we cannot exclude the presence of an important selection bias. However, the characteristics of those patients enrolled in the current study are similar to those reported for the main study.29 The normal values for exercise capacity, although derived in the 1980s, remain the only normal data for exercise testing.22 These values were not derived from a highly selected fitter group of study subjects. Since the purpose of this study is not to define exercise capacity but the association of physical activity levels with exercise capacity and functional status, the per cent predicted variables used are valid for these associations. Physician and parent‐imposed activity restrictions may have influenced patient self‐efficacy for physical activity and, therefore, influenced measured physical activity levels in our study. Activity recommendations are not reliably recorded in the medical notes and, thus, this information was not available for study.
What is already known about this topic
Children and adolescents after the Fontan procedure have diminished exercise capacity and functional status.
What this study adds
Objectively measured physical activity levels are low in children after the Fontan procedure.
Functional status is associated with exercise capacity; physical activity levels do not reflect functional status.
In conclusion, measured physical activity levels are reduced in children and adolescents after Fontan, are independent of exercise capacity and are associated with lower perceived general health but not other aspects of functional status. Rehabilitation interventions aimed at increasing exercise capacity and promoting self‐efficacy for physical activity may improve functional status and activity levels. These types of interventions should be developed and investigated.
Abbreviations
BMI - body mass index
CHAT - Congenital Heart Adolescent and Teenage questionnaire
CHQ - Child Health Questionnaire
METS - metabolic equivalent tasks
MPA - moderate physical activity
MVPA - moderate and vigorous physical activity
PHN - Pediatric Heart Network
VAT - ventilatory anaerobic threshold
Vco2 - carbon dioxide production
Vo2 - oxygen consumption
VPA - vigorous physical activity
Footnotes
References
- 1.Zajac A, Tomkiewicz L, Podolec P.et al Cardiorespiratory response to exercise in children after modified fontan operation. Scand Cardiovasc J 20023680–85. [DOI] [PubMed] [Google Scholar]
- 2.Driscoll D J. Cardiorespiratory responses to exercise after the Fontan operation. Circulation 1990812016–2017. [DOI] [PubMed] [Google Scholar]
- 3.Larsson E S, Eriksson B O, Sixt R. Decreased lung function and exercise capacity in Fontan patients. A long‐term follow‐up. Scand Cardiovasc J 20033758–63. [DOI] [PubMed] [Google Scholar]
- 4.Ohuchi H, Ohashi H, Takasugi H.et al Restrictive ventilatory impairment and arterial oxygenation characterize rest and exercise ventilation in patients after fontan operation. Pediatr Cardiol 200425513–521. [DOI] [PubMed] [Google Scholar]
- 5.Stromvall‐Larsson E, Eriksson B O, Holmgren D.et al Pulmonary gas exchange during exercise in Fontan patients at a long‐term follow‐up. Clin Physiol Funct Imaging 200424327–334. [DOI] [PubMed] [Google Scholar]
- 6.Fredriksen P M, Therrien J, Veldtman G.et al Lung function and aerobic capacity in adult patients following modified Fontan procedure. Heart 200185295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyairi T, Kawauchi M, Takamoto S.et al Oxygen utilization and hemodynamic response during exercise in children after Fontan procedure. Jpn Heart J 199839659–669. [DOI] [PubMed] [Google Scholar]
- 8.Troutman W B, Barstow T J, Galindo A J.et al Abnormal dynamic cardiorespiratory responses to exercise in pediatric patients after Fontan procedure. J Am Coll Cardiol 199831668–673. [DOI] [PubMed] [Google Scholar]
- 9.Durongpisitkul K, Driscoll D J, Mahoney D W.et al Cardiorespiratory response to exercise after modified Fontan operation: determinants of performance. J Am Coll Cardiol 199729785–790. [DOI] [PubMed] [Google Scholar]
- 10.Driscoll D J, Durongpisitkul K. Exercise testing after the Fontan operation. Pediatr Cardiol 19992057–59. [DOI] [PubMed] [Google Scholar]
- 11.Lunt D, Briffa T, Briffa N K.et al Physical activity levels of adolescents with congenital heart disease. Aust J Physiother 20034943–50. [DOI] [PubMed] [Google Scholar]
- 12.Fredriksen P M, Ingjer E, Thaulow E. Physical activity in children and adolescents with congenital heart disease. Aspects of measurements with an activity monitor. Cardiol Young 20001098–106. [DOI] [PubMed] [Google Scholar]
- 13.Bar‐Mor G, Bar‐Tal Y, Krulik T.et al Self‐efficacy and physical activity in adolescents with trivial, mild, or moderate congenital cardiac malformations. Cardiol Young 200010561–566. [DOI] [PubMed] [Google Scholar]
- 14.Bar‐Mor G, Zeevi B, Yaaron M.et al Use of the heart rate monitor to modulate physical activity in adolescents with congenital aortic stenosis: an innovative approach. J Pediatr Nurs 199914273–277. [DOI] [PubMed] [Google Scholar]
- 15.Brassard P, Poirier P, Martin J.et al Impact of exercise training on muscle function and ergoreflex in Fontan patients: a pilot study. Int J Cardiol 2006107(1)85–94. [DOI] [PubMed] [Google Scholar]
- 16.Opocher F, Varnier M, Sanders S P.et al Effects of aerobic exercise training in children after the Fontan operation. Am J Cardiol 200595150–152. [DOI] [PubMed] [Google Scholar]
- 17.Minamisawa S, Nakazawa M, Momma K.et al Effect of aerobic training on exercise performance in patients after the Fontan operation. Am J Cardiol 200188695–698. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes J, Curran T J, Camil L.et al Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics 20051161339–1345. [DOI] [PubMed] [Google Scholar]
- 19.van den Bosch A E, Roos‐Hesselink J W, Van Domburg R.et al Long‐term outcome and quality of life in adult patients after the Fontan operation. Am J Cardiol 2004931141–1145. [DOI] [PubMed] [Google Scholar]
- 20.Landgraf J M, Abetz L, Ware J E., JrChild Health Questionnaire. Boston: The Health Institute, New England Medical Center, 1996
- 21.Kendall L, Lewin R J, Parsons J M.et al Factors associated with self‐perceived state of health in adolescents with congenital cardiac disease attending paediatric cardiologic clinics. Cardiol Young 200111431–438. [DOI] [PubMed] [Google Scholar]
- 22.Cooper D M, Weiler‐Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis 1984129S47–S48. [DOI] [PubMed] [Google Scholar]
- 23.Eisenmann J C, Strath S J, Shadrick D.et al Validity of uniaxial accelerometry during activities of daily living in children. Eur J Appl Physiol 200491259–263. [DOI] [PubMed] [Google Scholar]
- 24.Trost S G, Ward D S, Moorehead S M.et al Validity of the computer science and applications (CSA) activity monitor in children. Med Sci Sports Exerc 199830629–633. [DOI] [PubMed] [Google Scholar]
- 25.Freedson P S, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 199830777–781. [DOI] [PubMed] [Google Scholar]
- 26.Trost S G, Pate R R, Sallis J F.et al Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc 200234350–355. [DOI] [PubMed] [Google Scholar]
- 27.Pate R R, Freedson P S, Sallis J F.et al Compliance with physical activity guidelines: prevalence in a population of children and youth. Ann Epidemiol 200212303–308. [DOI] [PubMed] [Google Scholar]
- 28.Strong W B, Malina R M, Blimkie C J.et al Evidence based physical activity for school‐age youth. J Pediatr 2005146732–737. [DOI] [PubMed] [Google Scholar]
- 29.McCrindle B W, Williams R V, Mitchell P D.et al Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation 20061131123–1129. [DOI] [PubMed] [Google Scholar]
- 30.Sallis J F. Age‐related decline in physical activity: a synthesis of human and animal studies. Med Sci Sports Exerc 2000321598–1600. [DOI] [PubMed] [Google Scholar]
- 31.Leitch C A, Karn C A, Ensing G J.et al Energy expenditure after surgical repair in children with cyanotic congenital heart disease. J Pediatr 2000137381–385. [DOI] [PubMed] [Google Scholar]
- 32.Riddoch C J, Bo A L, Wedderkopp N.et al Physical activity levels and patterns of 9‐ and 15‐yr‐old European children. Med Sci Sports Exerc 20043686–92. [DOI] [PubMed] [Google Scholar]
- 33.Craeynest M, Crombez G, De H J.et al Explicit and implicit attitudes towards food and physical activity in childhood obesity. Behav Res Ther 2005431111–1120. [DOI] [PubMed] [Google Scholar]
- 34.Robinson T N. Television viewing and childhood obesity. Pediatr Clin North Am 2001481017–1025. [DOI] [PubMed] [Google Scholar]
- 35.Viner R M, Cole T J. Television viewing in early childhood predicts adult body mass index. J Pediatr 2005147429–435. [DOI] [PubMed] [Google Scholar]
- 36.Must A, Tybor D J. Physical activity and sedentary behavior: a review of longitudinal studies of weight and adiposity in youth. Int J Obes (Lond) 200529(Suppl 2)S84–S96. [DOI] [PubMed] [Google Scholar]
- 37.Patrick K, Norman G J, Calfas K J.et al Diet, physical activity, and sedentary behaviors as risk factors for overweight in adolescence. Arch Pediatr Adolesc Med 2004158385–390. [DOI] [PubMed] [Google Scholar]
- 38.Robinson T N. Reducing children's television viewing to prevent obesity: a randomized controlled trial. JAMA 19992821561–1567. [DOI] [PubMed] [Google Scholar]
- 39.Stefan M A, Hopman W M, Smythe J F. Effect of activity restriction owing to heart disease on obesity. Arch Pediatr Adolesc Med 2005159477–481. [DOI] [PubMed] [Google Scholar]
- 40.Council on Sports Medicine and Fitness and Council on School Health Active healthy living: prevention of childhood obesity through increased physical activity. Pediatrics 20061171834–1842. [DOI] [PubMed] [Google Scholar]
- 41.Rhodes J, Curran T J, Camil L.et al Sustained effects of cardiac rehabilitation in children with serious congenital heart disease. Pediatrics 2006118e586–e593. [DOI] [PubMed] [Google Scholar]