Abstract
Objective
The validity of the rule of thumb that infants may have a weight loss of 10% in the first days after birth is unknown. We assessed the validity of this and other rules to detect breast‐fed infants with hypernatraemic dehydration.
Design
A reference chart for relative weight change was constructed by the LMS method. The reference group was obtained by a retrospective cohort study.
Participants
1544 healthy, exclusively breast‐fed infants with 3075 weight measurements born in the Netherlands and 83 cases of breast‐fed infants with hypernatraemic dehydration obtained from literature.
Results
The rule of thumb had a sensitivity of 90.4%, a specificity of 98.3% and a positive predictive value of 3.7%. Referring infants if their weight change is below −2.5 SDS (0.6th centile) in the reference chart in the first week of life and using the rule of thumb in the second week had a sensitivity of 85.5%, a specificity of 99.4% and a positive predictive value of 9.2%.
Conclusions
The rule of thumb is likely to produce too many false positive results, assuming that for screening purposes the specificity needs to be high. A chart for relative weight change can be helpful to detect infants with hypernatraemic dehydration.
Keywords: breast feeding, weight loss, growth monitoring, infancy, hypernatraemic dehydration
Exclusive breast feeding up to the sixth month of life is important for optimum infant development and growth as breast milk contains all the necessary nutrients in ideal proportions.1 Breast feeding protects against infections and allergies, and plays a major role in mother–infant bonding.2
In the Netherlands, 78% of mothers initiated breast feeding in the period 2001–2003. After 1 month 51% and after 4 months 25% of infants were fed primarily on human milk.3 The WHO and UNICEF started the “Baby Friendly Hospital Initiative” to promote breast feeding.4 In the Netherlands, this program is mainly focused on improvement of support and encouragement of breast feeding in general health care.
Almost all mothers are capable of breast feeding their infant successfully. However, in some cases initial milk supply is insufficient because of a poor start to milk production or transfer. If the infant's needs are not met for several days, dramatic weight loss and an increase in serum sodium concentration occur and the infant develops hypernatraemic dehydration.5,6,7 Hypernatraemic dehydration may cause serious complications, such as fits, disseminated intravascular coagulation and multiple cerebrovascular accidents, and may even result in death.8,9,10,11,12
A retrospective, population‐based study reported an incidence rate of hypernatraemic dehydration of 7.1 per 10 000 breast‐fed infants.6 This is probably the minimum incidence as cases may have been missed because they occurred in infants before initial discharge from hospital13,14 or because appropriate investigations were not performed. The clinical day of presentation of hypernatraemic dehydration is usually around 10 days of age.6
In clinical practice, weighing is an essential part of the assessment of an infant's growth and hydration status. However, there is no evidence‐based consensus for ”normal” and ”abnormal” early relative weight change (RWC). Several studies reported the normal (50th centile) or extreme (1st, 2.5th or 5th) centile RWC for exclusively breast‐fed infants.6,15 However, these centiles are not precisely described with respect to day of measurement nor shown on standard growth charts. Several authors propose different rules of thumbs for identifying “abnormal” RWC.16,17,18,19,20 It is suggested that many midwives use the rule of thumb that infants may have a weight loss of 10% ( = −10% RWC) and should regain birth weight by 10–14 days of life.5 To our knowledge, no evidence‐based referral rule is available to detect infants with hypernatraemic dehydration.
This study describes a reference chart for breast‐fed infants between postnatal days 2 and 11. This chart, together with reports of hypernatraemic dehydration obtained from the literature, will be used to define an evidence‐based referral rule. The centiles of the chart can be used as a test to detect infants with hypernatraemic dehydration. The test is considered positive if a breast‐fed infant's relative weight decreases below a chosen centile and negative if it stays above. Sensitivity, specificity and positive predictive value (PPV) will be used to optimise this rule. This test will be compared to the rule of thumb that infants may have a maximal weight loss of 10%.
Methods
Population
We selected a representative reference group of healthy, exclusively breast‐fed infants and a group of breast‐fed infants diagnosed with hypernatraemic dehydration. The reference group was obtained from a retrospective cohort study initiated in three primary care midwife practices in the Netherlands (metropolitan Amsterdam South‐East, rural Heerhugowaard and the country town of Veenendaal). In the Netherlands, a midwife either assists the delivery at home or in an outpatient clinic, or is involved in follow‐up care after hospital delivery by a gynaecologist. We selected 1544 infants born in 2002 with a weight measurement (in grams) at birth and at least one weight measurement between postnatal days 2 and 11. The infants were weighed at home by a midwife with a calibrated electronic scale. In this study, the midwife was instructed to weigh the infant routinely.
Infants hospitalised with hypernatraemic dehydration were identified by a literature search. Articles written in Dutch, English, French or German published between 1970 and 2005 that describe infants with hypernatraemic dehydration were obtained using the search program PubMed with the MESH terms “dehydration” and “breastfeeding”. References in these articles were used to increase the number of articles describing infants with hypernatraemic dehydration. We assumed that an infant had hypernatraemic dehydration when the author(s) of the article diagnosed the infant as such. In 47 articles we identified 129 cases of breast‐fed infants with hypernatraemic dehydration with a weight measurement at birth and day of presentation or a calculated RWC at day of presentation. A total of 83 literature cases had a day of presentation between 2 and 11 days of life and these were used in this study.6,9,11,14,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 Serum sodium concentration was known for 80 literature cases. All infants were born at term.
Statistical analysis
RWC was calculated as the difference in weight at day of presentation (w(t)) and birth weight (w(t0)) divided by birth weight in percentage, or in formula: 100%*(w(t)−w(t0))/w(t0)). Day of birth was represented by day 0. A reference chart for relative weight was obtained by the LMS method.43 The LMS method summarises the distribution of relative weight as it changes according to age by three curves representing the Box–Cox power (L‐curve), the median (M‐curve) and the coefficient of variation (S‐curve). The L‐, M‐, and S‐curves were used to convert data into standard normally distributed data. Such a data point is called a z score or standard deviation score (SDS). Normality of SDS was tested by so called “worm plots” for different age groups.44 A log power‐transformation was applied to age in the LMS method. Since the LMS method works only with positive values, an amount of 25% was added to relative weight and afterwards subtracted from the centiles. Each infant had multiple weight measurements. All weights were included in the analysis and were treated as independent as we did not find an association between the number of measurements and birth weight (t test, t = 1.14, p = 0.26).
The centiles of the curve and the 10% weight loss were used as a test. The specificity of the 10% rule was calculated as the mean of the percentiles of the reference chart that have a 10% weight loss for each day. To calculate PPV, we assumed that the incidence of hypernatraemic dehydration is 7.1 per 10 000 breast‐fed infants.6
Calculations for the LMS method were performed with LMS Light version 1.16 (Institute of Child Health, London, UK) compiled on 15 April 2002. All other analyses were performed with S‐plus version 6.2 (Insightful, Seattle, WA, USA).
Results
The characteristics of the reference infants are given in table 1. The number of measurements in reference infants and in those with hypernatraemic dehydration are shown in table 2.
Table 1 Characteristics of healthy, breast‐fed reference infants.
| Characteristics | Healthy, breast‐fed infants (n = 1544) |
|---|---|
| Maternal age in years | 30 (4.7) |
| Girls (%) | 49 |
| Gestation in weeks (n = 1543) | 39.5 (1.4) |
| Preterm <37 weeks (%) | 2.0 |
| Parity (%) | |
| First | 45 |
| Second | 36 |
| Third or more | 19 |
| Delivery (%) | |
| Spontaneous | 80 |
| Caesarean section | 10 |
| By vacuum extraction or forceps | 10 |
| Birth weight in kg | 3.44 (0.46) |
Data are means (SD) or percentages.
Table 2 Number of measurements between 2 and 11 days of life in healthy, breast‐fed infants and infants with hypernatraemic dehydration.
| Characteristics | Healthy, breast‐ fed infants | Infants with hypernatraemic dehydration |
|---|---|---|
| Number of infants | 1544 | 83 |
| Number of | ||
| measurements on | ||
| Day 2 | 9 | 0 |
| Day 3 | 505 | 9 |
| Day 4 | 263 | 9 |
| Day 5 | 618 | 4 |
| Day 6 | 128 | 15 |
| Day 7 | 287 | 10 |
| Day 8 | 272 | 11 |
| Day 9 | 864 | 6 |
| Day 10 | 93 | 16 |
| Day 11 | 36 | 3 |
RWC was not normally distributed (Shapiro–Wilk normality test: W = 0.975, p<0.01). To obtain normally distributed SDS for RWC, we used the LMS method with a Box–Cox power transformation of approximately 0.5. Normality of SDS was tested by worm plots of different age groups. The shape of the worm plots was reasonably flat, indicating that the data follow the assumed distribution in this age period.
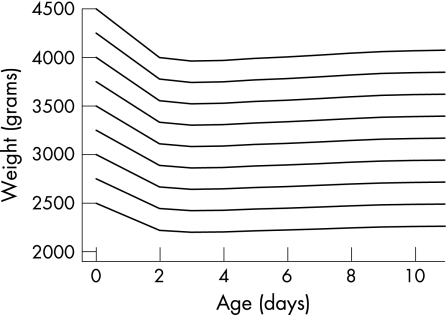
Figure 1 shows a reference chart with standard deviation lines of the RWC of healthy, breast‐fed infants as well as the RWC of 83 infants with hypernatraemic dehydration on the day of presentation. The rule of thumb of 10% weight loss is also indicated on the chart. The standard deviation lines or percentiles on this chart show which percentages of infants have the same RWC. For example, if a 5 day old infant weighs 3315 g and has a birth weight of 3750 g, then the calculated RWC is 100%*(3315−3750)/3750 = −11.6%. Notice that −11.6% RWC at day 5 on the chart corresponds to −2.6 SDS or the 0.5th percentile. This means that only 0.5% of 5 day old infants have a RWC less than this infant. To avoid the user calculating weight as a percentage, we converted the −2.5 SDS RWC centile to weights by age for a given birth weight. This converted −2.5 SDS centile is shown on fig 2 for different birth weights. The infant in the previous example has a birth weight of 3750 g. The −2.5 SDS centile for this infant is shown by the fourth line from the top, starting at 3750 at day 0. Follow this line until you reach day 5 and notice that 3315 g at day 5 is just below the line.
Figure 1 Reference chart with standard deviation lines of relative weight change ( = 100%*(weight−birth weight)/birth weight) for healthy, breast‐fed infants as well as relative weight change in 83 infants with hypernatraemic dehydration on day of presentation (day of birth is day 0) and showing the rule of thumb of 10% weight loss.
Figure 2 The −2.5 SDS relative weight change centile converted to weights by age for a given birth weight.
Maximal negative RWC for healthy, breast‐fed infants is at 3 days after birth, with a mean RWC of −6.0% (95% CI: −5.7% to −6.2%). The mean increases by approximately 1% per day from −6% at day 3 to 0% at day 8. However, even after 11 days about a third of these infants have not yet regained their birth weight; in contrast with healthy, breast‐fed infants, the mean of these patients is consistently declining. The mean RWC for the infants with hypernatraemic dehydration is −18.5% (95% CI: −17.0% to −19.9%). The mean decreases by approximately 2% per day from −10% at day 3 to −25% at day 10.
Notice that there were no cases of hypernatraemic dehydration before day 3, probably due to the fact that it takes some time before insufficient breast feeding leads to weight loss. We therefore applied the rules from 3 days up until 11 days after birth.
Table 3 shows sensitivity, specificity and PPV for several referral rules: the rule of thumb (10% test), the SDS rules and a combination of the −2.5 SDS test in the first week (3–6 days after birth) and the 10% test after the first week. All sensitivities for these tests were above 85% with less than 3% false positives. The sensitivity of the 10% test was similar to the that of the −2 SDS rule and specificity was slightly higher in the first week, although not significantly so (p>0.05). Combining the −2.5 SDS test in the first week with the 10% rule after the first week results in a sensitivity of 85.5% and a specificity of 99.4%; this is similar to the −2.5 SDS test for the first 2 weeks. This specificity is significantly higher (p<0.05) than that for the −2 SDS rule.
Table 3 Sensitivity, specificity and positive predictive value (PPV) for several referral rules for between postnatal days 3 and 11.
| Test | Sensitivity (%) | Specificity (%) | PPV (%) |
|---|---|---|---|
| 10% | 90.4 | 98.3 | 3.7 |
| −2.5 SDS | 85.5 | 99.4 | 9.2 |
| −2 SDS | 90.4 | 97.7 | 2.7 |
| −2.5 SDS 3–6 days | 85.5 | 99.4 | 9.2 |
| and 10% 7–11 days |
Cases with a positive −2 or −2.5 SDS test had a significantly higher mean serum sodium concentration (163 mM) compared to cases with a negative −2 SDS test (149 mM) (t = 2.6, df = 78, p = 0.01) and with a negative −2.5 SDS test (151 mM) (t = 3.0, df = 78, p = 0.004). Of the cases with a positive −2.5 SDS test, 89% had a concentration of >149 mM, so the test detects the more severe cases of dehydration. Of the cases with a concentration of >149 mM, 91% had a positive −2.5 SDS test and 97% a positive −2 SDS test, and of the cases with a concentration of >159 mM, all cases had a positive −2.5 SDS test (and therefore a positive −2 SDS test).
Eight cases of hypernatraemic dehydration had a very small RWC. Three cases had a RWC between −2 SDS and −1 SDS and five cases had a weight change above −1 SDS.19,35,36,42 Clinical information was given in some studies; only mild and transient symptoms in these infants were reported. Serum sodium concentration was reported for six cases: four cases had a concentration below 149 mM and two above 149 mM (both 157 mM).
Discussion
We developed a reference chart for breast‐fed infants between postnatal days 2 and 11. This chart, together with cases of hypernatraemic dehydration obtained from the literature, was used to define an evidence‐based referral rule. As far as we know, this is the first reference chart for RWC and the first evidence‐based investigation of referral rules to detect infants with hypernatraemic dehydration. Our results show that a reference chart for RWC can be helpful to detect infants with hypernatraemic dehydration.
The RWC chart shows that the mean maximal weight loss occurs 3 days after birth and is 6% for a healthy, breast‐fed infant. This is in agreement with several other studies which reported that breast‐fed infants may lose up to 6%45,46,47 or 7%15,19,48,49 of their birth weight during the first week of life. The American Academy of Pediatrics and others also reported that normal weight loss reaches its peak at 3–5 days after birth.50 Livingstone and the American Academy of Pediatrics Work Group on Breastfeeding suggested that a weight loss of greater than 7% of birth weight indicates possible breast feeding problems.19,50 Others suggested that a weight loss of 8% or more warrants further investigation.16,17,18
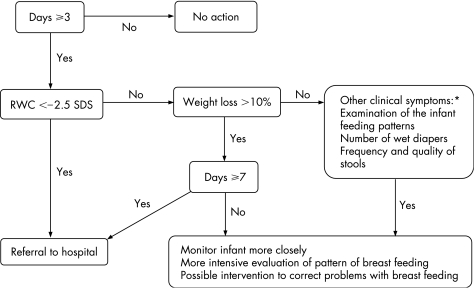
Most authors reported that many midwives use the rule of thumb that infants may lose up to 10% of birth weight. Our results show that most infants with hypernatraemic dehydration have a weight loss of 10%. However, referral to a hospital of all infants with a weight of loss of >10% would probably lead to many false positive results in the first week of life, assuming that for screening purposes the specificity needs to be sufficiently high. Therefore, we suggest applying the 0.6th centile (−2.5 SDS) as a criterion for referral to a hospital in the first week of life or using a weight loss of >10% after the first week of life. At the hospital, further diagnostic biochemical testing should be carried out. As it takes some time before insufficient breast feeding leads to weight loss, clinical differentiation between normal infants and those with hypernatraemic dehydration is not really possible in the first 2 days after birth. Infants with a weight loss of >10% (or <−2 SDS) in the first week after day 2, should be monitored closely and require more intensive evaluation of breast feeding and possible intervention to correct problems with breast feeding. Furthermore, referral may also be warranted in infants with other clinical symptoms of dehydration even if weight loss is not particularly high. Clinicians should combine RWC values with examination of the infant, knowledge of feeding patterns, and number of wet diapers and frequency and quality of stools. We suggest using the flowchart in fig 3.
Figure 3 Flowchart to detect dehydrated infants or infants at risk of dehydration. *Monitor the infant when mild clinical symptoms are present and refer the infant to hospital when severe clinical symptoms are present.
In addition to the 10% weight loss, another rule of thumb among midwives is that infants regain their birth weight by 10–14 days. The chart in this study shows that 50% of infants have regained their birth weight 8 days after birth, which is also consistent with other reports.6,15 This study also shows that even after 11 days, about a third of infants have not yet regained their birth weight. We also expect that at day 14 a high percentage of infants will not have regained their birth weight. Therefore, we assume that this rule will lead to many false positive results. Macdonald et al15 suggested a revised intervention criterion: offer additional breast feeding support to those losing 10% of their birth weight but still consider this as normal and only consider weight loss above 12.5% or failure to regain birth weight by 21 days as being abnormal and requiring medical assessment. We applied the 12.5% weight loss rule to our data with infants from birth to 11 days old and found a sensitivity of 83.1% and a specificity of 99.9%. This rule has a better specificity (+0.5%) at the cost of a lower sensitivity (−2.4%) compared to the −2.5 SDS rule. With the 12.5% weight loss rule, 2.4% of the cases are missed. We think that a decrease in sensitivity of 2.4% is high and we therefore recommend using the proposed flow chart. However, one could consider using the 12.5% weight loss rule at day 3 as the −2.5 SDS line almost reaches 12.5% at day 3.
In our study we used information from cases with hypernatraemic dehydration reported in the literature. We expected that this information is biased towards the more severe cases of hypernatraemic dehydration, since severe cases are more likely to be reported than mild cases. Recently Moritz et al51 found that only 17% of cases of hypernatraemic dehydration had non‐metabolic complications. Therefore, the sensitivity and PPV in this study are likely to be lower for all infants with hypernatraemic dehydration. On the other hand, PPV may also be an underestimate as this value was based on a minimum incidence rate of hypernatraemic dehydration. It would be very interesting in the future to test and possibly optimise our proposed referral rules using new cases with dehydration.
There is evidence that the degree of weight loss in babies born in a particular environment may be associated with the way that environment is managed.52,53 In populations with “baby friendly” care, the prevalence of hypernatraemic dehydration may be lower than in populations with care that is less baby friendly. We assumed that the prevalence of hypernatraemic dehydration is 7.1 per 10 000 breast‐fed infants. Based on this prevalence we calculated the PPV of several referral criteria. Since PPV is dependent on prevalence, in populations with a lower prevalence (perhaps due to baby friendly care) the PPV may be lower, whereas in populations with a higher prevalence the PPV of the same referral criteria will be higher.
We assumed that RWC expressed as a percentage is uncorrelated with birth weight. This means that a heavy child and a light child have the same distribution of RWC. However, this may not be true, as the degree, timing and variability of RWC may be quite different in small infants compared to large infants. We therefore tested the relationship between birth weight and RWC corrected for age using a linear mixed‐effects model (residual variance = 1.53, AIC = 15 864). We found that an infant with a birth weight of 2.5 kg has on average a 1% greater RWC than an infant with a birth weight of 4.5 kg. As this is a relatively small difference for a large difference in birth weight, we decided to use the methodology unconditional on birth weight. The latter approach is also more convenient in practice than, for instance, various RWC curves for different categories of birth weight.
What is already known on this topic?
Several authors have proposed different rules of thumbs for identifying abnormal relative weight change (RWC).
It is suggested that many midwives use the rule of thumb that infants may have a weight loss of 10% ( = −10% RWC) but should regain their birth weight by 10–14 days.
What this study adds
As far as we know, we have conducted the first evidence‐based investigation of referral rules for infants with hypernatraemic dehydration and have developed the first reference chart for relative weight change.
In this study, the weights of the infants were obtained in a research setting. The midwife was instructed to weigh the infant routinely. This means that the number of measurements should not depend on the status of the infant. To determine if this is indeed the case, using standard two‐sample t tests we tested the dependence of the number of measurements and the status of the infants by testing the difference in RWC each day between the infants whose weight was being measured for the first time (besides their birth weight) and those who were being reweighed. We refitted the LMS method without the cases which were possibly reweighed because of a high RWC, and found that the difference between the median RWC in the newly constructed growth chart and the reference chart based on all infants was negligible (< = −0.2%).
We conclude that the rule of thumb that infants may have a relative weight loss of 10% is excellent after the first week of life. However, in the first week of life this rule will produce too many false positive results. A chart for RWC can be helpful to detect infants with hypernatraemic dehydration.
Acknowledgements
We are grateful to Mieke Huisenga, Wendelien Roepke, Marleen Snabilie and the midwife practices for obtaining the reference group data. We thank Caren Lanting and Jan Maarten Wit for their suggestions for improvement.
Abbreviations
PPV - positive predictive value
RWC - relative weight change
SDS - standard deviation score
Footnotes
Competing interests: None declared.
References
- 1.World Health Organization The optimal duration of exclusive breastfeeding. Report of an expert consultation. Geneva: World Health Organization, 2001
- 2.Lawrence R A, Howard C R. Given the benefits of breastfeeding, are there any contraindications? Clin Perinatol 199926479–90, viii. [PubMed] [Google Scholar]
- 3.Lanting C I, Van Wouwe J P, Reijneveld S A. Infant milk feeding practices in the Netherlands and associated factors. Acta Paediatr 200594935–942. [DOI] [PubMed] [Google Scholar]
- 4.Naylor A J. Baby‐Friendly Hospital Initiative. Protecting, promoting, and supporting breastfeeding in the twenty‐first century. Pediatr Clin North Am 200148475–483. [DOI] [PubMed] [Google Scholar]
- 5.Laing I A, Wong C M. Hypernatraemia in the first few days: is the incidence rising? Arch Dis Child Fetal Neonatal Ed 200287F158–F162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oddie S, Richmond S, Coulthard M. Hypernatraemic dehydration and breast feeding: a population study. Arch Dis Child 200185318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke T A, Markarian M, Griswold W.et al Hypernatremic dehydration resulting from inadequate breast‐feeding. Pediatrics 197963931–932. [PubMed] [Google Scholar]
- 8.Arboit J M, Gildengers E. Breast‐feeding and hypernatremia. J Pediatr 198097335–336. [DOI] [PubMed] [Google Scholar]
- 9.Rowland T W, Zori R T, Lafleur W R.et al Malnutrition and hypernatremic dehydration in breast‐fed infants. JAMA 19822471016–1017. [PubMed] [Google Scholar]
- 10.Clarke A J, Sibert J R. Hypernatraemic dehydration and necrotizing enterocolitis. Postgrad Med J 19856165–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper W O, Atherton H D, Kahana M.et al Increased incidence of severe breastfeeding malnutrition and hypernatremia in a metropolitan area. Pediatrics 199596957–960. [PubMed] [Google Scholar]
- 12.Kaplan J A, Siegler R W, Schmunk G A. Fatal hypernatremic dehydration in exclusively breast‐fed newborn infants due to maternal lactation failure. Am J Forensic Med Pathol 19981919–22. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhary R, Twaddle S, Levi R.et al Hypernatremic dehydration in breast‐fed infants. Paediatrics Today 1998685–87. [Google Scholar]
- 14.Ng P C, Chan H B, Fok T F.et al Early onset of hypernatraemic dehydration and fever in exclusively breast‐fed infants. J Paediatr Child Health 199925585–587. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald P D, Ross S R, Grant L.et al Neonatal weight loss in breast and formula fed infants. Arch Dis Child Fetal Neonatal Ed 200388F472–F476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neifert M R. Clinical aspects of lactation. Promoting breastfeeding success. Clin Perinatol 199926281–306, vvi. [PubMed] [Google Scholar]
- 17.Neifert M R. Prevention of breastfeeding tragedies. Pediatr Clin North Am 200148273–297. [DOI] [PubMed] [Google Scholar]
- 18.Simoes E A, Pereira S M. The growth of exclusively breastfed infants. Ann Trop Paediatr 1986617–21. [DOI] [PubMed] [Google Scholar]
- 19.Livingstone V H, Willis C E, Abdel‐Wareth L O.et al Neonatal hypernatremic dehydration associated with breast‐feeding malnutrition: a retrospective survey. CMAJ 2000162647–652. [PMC free article] [PubMed] [Google Scholar]
- 20.DeMarzo S, Seacat J, Neifert M. Initial weight loss and return to birth weight criteria for breast‐fed infants: challenging the ‘rule of thumb'. Am J Dis Children 1991145402 [Google Scholar]
- 21.Bajpai A. Hypernatremic dehydration in a neonate. Indian Pediatr 200239599–600. [PubMed] [Google Scholar]
- 22.Becker P G, Conard J A. Breast‐feeding failure. Indiana Med 199083648–650. [PubMed] [Google Scholar]
- 23.Boumahni B, Pyaraly S, Randrianaly H.et al Hypernatremic dehydration and breastfeeding. Arch Pediatr 20018731–733. [DOI] [PubMed] [Google Scholar]
- 24.Canet E, Canet J, Perrain M F. Quel est votre diagnostic? Concours Med 19841061689–1691. [Google Scholar]
- 25.Chilton L A. Prevention and management of hypernatremic dehydration in breast‐fed infants. West J Med 199516374–76. [PMC free article] [PubMed] [Google Scholar]
- 26.Gebara B M, Everett K O. Dural sinus thrombosis complicating hypernatremic dehydration in a breastfed neonate. Clin Pediatr (Phila) 20014045–48. [DOI] [PubMed] [Google Scholar]
- 27.Harding D, Moxham J, Cairns P. Weighing alone will not prevent hypernatraemic dehydration. Arch Dis Child Fetal Neonatal Ed 200388F349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilliard T N, Marsh M J, Malcolm P.et al Radiological case of the month. Sagittal sinus thrombosis in hypernatremic dehydration. Arch Pediatr Adolesc Med 19981521147. [DOI] [PubMed] [Google Scholar]
- 29.Jaramillo I, Lopez G, Hernandez H. Hypernatremic dehydration and death in an infant. Pediatr Emerg Care 20031962–63. [DOI] [PubMed] [Google Scholar]
- 30.Kini N, Zahn S, Werlin S L. Hypernatremic dehydration in breast‐fed infants. Wis Med J 199594143–145. [PubMed] [Google Scholar]
- 31.Macdonald P D, Grant L, Ross S R. Hypernatraemia in the first few days: a tragic case. Arch Dis Child Fetal Neonatal Ed 200388F350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marino R, Gourji S, Rosenfeld W. Neonatal metabolic casebook. Hypernatremia and breast feeding. J Perinatol 19899451–453. [PubMed] [Google Scholar]
- 33.Mercier J C, Outin S, Paradis K.et al [Breast feeding and hypernatremic dehydration. 3 case studies]. Arch Fr Pediatr 198643465–470. [PubMed] [Google Scholar]
- 34.Molteni K H. Initial management of hypernatremic dehydration in the breastfed infant. Clin Pediatr (Phila) 199433731–740. [DOI] [PubMed] [Google Scholar]
- 35.Niestijl A L, Sauer P J. Breast feeding during the first few days after birth: sometimes insufficient. Ned Tijdschr Geneeskd 20031472405–2407. [PubMed] [Google Scholar]
- 36.Pascale J A, Brittian L, Lenfestey C C.et al Breastfeeding, dehydration, and shorter maternity stays. Neonatal Netw 19961537–43. [PubMed] [Google Scholar]
- 37.Rand S E, Kolberg A. Neonatal hypernatremic dehydration secondary to lactation failure. J Am Board Fam Pract 200114155–158. [PubMed] [Google Scholar]
- 38.Roddey O F, Martin E S, Swetenburg R L. Critical weight loss and malnutrition in breast‐fed infants. Am J Dis Child 1981135597–599. [DOI] [PubMed] [Google Scholar]
- 39.Rosenfeld W, deRomana G L, Kleinman R.et al Improving the clinical management of hypernatremic dehydration. Observations from a study of 67 infants with this disorder. Clin Pediatr (Phila) 197716411–417. [DOI] [PubMed] [Google Scholar]
- 40.Sofer S, Ben Ezer D, Dagan R. Early severe dehydration in young breast‐fed newborn infants. Isr J Med Sci 19932985–89. [PubMed] [Google Scholar]
- 41.van der Heide P A, Toet M C, van Diemen‐Steenvoorde J A.et al Hypertonic dehydration in “silent” malnutrition of breast‐fed infants. Ned Tijdschr Geneeskd 1998142993–995. [PubMed] [Google Scholar]
- 42.Willis C E, Livingstone V. Infant insufficient milk syndrome associated with maternal postpartum hemorrhage. J Hum Lact 199511123–126. [DOI] [PubMed] [Google Scholar]
- 43.Cole T J, Green P J. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992111305–1319. [DOI] [PubMed] [Google Scholar]
- 44.van Buuren S, Fredriks M. Worm plot: a simple diagnostic device for modelling growth reference curves. Stat Med 2001201259–1277. [DOI] [PubMed] [Google Scholar]
- 45.Maisels M J, Gifford K. Breast‐feeding, weight loss and jaundice. J Pediatr 1983102117–118. [DOI] [PubMed] [Google Scholar]
- 46.Marchini G, Fried G, Ostlund E.et al Plasma leptin in infants: relations to birth weight and weight loss. Pediatrics 1998101429–432. [DOI] [PubMed] [Google Scholar]
- 47.Marchini G, Stock S. Thirst and vasopressin secretion counteract dehydration in newborn infants. J Pediatr 1997130736–739. [DOI] [PubMed] [Google Scholar]
- 48.Maisels M J, Gifford K, Antle C E.et al Jaundice in the healthy newborn infant: a new approach to an old problem. Pediatrics 198881505–511. [PubMed] [Google Scholar]
- 49.Thureen P J, Hay W W., Jr Advice for selected breast‐feeding issues. Compr Ther 199622802–805. [PubMed] [Google Scholar]
- 50.American Academy of Pediatrics Section on Breastfeeding Breastfeeding and the use of human milk. Pediatrics 2005115496–506.15687461 [Google Scholar]
- 51.Moritz M L, Manole M D, Bogen D L.et al Breastfeeding‐associated hypernatremia: are we missing the diagnosis? Pediatrics 2005116343–347. [DOI] [PubMed] [Google Scholar]
- 52.Mikiel‐Kostyra K, Mazur J. Hospital policies and their influence on newborn body weight. Acta Paediatr 19998872–75. [PubMed] [Google Scholar]
- 53.Avoa A, Fischer P R. The influence of perinatal instruction about breast‐feeding on neonatal weight loss. Pediatrics 199086313–315. [PubMed] [Google Scholar]