Abstract
Context:
Evidence suggests that babies' fat mass at birth is greater if their mothers were themselves fatter during pregnancy, but it is unclear whether this association persists into childhood.
Objective:
To examine the relation between maternal size in pregnancy, early growth and body composition in children.
Design:
Prospective cohort study
Setting:
Southampton, UK.
Participants:
216 nine-year-old children whose mothers had participated in a study of nutrition during pregnancy.
Main outcome measures:
Fat mass and lean mass measured by dual-energy X-ray absorptiometry, adjusted for height (“fat mass index” and “lean mass index”).
Results:
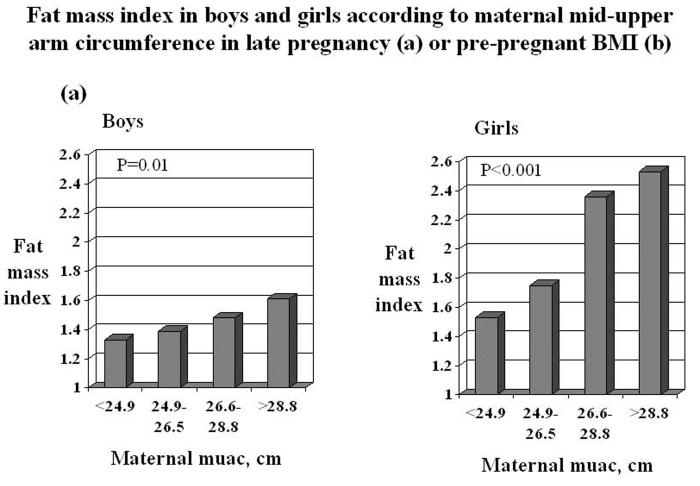
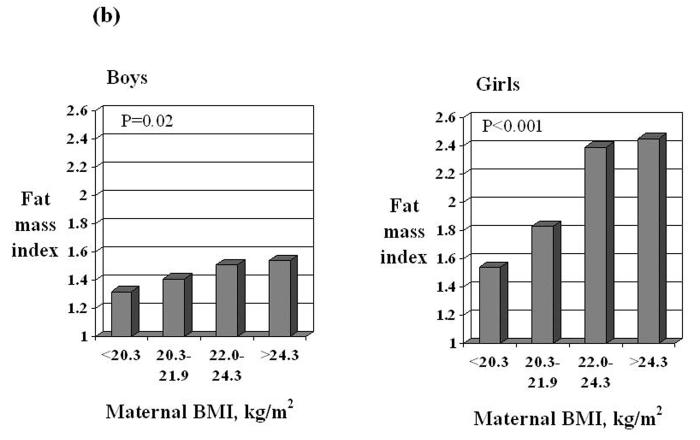
Fat mass index at age nine years was greater in children whose mothers had a larger mid-upper arm circumference in late pregnancy or a higher pre-pregnant body mass index. For one standard deviation (SD) increase in maternal mid-upper arm circumference in late pregnancy, fat mass index rose by 0.26 (95% CI 0.06-0.46) SD in boys and by 0.44 (95% CI 0.31-0.57) SD in girls. For one SD increase in maternal pre-pregnant BMI, fat mass index rose by 0.26 (95% CI 0.04-0.48) SD in boys and by 0.42 (95% CI 0.29-0.56) SD in girls.
Conclusions:
Mothers with a higher pre-pregnant body mass index or a larger mid-upper arm circumference during pregnancy tend to have children with greater adiposity at age nine. The extent to which this is attributable to genetic factors, the influence of maternal lifestyle on that of her child, or maternal adiposity acting specifically during pregnancy on the child's fat mass cannot be determined in this study.
Keywords: child, body composition, pregnancy, birth weight, weight gain, infancy
Introduction
The increasing prevalence of childhood overweight and obesity in the developed world is widely recognized as a major public health problem.(1) Being obese in childhood or adolescence is associated with higher blood pressure,(2) insulin resistance,(3) increased atherogenesis(4) and a greater risk of obesity in adult life.(5) The observation that persistent obesity is largely established by age 11 years suggests that early life may be a critical time for primary prevention.(6;7)
There is considerable evidence that infancy and early childhood are important periods for determining future risk of obesity. Infants who are at the highest end of the weight or body mass index (BMI) distribution or who grow rapidly in the first two years of life are at increased risk of subsequent obesity.(8) Recent findings that rapid weight gain in infancy and between the ages of three and six years predict increased adiposity in young adults imply that the changes in body composition linked with faster early weight gain may be long-term.(9) Evidence from several systematic reviews suggest that breastfeeding may protect against later obesity, possibly by slowing weight gain in infancy,(10;11) though there is little data on its relation with direct measures of childhood adiposity, such as those obtained by dual-energy X-ray absorptiometry (DXA).(12)
The importance of the prenatal environment in influencing later obesity is still unclear. Although many studies have shown associations between high birthweight and greater BMI in later life,(13) birthweight provides only a crude indicator of prenatal experience and BMI depends on both fat and lean tissue mass so may provide a misleading indicator of adiposity.(14) In studies of children and adolescents using direct measurements of body composition, increased birthweight is associated with greater lean mass but not with fat mass.(15;16) New research, however, suggests that children's adiposity may be influenced by their mother's own fatness during pregnancy. Few studies have examined the long-term consequences of maternal overnutrition on fat mass in the offspring.(17) Some evidence of its potential effect has come from an Australian cohort where a higher maternal BMI prior to pregnancy was linked with a raised BMI in the offspring at age 14,(18) but BMI cannot differentiate between lean and fat mass. In a study of women with normal glucose tolerance levels, those who were overweight or obese in pregnancy had offspring with increased fat mass at birth.(19) If this relation persists into later childhood it could have important implications for preventing obesity.
We investigated the relations between maternal size in pregnancy, early growth and body composition at the age of nine years in a cohort of children whose mothers had participated in a study of nutrition during pregnancy.
Materials and methods
In 1991-2 Caucasian women aged ≥16 years with singleton pregnancies of <17 weeks' gestation were recruited at the Princess Anne Maternity Hospital in Southampton, UK; diabetics and those who had undergone hormonal treatment to conceive were excluded. In early (15 weeks of gestation) and late (32 weeks of gestation) pregnancy, we administered a lifestyle questionnaire to the women which included questions about their smoking habits, their weight before pregnancy and whether they took strenuous exercise, defined as sporting or other activity during which they got out of breath or sweaty. We measured their height in early pregnancy using a stadiometer. In late pregnancy, we measured mid-upper arm circumference on the left arm halfway between the acromion process of the humerus and the olecranon process of the ulna, with the arm relaxed alongside the body.(20) Measurements were taken to the nearest 0.1 cm. Weight was measured at 37 weeks' gestation. We used this information and data on pre-pregnant weight to calculate weight gain during pregnancy. We also categorized women as having gained adequate, inadequate or excessive weight according to Institute of Medicine guidelines.(21) These guidelines recommend that women with a normal pre-pregnant BMI (19.8-26.0 kg/m2) should gain 12.5-18 kg, that women with a BMI of <19.8 kg/m2 should gain 12.5-18kg, that women with a BMI of 26.0-29.0 kg/m2 should gain 7-11kg and that women with a BMI >29.0 kg/m2 should gain at least 6.0kg. We used 11.5kg as the upper limit for this latter group of women,(22) as was done in a recent study of gestational weight gain.(23) Anthropometric data on the child were collected at birth. Gestational age was estimated from menstrual history and scan data. 559 children were followed-up at age nine months, when data on anthropometry and infant feeding were recorded.
When these children approached age nine years, we wrote to the parents of those still living in Southampton inviting the children to participate in a further study. Of 461 invited, 216 (47%) agreed to attend a clinic. Height was measured using a stadiometer and weight using digital scales (SECA Model No.835). The children underwent measurements of body composition by DXA (dual energy x-ray absorptiometry; Lunar DPX-L instrument using specific paediatric software; version 4.7c, GE Corporation, Madison, WI, USA). The instrument was calibrated every day and all scans were done with the children wearing light clothing. The short-term and long-term coefficients of variation of the instrument were 0.8% and 1.4% respectively.
Statistical analysis
Fat mass and lean mass were adjusted for height by calculating fat mass index and lean mass index. Following log-log regression analysis, (24) fat mass index was calculated as total body fat (kg)/(height (m))n and lean mass index as total lean body mass (kg)/(height (m))n where the power of n was 4.9 (fat mass index) and 2.2 (lean mass index) respectively. These values of n are those that gave the least correlation between the respective indices and height. To examine the influence of infant growth with the effect of birthweight removed, we created a conditional infant weight gain variable, unrelated to birthweight, by using linear regression with weight at age nine months as the dependent variable and birthweight as the independent variable and saving the standardised residuals. All anthropometric variables for mother and child are expressed as standard deviation scores (SDS), which were internally referenced and calculated for each sex separately. Birthweight SDS were adjusted for gestational age at birth.
We used t or χ2-tests to examine the characteristics of the participants at the nine-year follow-up. Linear regression was used to examine the relation between maternal and child characteristics and fat mass and lean mass indices, adjusting for age at examination. Where necessary variables were transformed using logarithms to satisfy statistical assumptions of normality.
The Local Research Ethics Committee approved the study. The children and their parents gave written informed consent.
Results
Table 1 shows the characteristics of the 216 participants who attended a clinic for examination compared to those who took part in the previous follow-up when they were aged nine months but were non-participants in the nine-year follow-up. Participants were similar to non-participants as regards sex, proportion born before 37 weeks' completed gestation, breast-feeding, maternal age, educational attainment, parity, smoking and exercise habits, body size and weight gain during pregnancy. Compared to non-participants, participants tended to have a lower mean weight at birth (3.31 kg vs 3.41 kg, p=0.06) and at age nine months (8.96 kg vs 9.16 kg, p=0.06); a lower proportion of them had mothers in manual occupational social classes (24.7% vs 35%, p=0.03), or had mothers who had pre-eclampsia during pregnancy (7.4% vs 7.9%, p=0.02).
Table 1.
Distribution of maternal and early life characteristics among children who were examined at age 9 years compared to those who were not examined
| Examined | Not examined | |||
|---|---|---|---|---|
| Characteristic | No, % or Mean, SD | No, % or Mean, SD | ||
| Mother | ||||
| Maternal social class | ||||
| Manual | 55 | 24.7 | 120 | 35.0 |
| Non-manual | 168 | 75.3 | 223 | 65.0 |
| Maternal education | ||||
| < A levels | 128 | 58.8 | 220 | 64.3 |
| ≥ A levels | 89 | 41.2 | 122 | 35.7 |
| Primiparous | ||||
| Yes | 122 | 56.5 | 173 | 50.4 |
| No | 94 | 43.5 | 170 | 49.6 |
| Smoked in early pregnancy | ||||
| Yes | 48 | 22.2 | 91 | 26.5 |
| No | 168 | 77.8 | 252 | 73.5 |
| Smoked in late pregnancy | ||||
| Yes | 40 | 18.8 | 83 | 24.3 |
| No | 173 | 81.2 | 259 | 75.7 |
| Exercised strenuously in early pregnancy | ||||
| Yes | 37 | 17.2 | 66 | 19.2 |
| No | 178 | 82.8 | 277 | 80.8 |
| Exercised strenuously in late pregnancy | ||||
| Yes | 48 | 22.5 | 65 | 19.0 |
| No | 165 | 77.5 | 277 | 81.0 |
| Pre-eclampsia in pregnancy | ||||
| Yes | 16 | 7.40 | 29 | 7.90 |
| No | 200 | 92.6 | 338 | 92.1 |
| Age, y | 26.8 | 4.7 | 26.2 | 4.9 |
| Height, m | 1.63 | 0.66 | 1.64 | 0.64 |
| Prepregnancy BMI, kg/m2 | 22.7 | 3.88 | 23.3 | 4.55 |
| Pregnancy weight gain, kg | 13.4 | 6.06 | 13.6 | 6.18 |
| Pregnancy weight gain category (Institute of Health recommendations) |
||||
| Inadequate | 95 | 44.2 | 143 | 39.6 |
| Adequate | 60 | 27.9 | 111 | 33.0 |
| Excessive | 60 | 29.3 | 99 | 27.4 |
| Mid-upper arm circumference in late pregnancy, cm |
27.1 | 3.23 | 27.5 | 3.38 |
| Child | ||||
| Sex | ||||
| Male | 113 | 52.3 | 188 | 54.8 |
| Female | 103 | 47.7 | 155 | 45.2 |
| Duration of breast-feeding | ||||
| Never | 65 | 30.1 | 103 | 30.0 |
| ≤ 4 months | 96 | 44.4 | 141 | 41.1 |
| > 4 months | 55 | 25.5 | 99 | 28.9 |
| Premature | 16 | 7.41 | 22 | 5.95 |
| Birthweight, kg | 216 | 3.31 (6.25) | 343 | 3.41 (5.31) |
| Weight at 9 m, kg | 215 | 8.96 (1.18) | 343 | 9.16 (1.13) |
The anthropometric characteristics of the participants at the time of the nine-year follow-up are described in Table 2. Boys differed significantly from girls in being taller, having a higher lean mass and a lower fat mass (all p<0.01). There was no difference between them in weight.
Table 2.
Anthropometric characteristics of the participants
| Boys (n=115) | Girls (n=101) | |
|---|---|---|
| Height, m* | 1.31 (0.06) | 1.29 (0.06) |
| Weight, kg§ | 27.7 (25.5-31.6) | 28.1 (25.1-32.5) |
| Lean mass, kg§ | 22.2 (20.6-24.4) | 20.0 (18.5-21.6) |
| Fat mass, kg§ | 4.73 (3.62-6.83) | 6.97 (5.22-9.43) |
| Lean mass index (kg/m2)* | 12.3 (0.87) | 11.5 (0.85) |
| Fat mass index (kg/m5)§ | 1.25 (1.01-1.69) | 1.96 (1.48-2.57) |
| Age, y* | 8.89 (0.29) | 8.85 (0.28) |
values are means (standard deviation)
values are medians (interquartile range)
Children who were taller at the age of nine years had a higher fat and lean mass. The correlation between height and fat mass was r=0.49 in boys and r=0.44 in girls, while that between height and lean mass was r=0.84 and r=0.78, respectively, all p<0.001). Height at age nine was not associated with maternal mid-upper arm circumference, pre-pregnant BMI or with the total amount of weight put on during pregnancy, but it did vary according to the gestational weight gain categories recommended by the Institute of Health: mean (SD) height of children whose mothers had put on an inadequate amount of weight given their pre-pregnant BMI, was 1.29 (0.6) m compared to 1.32 (0.7) m in those whose mothers had put on an excessive amount of weight given their pre-pregnant BMI (p=0.03). Children with a greater weight gain in infancy, conditional on their weight at birth, also tended to be taller (r=0.43 in boys, r=0.30 in girls, p<0.001 and p=0.002, respectively). In order to evaluate the relations between maternal size and early growth on the children's body composition uninfluenced by their current height, we therefore used height-adjusted indices of fat and lean mass in the following analyses.
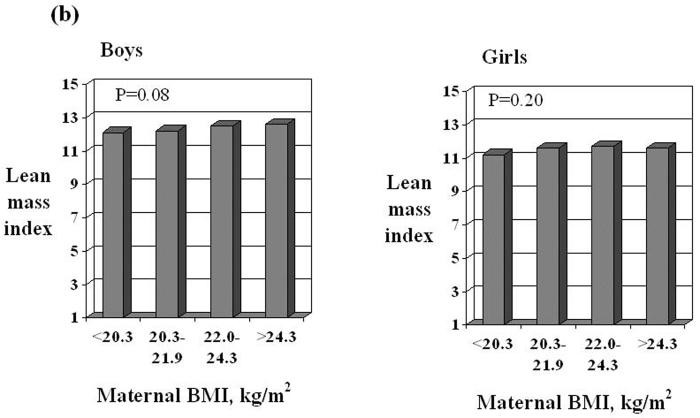
In age-adjusted linear regression analyses of boys and girls separately, fat mass index tended to be greater in children whose mothers had a larger mid-upper arm circumference in late pregnancy or a greater pre-pregnant BMI (Table 3). Lean mass index tended to be greater in those whose mother had a larger mid-upper arm circumference or a greater pre-pregnant BMI. We found no significant associations in either sex between fat or lean mass indices and maternal height or the amount of weight women gained during pregnancy, whether expressed as a continuous variable or examined according to Institute of Medicine categories. In boys, but not in girls (p for interaction term = 0.018), lean mass index was greater in those who had weighed more at birth. There was a similar, though weaker, association in boys between lean mass index and weight at age 9 months, though weight gain by 9 months conditional on birthweight was not associated with lean mass index. Maternal smoking either in early or late pregnancy was associated with a lower lean mass index in boys. In girls, these relations were weaker, though interaction terms were not statistically significant. In girls only, being breastfed for over four months was associated with significant reductions in fat mass index compared to children who were never breastfed. No such relation was seen in boys (p for interaction term = 0.03). There were no statistically significant associations in either sex between body composition and maternal social class, educational qualifications or exercise during pregnancy (data not shown).
Table 3.
Age-adjusted associations between maternal and early life characteristics and body composition at age 9 years
| Fat mass index SDS | Lean mass index SDS | |
|---|---|---|
| Boys | ||
| Maternal height SDS§ | −0.03 (−0.20 to 0.13) | 0.11 (−0.05 to 0.26) |
| Mid-upper arm circ. SDS | 0.24 (0.05 to 0.43)* | 0.16 (−0.02 to 0.34) |
| Pre-pregnant BMI SDS | 0.16 (−0.04 to 0.35) | 0.22 (0.04 to 0.41)* |
| Weight gain in pregnancy SDS | 0.07 (−0.11 to 0.25) | −0.06 (−0.23 to 0.11) |
| Pregnancy weight gain categories | ||
| Inadequate | Ref | Ref |
| Adequate | 0.11 (−0.34 to 0.55) | −0.19 (−0.61 to 0.23) |
| Excessive | 0.18 (−0.25 to 0.61) | −0.12 (−0.53 to 0.29) |
| Smoked | ||
| early pregnancy | 0.35 (−0.12 to 0.82) | −0.45 (−0.89 to −0.01)* |
| late pregnancy | 0.24 (−0.16 to 0.65) | −0.39 (−0.77 to −0.02)* |
| Birthweight SDS | 0.09 (−0.10 to 0.28) | 0.37 (0.20 to 0.53)*** |
| Weight at 9 m SDS | 0.07 (−0.01 to 0.15) | 0.23 (0.07 to 0.39)** |
| Conditional infant weight gain SDS | 0.16(−0.01 to 0.34) | 0.08 (−0.08 to 0.24) |
| Breastfed | ||
| Never | Ref | Ref |
| ≤ 4 months | 0.09 (−0.36 to 0.54) | 0.29 (−0.13 to 0.72) |
| > 4 months | 0.06 (−0.43 to 0.55) | 0.23 (−0.23 to 0.69) |
| Girls | ||
| Maternal height SDS | −0.11 (−0.29 to 0.08) | −0.05 (−0.25 to 0.14) |
| Mid-upper arm circ. SDS | 0.42 (0.28 to 0.55)*** | 0.18 (0.01 to 0.34)* |
| Pre-pregnant BMI SDS | 0.39 (0.26 to 0.53)*** | 0.15 (−0.1 to 0.32) |
| Weight gain in pregnancy SDS | 0.01 (−0.17 to 0.17) | −0.02 (−0.20 to 0.15) |
| Pregnancy weight gain categories | ||
| Inadequate | Ref | Ref |
| Adequate | 0.06 (−0.34 to 0.45) | −0.16 (−0.59 to 0.26) |
| Excessive | 0.15 (−0.27 to 0.56) | −0.01 (−0.44 to 0.44) |
| Smoked | ||
| early pregnancy | −0.06(−0.45 to 0.32) | −0.03 (−0.44 to 0.38) |
| late pregnancy | −0.09 (−0.43 to 0.24) | −0.15 (−0.51 to 0.21) |
| Birthweight SDS | −0.08 (−0.24 to 0.08) | 0.08 (−0.09 to 0.25) |
| Weight at 9 m SDS | −0.06 (−0.25 to 0.14) | −0.04 (−0.25 to 0.16) |
| Conditional infant weight gain SDS | 0.01 (−0.18 to 0.21) | −0.14 (−0.35 to 0.07) |
| Breastfed | ||
| Never | Ref | Ref |
| ≥ 4 months | −0.14 (−0.51 to 0.24) | −0.06 (−0.48 to 0.35) |
| > 4 months | −0.66 (−1.11 to 0.22)** | −0.48 (−0.97 to 0.01) |
p<0.001
p<0.01
p<0.05
SDS=standard deviation scores.
Maternal mid-upper arm circumference in late pregnancy was highly correlated with maternal pre-pregnant BMI (in boys, r=0.79, p<0.001, n=115, in girls, r=0.82, p<0.001, n=101). To avoid problems with collinearity, we performed multivariate linear regression analyses of these variables separately (Table 4). Model 1 includes mid-upper arm circumference in late pregnancy. Model 2 includes pre-pregnant BMI. Regression coefficients are shown adjusted for age and for all other variables in the model.
Table 4.
Multivariate-adjusted associations between maternal and early life characteristics and body composition at age 9 years. Model 1 includes maternal mid-upper arm circumference in late pregnancy. Model 2 includes maternal pre-pregnant BMI
| Fat mass index SDS | Lean mass index SDS | |
|---|---|---|
| Characteristic | Regression coefficient (95% CI) adjusted for age and all characteristics in the model |
|
| Model 1 | ||
| Boys | ||
| Mid-upper arm circ. SDS | 0.26 (0.06 to 0.46)* | 0.11 (−0.08 to 0.31) |
| Maternal height SDS§ | −0.12 (−0.28 to 0.05) | 0.01 (−0.16 to 0.16) |
| Weight gain in pregnancy SDS | −0.01 (−0.18 to 0.18) | −0.06 (−0.24 to 0.11) |
| Smoked in pregnancy | 0.57 (0.10 to 1.50)* | −0.15 (−0.61 to 0.30) |
| Birthweight SDS | 0.14 (−0.06 to 0.33) | 0.32 (0.13 to 0.50)*** |
| Conditional infant weight gain SDS | 0.17 (0.01 to 0.34* | 0.06 (−0.10 to 0.22) |
| Breastfed | ||
| Never | Ref | Ref |
| ≤ 4 months | 011 (−0.32 to 0.54) | 0.35 (−0.06 to 0.77) |
| > 4 months | 0.12 (−0.36 to 0.60) | 0.29 (−0.17 to 0.75) |
| Girls | ||
| Mid-upper arm circ. SDS | 0.44 (0.31 to 0.57)*** | 0.14 (−0.03 to 0.32) |
| Maternal height SDS | −0.19 (−0.37 to −0.01)* | −0.18 (−0.41 to 0.06) |
| Weight gain in pregnancy SDS | −0.09 (−0.24 to 0.06) | −0.09 (−0.29 to 0.10) |
| Smoked in pregnancy | 0.04 (−0.28 to 0.35) | 0.13 (−0.29 to 0.54) |
| Birthweight SDS | −0.04 (−0.21 to 0.13) | 0.19 (−0.03 to 0.42) |
| Conditional infant weight gain SDS | −0.08 (−0.26 to 0.09) | −0.17 (−0.40 to 0.06) |
| Breastfed | ||
| ≤ 4 months | 0.05 (−0.29 to 0.39) | 0.16 (−0.29 to 0.61) |
| > 4 months | −0.52 (−0.92 to −0.12)* | −0.34 (−0.86 to 0.18) |
| Model 2 | ||
| Boys | ||
| Pre-pregnant BMI SDS | 0.26 (0.04 to 0.48)* | 0.19 (−0.02 to 0.40) |
| Maternal height SDS§ | −0.07 (−0.25 to 0.10) | 0.03 (−0.13 to 0.20) |
| Weight gain in pregnancy SDS | 0.09 (−0.09 to 0.26) | −0.05 (−0.24 to 0.13) |
| Smoked in pregnancy | 0.60 (0.10 to 1.10)* | −0.10 (−0.58 to 0.37) |
| Birthweight SDS | 0.16 (−0.04 to 0.35) | 0.33 to (0.14 to 0.52)*** |
| Conditional infant weight gain SDS | 0.20 (0.02 to 0.38)* | 0.09 (−0.08 to 0.25) |
| Breastfed | ||
| Never | Ref | Ref |
| ≤ 4 months | 0.11 (−0.33 to 0.54) | 0.35 (−0.06 to 0.76) |
| > 4 months | 0.14 (−0.35 to 0.62) | 0.31 (−0.15 to 0.77) |
| Girls | ||
| Pre-pregnant BMI SDS | 0.42 (0.29 to 0.56)*** | 0.12 (−0.06 to 0.30) |
| Maternal height SDS | −0.06 (−0.24 to 0.11) | −0.05 (−0.28 to 0.17) |
| Weight gain in pregnancy SDS | 0.11 (−0.04 to 0.26) | −0.01 (−0.20 to 0.19) |
| Smoked in pregnancy | 0.05 (−0.28 to 0.37) | 0.05 (−0.38 to 0.47) |
| Birthweight SDS | −0.08 (−0.23 to 0.06) | 0.08 (−0.11 to 0.27) |
| Conditional infant weight gain SDS | −0.08 (−0.26 to 0.09) | −0.19 (−0.42 to 0.04) |
| Breastfed | ||
| Never | Ref | Ref |
| ≤ 4 months | 0.08 (−0.27 to 0.43) | 0.01 (−0.44 to 0.46) |
| > 4 months | −0.53 (−0.93 to −0.12)* | −0.48 (−1.01 to 0.05) |
p<0.001
p<0.01
p<0.05
SDS=standard deviation scores.
After adjustment for age, birthweight, conditional infant weight gain, duration of breastfeeding, maternal height, weight gain and smoking in pregnancy, a larger maternal mid-upper arm circumference in late pregnancy remained an independent predictor of greater fat mass index in both boys and girls (Table 4, model 1). For one SD increase in maternal mid-upper arm circumference in late pregnancy, fat mass index rose by 0.26 (95% CI 0.06 to 0.46) of a SD in boys and by 0.44 (95% CI 0.31 to 0.57) of a SD in girls. When maternal pre-pregnant BMI was used in the analysis instead of mid-upper arm circumference in late pregnancy (Table 4, model 2), the results were similar: for a one SD increase in pre-pregnant BMI, fat mass rose by 0.26 (95% CI 0.04 to 0.48) of an SD in boys and by 0.42 (95% CI 0.29 to 0.56) of an SD in girls.
In boys, other independent predictors of a greater fat mass index at age 9 years were greater conditional weight gain by age nine months and being exposed to maternal smoking in pregnancy. The relation in boys between lean mass and maternal smoking in pregnancy ceased to be statistically significant in multivariate analysis due to adjustment for birthweight. In girls, being breastfed for over four months remained a significant independent predictor of a lower fat mass index compared to girls who were never breastfed. In both sexes, greater lean mass index at age nine years was associated with higher birthweight, though this relation was of borderline significance in girls. In total, 15 children had a birthweight of <2.5kg. There were weak inverse associations of borderline significance between low birthweight and both maternal pre-pregnant BMI and mid-upper arm circumference in late pregnancy. In sex-adjusted analyses, low birth weight was associated with lower lean mass index at age 9 years (−0.50, 95% CI −0.95 to −0.05 of a SD), but was not associated with fat mass index.
Exclusion of children who were premature (n=16) or whose mothers had pre-eclampsia during pregnancy (n=16) had little effect on the results.
We found no associations in the multivariate analyses between the height-adjusted fat or lean mass indices and maternal weight gain in pregnancy, whether expressed as a continuous variable (shown in Table 4) or as the Institute of Medicine weight gain categories (data not shown). However, there was evidence that women who put on an excessive amount of weight in pregnancy, according to the Institute of Medicine weight gain categories, had a considerably larger mid-upper arm circumference in late pregnancy – itself a strong predictor of fat mass in the child. Compared to women whose weight gain was inadequate during pregnancy, women whose weight gain was excessive had a mid-upper arm circumference in late pregnancy that was 0.95 (95% CI 0.65 to 1.24) of a standard deviation higher (p<0.0001). In order to gauge whether the associations found here between mid-upper arm circumference in late pregnancy and fat mass index in the child were dependent on those children whose mothers gained an excessive amount of weight during pregnancy, we repeated our analysis removing these individuals. This exclusion had little effect.
In summary, fat mass index was greater in children whose mothers had a larger mid-upper arm circumference in late pregnancy or a higher pre-pregnant body mass index (Figure 1). Other independent predictors of greater fat mass were smoking in pregnancy and greater conditional weight gain in infancy (in boys) and not being breastfed (in girls). Lean mass was greater in children who had weighed more at birth.
Figure 1 legend.
Fat mass index and lean mass index in boys and girls according to quarters of the distribution of maternal mid-upper arm circumference in late pregnancy or pre-pregnant BMI. Values are adjusted for age, duration of breastfeeding, birthweight, conditional infant weight gain, maternal height, smoking and total weight gain in pregnancy. P values were calculated in multiple linear regression.
Discussion
In this cohort of children whose normal, non-diabetic mothers had been studied during pregnancy, fat mass index at age nine years was greater in children whose mothers had a larger mid-upper arm circumference in late pregnancy or a higher pre-pregnant BMI. Mothers who gained an excessive amount of weight in pregnancy, (21)had a larger mid-upper arm circumference in late pregnancy, but excessive weight gain in pregnancy did not explain the association between maternal mid-upper arm circumference and offspring fat mass index. Greater weight gain during pregnancy has recently been linked with increased adiposity in the child,(23) but we found no evidence that weight gain during pregnancy was associated with childhood fat mass, independently of current height. Our results suggest that maternal overnutrition before and during pregnancy - but not excessive gestational weight gain - may have a persisting and long-term influence on the offspring's adiposity.
One explanation for these findings may be genetic. Evidence from twin studies suggest that genetic influences account for some of the phenotypic variation in BMI(25) and percent body fat. (26) Another explanation may lie in the maternal metabolic environment during pregnancy. Women who are overweight or obese before conception have been shown to experience a marked decrease in insulin sensitivity during pregnancy.(27) This results in greater availability of lipids and glucose for the fetus and could lead to higher fetal concentrations of insulin(28;29) and leptin.(30;31) Fetuses with higher cord concentrations of insulin and leptin tend to have a higher fat mass at birth.(32;33) The long-term effects on offspring adiposity of exposure to maternal overnutrition during pregnancy are not yet clear, but there is some evidence from experimental studies in animals to suggest that increases in fetal nutrient supply may influence the development of appetite regulation and adipocyte metabolism.(31)
The mean pre-pregnancy BMI of the mothers in this study, recruited in 1991/2, was at 22.7 kg/m2 similar to that found in a prospective study of pregnancy in Australia where women were recruited between 1981 and 1984.(18) However, rates of obesity among women at the start of pregnancy have increased markedly in recent years.(34;35) Contemporary cohorts of pregnant women contain far higher proportions of women who are overweight or obese at the start of pregnancy(23) than our comparatively healthy sample.
Although evidence from several systematic reviews suggests that breastfeeding may protect against later obesity,(10;11) the few studies that have examined its relation with direct measures of childhood adiposity, obtained by DXA, found no statistically significant associations.(12;36) In the present study, we found that girls who had been breastfed for over four months had significantly lower fat mass at the age of nine years than those who were never breastfed, but no such relation was present in boys. Although in these data there was no association between conditional weight gain in infancy and duration of breastfeeding, there was a trend in girls towards reduced weight gain with increasing duration of breastfeeding. Consistent with other studies,(8;9;15) we found that children with a lower weight gain in the first nine months after birth tended to have a lower fat mass at age nine years. This association was only statistically significant in boys, but similar, though weaker, trends were seen in girls. Greater weight at birth was associated with higher lean mass at age nine years. Similar observations have been reported both in other cohorts of children(37) and in older populations.(38;39)
Although some studies of children have found a higher percent body fat in boys than in girls,(40)findings from other studies have found the reverse. In the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort at age 9,(37), Project Heartbeat at age 8,(41) and in studies of 6-year old children in Denmark(42) and 8-year olds in Sweden.(43) fat mass and percent body fat were consistently greater in girls than in boys. There was a similar pattern in the present study.
Our study has a number of limitations. Firstly, we are not able to identify with certainty whether the relations we found between measures of maternal size in pregnancy – pre-pregnant BMI and mid-upper arm circumference - and fat mass in childhood were in fact due to maternal adiposity during pregnancy. Both measures provide an indicator of nutritional status,(44) but they reflect both lean and fat mass. It is also possible that the associations found were due not to maternal nutritional status during pregnancy, but rather to maternally-transmitted genes, or to the influence of maternal lifestyle and diet on that of her child. Secondly, our data on weight prior to pregnancy was self-reported. It is possible that these self-reports may have introduced some inaccuracy into our measures of pre-pregnant BMI and weight gain during pregnancy. Thirdly, we were unable to follow up all the children in the cohort. Some had moved away from the area and some declined to participate. Children who took part in the nine-year follow-up were more likely to have mothers from non-manual occupational classes, but mean maternal mid-upper arm circumference and BMI were similar in the groups who did and did not participate. We think it unlikely that bias will have been introduced. Another limitation was the size of our study (115 boys and 101 girls). It is possible that the differing strength of some associations between the sexes was due to lack of statistical power. Finally, we had no information on pubertal status. It is possible that the earlier onset of puberty in girls may help to account for the differences in findings by sex.
One explanation for our findings that fat mass in nine-year-old children was increased in those whose mothers had a greater mid-upper arm circumference in late pregnancy or a higher pre-pregnant BMI is that maternal overnutrition before and during pregnancy – though not excessive weight gain - may have a long-term, persisting influence on the adiposity of the child. However, maternally transmitted genetic factors and the effect of maternal lifestyle on that of her child could also explain our results. The extent to which the association is due to maternal adiposity acting specifically during pregnancy cannot be determined in this study.
Acknowledgments
We thank the children and their families for participating in the study and the research nurses who collected the data. We are also grateful to the reviewers of this paper for their helpful and constructive comments.
Sources of funding
The study was funded by the Medical Research Council, WellChild (previously Children Nationwide), the Cohen Trust, the Arthritis Research Campaign and the National Osteoporosis Society.
Footnotes
Disclosure summary: The authors have nothing to declare
References
- 1.Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev. 2004;5(Suppl 1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 2.Falkner B, Gidding SS, Ramirez-Garnica G, Wiltrout SA, West D, Rappaport EB. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195–200. doi: 10.1016/j.jpeds.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Lawlor DA, Riddoch CJ, Page AS, Anderssen SA, Froberg K, Harro M, Stansbie D, Smith GD. The association of birthweight and contemporary size with insulin resistance among children from Estonia and Denmark: findings from the European Youth Heart Study. Diabet Med. 2005;22:921–930. doi: 10.1111/j.1464-5491.2005.01551.x. [DOI] [PubMed] [Google Scholar]
- 4.Gale CR, Jiang B, Robinson SM, Godfrey KM, Law CM, Martyn CN. Maternal diet during pregnancy and carotid intima-media thickness in children. Arterioscler Thromb Vasc Biol. 2006;26:1877–1882. doi: 10.1161/01.ATV.0000228819.13039.b8. [DOI] [PubMed] [Google Scholar]
- 5.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 6.Lawlor DA, Chaturvedi N. Treatment and prevention of obesity--are there critical periods for intervention? Int J Epidemiol. 2006;35:3–9. doi: 10.1093/ije/dyi309. [DOI] [PubMed] [Google Scholar]
- 7.Wardle J, Brodersen NH, Cole TJ, Jarvis MJ, Boniface DR. Development of adiposity in adolescence: five year longitudinal study of an ethnically and socioeconomically diverse sample of young people in Britain. BMJ. 2006;332:1130–1135. doi: 10.1136/bmj.38807.594792.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekelund U, Ong K, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 10.Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity--a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247–1256. doi: 10.1038/sj.ijo.0802758. [DOI] [PubMed] [Google Scholar]
- 11.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162:397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 12.Burdette HL, Whitaker RC, Hall WC, Daniels SR. Breastfeeding, introduction of complementary foods, and adiposity at 5 y of age. Am J Clin Nutr. 2006;83:550–558. doi: 10.1093/ajcn.83.3.550. [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 14.Kensara OA, Wootton SA, Phillips DI, Patel M, Jackson AA, Elia M. Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr. 2005;82:980–987. doi: 10.1093/ajcn/82.5.980. [DOI] [PubMed] [Google Scholar]
- 15.Wells JC, Hallal PC, Wright A, Singhal A, Victora CG. Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. Int J Obes (Lond) 2005;29:1192–1198. doi: 10.1038/sj.ijo.0803054. [DOI] [PubMed] [Google Scholar]
- 16.Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003;77:726–730. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]
- 17.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–298. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- 18.Lawlor DA, Smith GD, O'Callaghan M, Alati R, Mamun AA, Williams GM, Najman JM. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165:418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 19.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Godfrey KM, Barker DJ, Robinson S, Osmond C. Maternal birthweight and diet in pregnancy in relation to the infant's thinness at birth. Br J Obstet Gynaecol. 1997;104:663–667. doi: 10.1111/j.1471-0528.1997.tb11975.x. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine . Nutrition during pregnancy. Washington DC: National Academy Press; 1990. [Google Scholar]
- 22.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 23.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322–328. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells JC, Cole TJ. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord. 2002;26:947–952. doi: 10.1038/sj.ijo.0802027. [DOI] [PubMed] [Google Scholar]
- 25.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 26.Faith MS, Pietrobelli A, Nunez C, Heo M, Heymsfield SB, Allison DB. Evidence for independent genetic influences on fat mass and body mass index in a pediatric twin sample. Pediatrics. 1999;104:61–67. doi: 10.1542/peds.104.1.61. [DOI] [PubMed] [Google Scholar]
- 27.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 28.Godfrey KM, Robinson S, Hales CN, Barker DJ, Osmond C, Taylor KP. Nutrition in pregnancy and the concentrations of proinsulin, 32-33 split proinsulin, insulin, and C-peptide in cord plasma. Diabet Med. 1996;13:868–873. doi: 10.1002/(SICI)1096-9136(199610)13:10<868::AID-DIA261>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Soltani K, Bruce C, Fraser RB. Observational study of maternal anthropometry and fetal insulin. Arch Dis Child Fetal Neonatal Ed. 1999;81:F122–F124. doi: 10.1136/fn.81.2.f122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cripps RL, Martin-Gronert MS, Ozanne SE. Fetal and perinatal programming of appetite. Clin Sci (Lond) 2005;109:1–11. doi: 10.1042/CS20040367. [DOI] [PubMed] [Google Scholar]
- 31.McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth: implications for the early programming of adult obesity. Reproduction. 2006;131:415–427. doi: 10.1530/rep.1.00303. [DOI] [PubMed] [Google Scholar]
- 32.Javaid MK, Godfrey KM, Taylor P, Robinson SM, Crozier SR, Dennison EM, Robinson JS, Breier BR, Arden NK, Cooper C. Umbilical cord leptin predicts neonatal bone mass. Calcif Tissue Int. 2005;76:341–347. doi: 10.1007/s00223-004-1128-3. [DOI] [PubMed] [Google Scholar]
- 33.Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, Hsu YW, Ho SC, Chu CH. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol (Oxf) 2004;61:88–93. doi: 10.1111/j.1365-2265.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- 34.Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG. 2007;114:187–194. doi: 10.1111/j.1471-0528.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in Pre-pregnancy Obesity in Nine States, 1993-2003. Obesity (Silver Spring) 2007;15:986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 36.Tulldahl J, Pettersson K, Andersson SW, Hulthen L. Mode of infant feeding and achieved growth in adolescence: early feeding patterns in relation to growth and body composition in adolescence. Obes Res. 1999;7:431–437. doi: 10.1002/j.1550-8528.1999.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 37.Rogers IS, Ness AR, Steer CD, Wells JC, Emmett PM, Reilly JR, Tobias J, Smith GD. Associations of size at birth and dual-energy X-ray absorptiometry measures of lean and fat mass at 9 to 10 y of age. Am J Clin Nutr. 2006;84:739–747. doi: 10.1093/ajcn/84.4.739. [DOI] [PubMed] [Google Scholar]
- 38.Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab. 2001;86:267–272. doi: 10.1210/jcem.86.1.7155. [DOI] [PubMed] [Google Scholar]
- 39.Sayer AA, Syddall HE, Dennison EM, Gilbody HJ, Duggleby SL, Cooper C, Barker DJ, Phillips DI. Birth weight, weight at 1 y of age, and body composition in older men: findings from the Hertfordshire Cohort Study. Am J Clin Nutr. 2004;80:199–203. doi: 10.1093/ajcn/80.1.199. [DOI] [PubMed] [Google Scholar]
- 40.Washino K, Takada H, Nagashima M, Iwata H. Significance of the atherosclerogenic index and body fat in children as markers for future, potential coronary heart disease. Pediatr Int. 1999;41:260–265. doi: 10.1046/j.1442-200x.1999.01065.x. [DOI] [PubMed] [Google Scholar]
- 41.Dai S, Labarthe DR, Grunbaum JA, Harrist RB, Mueller WH. Longitudinal analysis of changes in indices of obesity from age 8 years to age 18 years. Project HeartBeat! Am J Epidemiol. 2002;156:720–729. doi: 10.1093/aje/kwf109. [DOI] [PubMed] [Google Scholar]
- 42.Hasselstrom H, Karlsson KM, Hansen SE, Gronfeldt V, Froberg K, Andersen LB. Sex differences in bone size and bone mineral density exist before puberty. The Copenhagen School Child Intervention Study (CoSCIS) Calcif Tissue Int. 2006;79:7–14. doi: 10.1007/s00223-006-0012-8. [DOI] [PubMed] [Google Scholar]
- 43.Dencker M, Thorsson O, Karlsson MK, Linden C, Eiberg S, Wollmer P, Andersen LB. Daily physical activity related to body fat in children aged 8-11 years. J Pediatr. 2006;149:38–42. doi: 10.1016/j.jpeds.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, Illinois: Human Kinetics Publishers, Inc.; 1988. [Google Scholar]