Summary
The nematode Caenorhabditis elegans provides numerous experimental advantages for developing an integrative molecular understanding of physiological processes and has proven to be a valuable model for characterizing Ca2+ signaling mechanisms. This review will focus on the role of Ca2+ release activated Ca2+ (CRAC) channel activity in function of the worm gonad and intestine. Inositol 1,4,5-trisphosphate (IP3)-dependent oscillatory Ca2+ signaling regulates contractile activity of the gonad and rhythmic posterior body wall muscle contraction (pBoc) required for ovulation and defecation, respectively. The C. elegans genome contains a single homolog of both STIM1 and Orai1, proteins required for CRAC channel function in mammalian and Drosophila cells. C. elegans STIM-1 and ORAI-1 are coexpressed in the worm gonad and intestine and give rise to robust CRAC channel activity when coexpressed in HEK293 cells. STIM-1 or ORAI-1 knockdown causes complete sterility demonstrating that the genes are essential components of gonad Ca2+ signaling. Knockdown of either protein dramatically inhibits intestinal cell CRAC channel activity, but surprisingly has no effect on pBoc, intestinal Ca2+ oscillations or intestinal ER Ca2+ store homeostasis. CRAC channels thus do not play obligate roles in all IP3-dependent signaling processes in C. elegans. Instead, we suggest that CRAC channels carry out highly specialized and cell specific signaling roles and that they may function as a failsafe mechanism to prevent Ca2+ store depletion under pathophysiological and stress conditions.
1. Introduction
Non-mammalian model organisms such as E. coli, Saccharomyces, Caenorhabditis elegans, Drosophila, zebrafish and the plant Arabidopsis provide numerous experimental advantages for defining the molecular bases of complex physiological processes. C. elegans provides a particularly striking example of the experimental utility of non-mammalian model organisms [1;2]. Worms have a short life cycle, produce large numbers of offspring by sexual reproduction and can be cultured easily and inexpensively in the laboratory. Sexual reproduction occurs by self-fertilization in hermaphrodites or by mating with males. The reproductive and laboratory culture characteristics of C. elegans make it an exceptionally powerful model system for forward genetic analysis.
In addition to forward genetic tractability, C. elegans also has a fully sequenced and well-annotated genome. Genomic sequence and virtually all other biological data on this organism are assembled in readily accessible public databases (e.g., WormBase; http://www.wormbase.org). Numerous reagents including mutant worm strains and cosmid and YAC clones spanning the genome are freely available through public resources. Creation of transgenic worms is relatively easy, inexpensive and rapid. C. elegans gene expression can be specifically and potently targeted for knockdown using RNA interference (RNAi). Finally, C. elegans is a highly differentiated animal but is comprised of <1000 somatic cells. This relatively simple anatomy greatly facilitates the study of complex physiological processes.
2. IP3-dependent Ca2+ signaling processes in C. elegans
C. elegans has proven to be a valuable model for characterizing Ca2+ signaling mechanisms that control diverse physiological processes (reviewed in [3]). For the purpose of this review, we will focus on IP3-dependent Ca2+ signaling in the worm gonad and intestine, and on the role of Ca2+ release activated Ca2+ (CRAC) channel activity in these signaling pathways.
2.1 Gonad function
The gonad of adult hermaphrodite worms consists of two identical U-shaped arms connected via spermatheca to a common uterus [4;5](Figure 1A). Gonad arms are surrounded by thin, smooth muscle-like myoepithelial sheath cells. The distal portion of each arm contains germline nuclei that differentiate into either sperm or oocytes.
Figure 1.
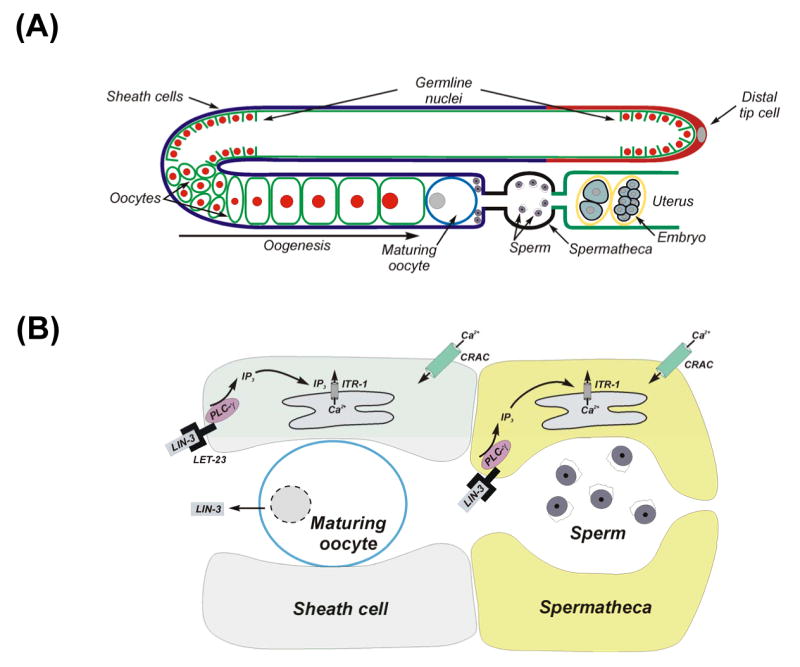
1,4,5-trisphosphate-dependent Ca2+ signaling regulates gonad function and fertility in C. elegans. (A) Schematic diagram of the gonad in an adult hermaphrodite worm. (B) Model illustrating IP3 and Ca2+ signaling events that regulate ovulation in C. elegans. Oocyte undergoing meiotic maturation releases LIN-3, an EGF-like molecule that binds to the LET-23 receptor expressed in sheath and spermatheca cells. LIN-3/LET-23 binding activates PLC-γ producing IP3 that in turn activates the IP3 receptor ITR-1. Release of ER Ca2+ and plasma membrane Ca2+ influx through CRAC channels encoded by orai-1 and regulated by stim-1 trigger sheath cell ovulatory contractions and opening of the gonad-spermatheca valve. Contracting sheath cells pull the spermatheca over the maturing oocyte.
During the fourth larval stage, germline nuclei develop into sperm that are stored in the spermatheca (Figure 1A). In adult worms, germline nuclei differentiate into oocytes. Newly formed oocytes undergo oogenesis, which is a period of intense biosynthetic activity and rapid and massive growth. Oocytes accumulate in the proximal gonad arm in a single-file row of graded developmental stages. These oocytes remain in diakinesis of prophase I of the meiotic cell cycle until they reach the most proximal position in the gonad arm. During the late stage of oogenesis, an oocyte located immediately adjacent to the spermatheca re-enters the meiotic cell cycle, a process termed meiotic maturation (Figure 1A). Within 5-6 min after maturation is initiated, the oocyte is ovulated into the spermatheca where it is fertilized.
Prior to ovulation, sheath cells contract weakly at a basal rate of 7-8 contractions/min. Basal sheath contractions are triggered by release of major sperm protein (MSP) from sperm stored in the spermatheca. MSP also triggers meiotic maturation in the most proximally located oocyte [6]. The EGF-like protein LIN-3 is released from the maturing oocyte and binds to its receptor LET-23 located on sheath and spermatheca cells. This in turn activates PLCγ and the resulting IP3 and Ca2+ signals induce ovulation by increasing the rate and force of sheath cell contractions and by triggering opening of the distal spermatheca [7-12] (Figure 1B).
2.2 Defecation rhythm
Defecation in C. elegans is a rhythmic process that occurs every 45-50 sec with little variation while the animal is feeding [13;14]. Defecation is mediated by sequential contraction of the posterior body wall muscles, anterior body wall muscles and enteric muscles.
In an effort to identify genes that regulate rhythmic biological processes, Iwasaki et al. [15] mutagenized worms and screened for animals with abnormal defecation cycles. Twelve dec (defecation cycle defective) genes were identified that when mutated cause the cycle to slow down or speed up. dec-4 encodes the single C. elegans IP3 receptor [16]. This gene is also termed itr-1 (inositol trisphosphate receptor). Loss-of-function mutations in itr-1 slow or eliminate the defecation cycle [16-18] whereas overexpression of the gene increases defecation rate [16].
Dal Santo et al. [16] microinjected fura-2 into intestinal epithelial cells and demonstrated that an intracellular Ca2+ spike occurs just prior to the initiation of posterior body wall muscle contraction (pBoc). Calcium oscillations are slowed or absent in animals with loss-of-function mutations in itr-1. Rhythmic, IP3–dependent intestinal Ca2+ oscillations are also observed in transgenic worms expressing cameleon protein in the intestine and in isolated intestines loaded with Ca2+ sensitive fluorescent probes [17;18]. Dal Santo et al. [16] have proposed that IP3-dependent Ca2+ oscillations in intestinal cells control the secretion of a signal that regulates contraction of the posterior body wall muscles that initiate the defecation cycle (Figure 2).
Figure 2.
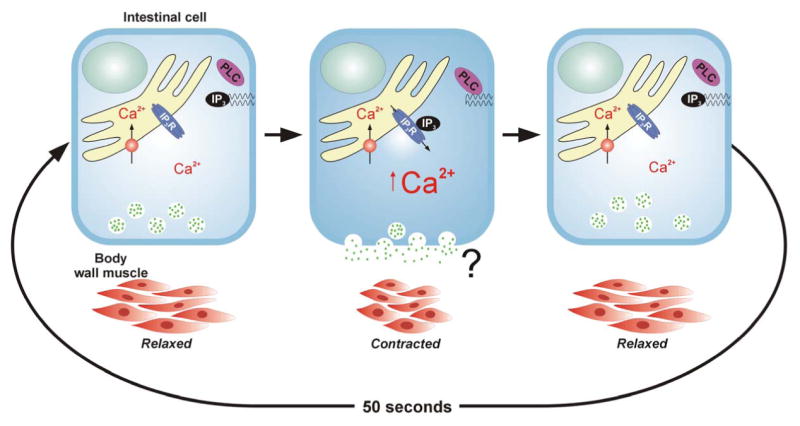
Model illustrating role of intestinal cell IP3 and intracellular Ca2+ in regulating defecation cycle. Cyclical elevation of cytoplasmic Ca2+ levels driven in part by IP3-dependent release of Ca2+ from intracellular stores triggers exocytic secretion from intestinal cells of an unidentified messenger that induces posterior body wall muscle contraction (pBoc).
3. Identification of a CRAC-like current in C. elegans intestinal cells
Our interest in the C. elegans intestine began with the recognition that it could potentially provide a powerful experimental system in which to develop an integrated genetic and molecular understanding of an oscillatory Ca2+ signaling pathway. Patch clamp electrophysiology is an important tool for defining the role of ion channels in generating Ca2+ oscillations. However, electrophysiological characterization of somatic cells in C. elegans is difficult due to the small size of the animal and the presence of a tough, pressurized cuticle that limits access. To circumvent this problem, we developed methods for primary culture of nematode embryo cells [19;20]. When placed in culture, embryo cells undergo terminal differentiation within 24 h. Individual cell types in culture are identified by isolating embryo cells from transgenic worms expressing cell specific GFP reporters.
Cultured intestinal cells express two Ca2+ currents [21]. One of these currents, ORCa (Outwardly Rectifying Calcium), displays strong outward rectification, inhibition by intracellular Mg2+, and insensitivity to intracellular Ca2+ store depletion. IORCa resembles the Mg2+-inhibited current (MIC), Mg2+-nucleotide regulated metal ion current (MagNUM), and TRPM7 currents studied in mammalian cells (e.g., [22-25]).
C. elegans intestinal cells also express a store-operated Ca2+ channel (SOCC) current [21]. SOCC shares many characteristics with vertebrate and Drosophila CRAC channels (reviewed in [26]). These characteristics include activation by passive and active depletion of endoplasmic reticulum (ER) Ca2+ stores, strong inward rectification, high selectivity for Ca2+ over monovalent cations, and lack of voltage dependent gating (Figure 3).
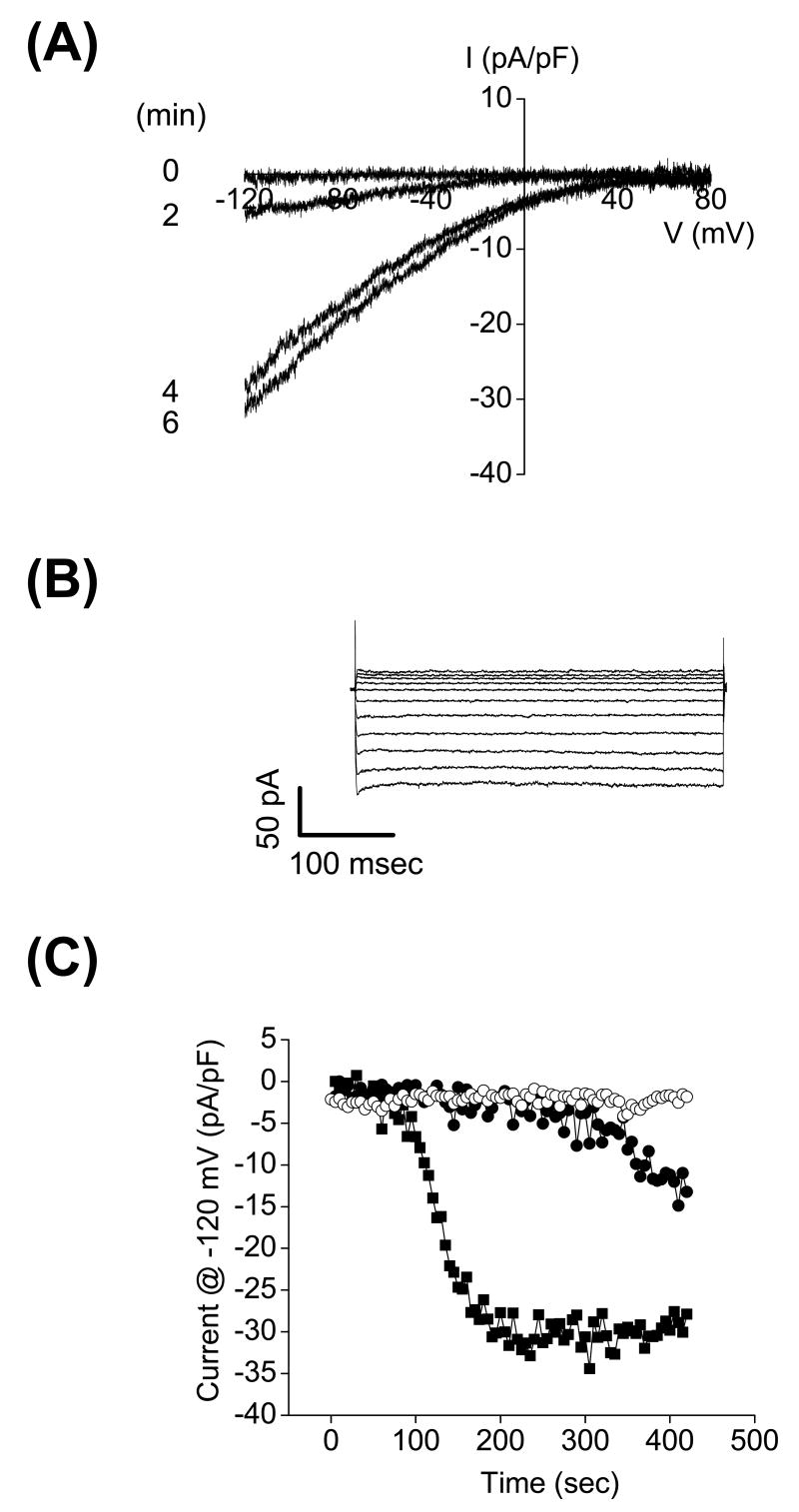
Figure 3.
Activation of CRAC-like current in cultured C. elegans intestinal cells. (A) An inwardly rectifying CRAC-like current activates when Ca2+ stores are depleted by inclusion of 10 μM IP3 in the patch pipette solution. Currents were elicited by ramping membrane voltage from −120 mV to +80 mV at 200 mV/sec every 5 sec. (B) Inwardly rectifying whole-cell currents elicited by stepping membrane voltage from −120 mV to +80 mV from a holding potential of 0 mV. Steps were 400 msec in duration. Currents were measured after store depletion induced activation was complete. (C) Changes in whole cell current observed in the absence of store depletion (open circles), during passive store depletion (solid circles), and during active store depletion induced by IP3 (solid squares).
4. STIM1 and Orai1 homologs in C. elegans
Two recent discoveries have dramatically advanced our understanding of the molecular basis of CRAC channel function and store-operated Ca2+ entry (SOCE). RNA interference screening in Drosophila S2 cells first identified stromal interaction molecule 1 (STIM1) as an essential component of CRAC channel activation [27]. Studies from several laboratories have established that Drosophila and human STIM1 homologs function as ER Ca2+ sensors [28-31]. In response to Ca2+ store depletion, STIM1 undergoes redistribution from a diffuse ER localization to a punctate localization [28;30;32-34] that corresponds to sites of ER-plasma membrane contact [32]. This redistribution in turn activates CRAC channels and SOCE [28-32;35]. The sites of punctate STIM1 localization also appear to be sites of localized Ca2+ influx and CRAC channel activity [35].
In an elegant study, Feske et al. [36] used linkage analysis and a Drosophila S2 cell genome-wide RNAi screen to identify Orai1 as an essential component of CRAC channel activity and as the gene mutated in patients with a rare form of severe combined immunodeficiency syndrome (SCID) (see also [37;38]). Immune cells of these patients lack functional CRAC channels and SOCE. Work from several laboratories has demonstrated that Orai1 homologs are essential components of the CRAC channel and likely function as pore subunits [39-41]. Coexpression of STIM1 and Orai1 homologs dramatically increases SOCE and CRAC channel activity [39-44]. During SOCE/CRAC channel activation, Orai1 redistributes from a diffuse localization pattern in the plasma membrane and colocalizes with STIM1 puncta [34;35]. Coimmunoprecipitation studies suggest that STIM1 and Orai1 homologs bind to each other directly or through intermediary proteins [39;40]. Together, these observations have led to the hypothesis that redistribution and subsequent coassociation of STIM1 and Orai1 homologs in response to ER Ca2+ depletion activates CRAC channels and SOCE.
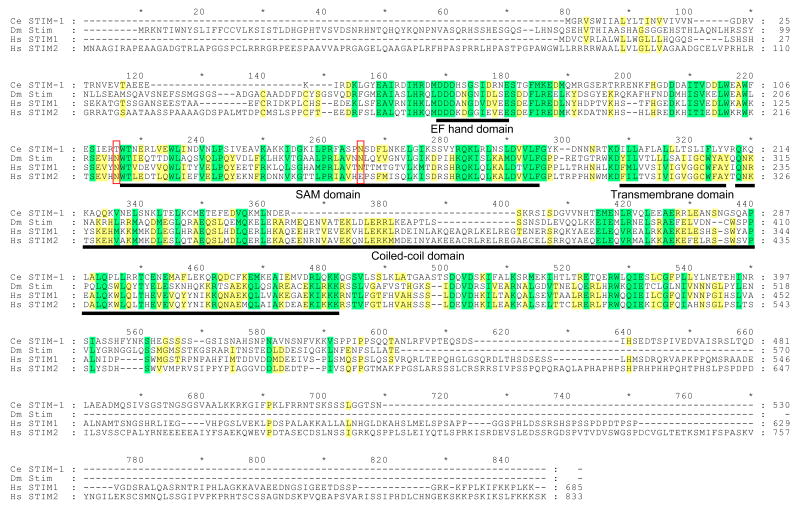
The C. elegans genome contains a single homolog of both STIM1 and Orai1 (Figures 4-5). C. elegans stim-1 encodes a 530 amino acid protein that is most similar to human STIM1 versus STIM2. Human STIM1 and Drosophila Stim possess several conserved domains including an N-terminal signal peptide, an EF-hand Ca2+ binding motif, a SAM domain, a single predicted transmembrane domain, and a large C-terminal region predicted to encode a coiled-coil domain [45]. These motifs are conserved in C. elegans STIM-1 (Figure 4).
Figure 4.
Amino acid sequence alignment of C. elegans (Ce) STIM-1 with Drosophila (Dm) Stim and human (Hs) STIM1 and STIM2 homologs. Yellow and green shading indicates sequence identity and conserved amino acid substitutions, respectively. Conserved domains are underlined in black. Red boxes show location of N-linked glycosylation sites in human STIM1. Percent similarity and identity of EF-hand, SAM, transmembrane and coiled-coil domains are 58.3% and 50.0%, 50.7% and 37.7%, 27.8% and 5.6%, and 57.4% and 21.3%, respectively. Alignment was performed using Vector NTI software (InforMax, Bethesda, MD). Protein domains were identified by SMART (http://smart.embl-heidelberg.de/smart/change_mode.pl).
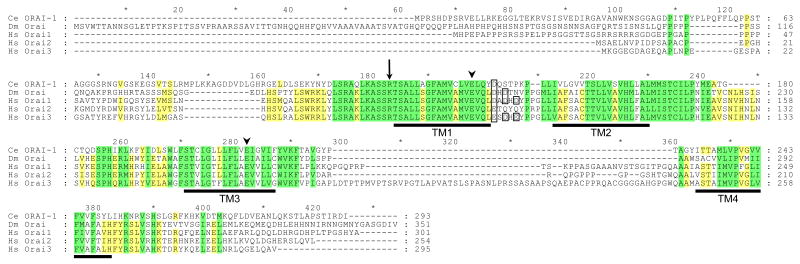
Figure 5.
Amino acid sequence alignment of C. elegans (Ce) ORAI-1 with Drosophila (Dm) Orai and human (Hs) Orai1, Orai2 and Orai3. Yellow and green shading indicates sequence identity and conserved amino acid substitutions, respectively. Transmembrane (TM) domains of ORAI-1 predicted by TMpred (http://www.ch.embnet.org/software/TMPRED_form.html#) are underlined in black. A conserved arginine residue mutated in SCID patients lacking lymphocyte CRAC activity is denoted by the arrow. Conserved glutamate residues located in TM1 and TM3 that contribute to channel ionic selectivity are denoted by arrowheads. Alignment was performed using Vector NTI software (InforMax, Bethesda, MD). Percent identity and similarity of C. elegans ORAI-1 to Drosophila Orai, human Orai1, human Orai2 and human Orai3 were 34% and 55%, 34% and 54%, 38% and 59%, and 35% and 59%, respectively. Acidic residues in the extracellular loop between TM1 and TM2 that may control channel selectivity are outlined in black.
C. elegans orai-1 encodes a 293 amino acid protein that shares 34-38% amino acid identity with Drosophila and human Orai1 homologs. Sequence alignment of worm, fly and human Orai homologs reveals that the four predicted transmembrane (TM) domains show strong conservation of primary structure in all five proteins. In addition, the predicted intracellular loop between TM2 and TM3 is highly conserved. Glutamate residues located in TM1 and TM3 have recently been shown to play key roles in controlling CRAC channel ion selectivity [39;40;46] and are fully conserved in worm, fly and human Orai homologs. Mutation of an arginine residue at the beginning of TM1 in human Orai1 is responsible for the loss of CRAC channel activity in lymphocytes of a subset of SCID patients [36]. This residue is conserved in worm and human Orai proteins and exhibits a conserved substitution with lysine in fly Orai (Figure 5).
Mutagenesis studies on Drosophila Orai [39] and human Orai1 [40] suggest that 2-3 acidic amino acid residues in the extracellular loop between TM1 and TM2 may function to attract polyvalent cations towards the channel pore and control channel selectivity. Interestingly, only one of these residues is conserved in C. elegans ORAI-1 and human Orai2 suggesting that these channels may exhibit selectivity properties distinct from other Orai homologs.
To determine where stim-1 and orai-1 are expressed in C. elegans, we generated transgenic worms expressing full-length STIM-1 and ORAI-1 proteins fused to either GFP or DsRed. Expression of the transgenes was driven by 1.9 kb and 4 kb of stim-1 and orai-1 promoter sequence, respectively. STIM-1 and ORAI-1 reporters are coexpressed in the intestine, gonad sheath cells and spermatheca. ORAI-1 appears to be localized primarily to the plasma membrane and/or a submembrane region while STIM-1 is expressed largely in an intracellular compartment [47].
5. C. elegans STIM-1 and ORAI-1 reconstitute CRAC channel activity
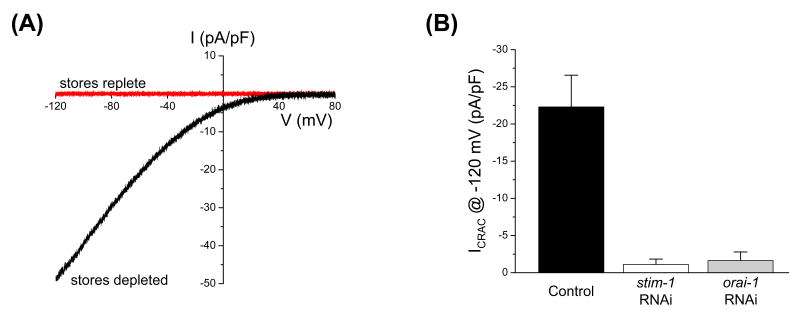
Coexpression of STIM1 and Orai1 homologs dramatically increases SOCE and CRAC channel activity in cultured mammalian and Drosophila cells [39-44]. Similarly, coexpression of C. elegans STIM-1 and ORAI-1 in HEK293 cells induces large SOCC currents (Figure 6A) [47]. These currents show strong inward rectification, reverse at membrane voltages >+80 mV and are not activated by membrane voltage.
Figure 6.
C. elegans CRAC channel activity is mediated by STIM-1 and ORAI-1. (A) Current-to-voltage relationships of currents observed in a HEK293 with replete stores coexpressing ORAI-1 and STIM-1 and in an ORAI-1/STIM-1 coexpressing HEK293 cell in which stores were depleted. Current-to-voltage relationships were plotted for currents measured 5 min after obtaining whole cell access. Store depletion was induced by dialyzing cells with a pipette solution containing 10 mM BAPTA, 10 μM IP3 and ∼18 nM free-Ca2+. Depletion of stores was prevented using a pipette solution containing 10 mM BAPTA, 2 mM ATP and ∼140 nM free-Ca2+. (B) Effect of stim-1 or orai-1 RNAi on endogenous CRAC channel activity measured in cultured C. elegans intestinal cells. Values are means ± S.E. (n=7-21)
The C. elegans intestinal cell SOCC current shares many similarities with mammalian ICRAC. However, the current also exhibits some distinct differences. Unlike Drosophila and mammalian ICRAC, C. elegans ISOCC is not activated by low concentrations of 2-APB, has a relatively high Cs+ permeability and shows much slower rundown or “depotentiation” when exposed to divalent cation-free (DVF) extracellular solution [21;26]. To determine whether heterologous expression of STIM-1 and ORAI-1 recapitulates the properties of the endogenous intestinal ISOCC, we characterized 2-APB sensitivity, Cs+ permeability and depotentiation.
As was observed in cultured intestinal cells [21], low concentrations of 2-APB had no effect on the STIM-1/ORAI-1 induced ISOCC. The effect of DVF bath on whole cell current was complex. Two cells with SOCC current densities of 6-7 pA/pF exhibited the typical response to DVF bath; upon removal of extracellular divalent cations, current amplitude increased rapidly and then underwent slow depotentiation. In one cell, bath Na+ was successfully replaced with Cs+. The calculated Cs+ permeability relative to Na+ (i.e., PCs/PNa) in this cell was 0.7 [47]. Both the rate of depotentiation and relative Cs+ permeability are remarkably similar to what we have observed previously for the native SOCC expressed in cultured worm intestinal cells [21].
The two cells that exhibited the typical response to DVF bath had relatively low SOCC currents. We refer to these cells as “low current” cells. All other cells in which DVF experiments were performed successfully had current densities of 30-60 pA/pF and we refer to these as “high current” cells. The response to DVF bath in high current cells contrasted sharply with that of low current cells. Upon removal of bath divalent cations, whole cell current in high current cells was rapidly and nearly completely inhibited. Current inhibition was followed by slow current reactivation. The calculated PCs/PNa for high current cells was 0.3 [47].
Several observations on high current cells suggested the possibility that the anomalous response to DVF bath might be due to high rates of Ca2+ influx and intracellular Ca2+ dependent channel regulatory mechanisms. Regulation of CRAC channels by intracellular Ca2+ concentration has been well described (e.g., [48;49]). To test the possible effects of high rates of Ca2+ influx on the response to bath divalent cation removal, cells were bathed in an extracellular solution containing 0.25 mM Ca2+ before being exposed to DVF medium. We refer to these cells as “low Ca2+” cells. In 10 out of 10 low Ca2+ cells, exposure to DVF bath caused an immediate increase in whole cell current. This current typically continued to activate slowly and then stabilized. Rapid rundown or “depotentiation” of the current was never observed. The mean PCs/PNa in low Ca2+ cells was 0.2 [47].
To summarize, the ORAI-1/STIM-1 induced Ca2+ current has biophysical properties similar to those of the native SOCC current. The ORAI-1/STIM-1 induced SOCC is not activated by 5 μM 2-APB and has a PCs/PNa 2-7-fold higher than that reported for mammalian and Drosophila CRAC channels. In addition, whole cell currents that showed a normal response to DVF bath underwent depotentiation at a rate remarkably similar to that of the native channel. We conclude from these results and studies on Drosophila and human Orai homologs [39-41], that C. elegans expresses a bona fide CRAC channel encoded by orai-1 and regulated by STIM-1.
The anomalous response of high current cells to DVF bath suggests that high rates of Ca2+ influx alter CRAC channel function and regulation. The underlying mechanism by which this occurs is unclear at present. However, further study of this phenomenon may shed light on the mechanisms by which both extracellular and intracellular Ca2+ regulate CRAC channel activity. Our findings also raise a cautionary note. We are unaware of studies showing that native CRAC channels undergo rapid inhibition in response to DVF extracellular solutions. Thus, it is likely that heterologous overexpression alters channel structure/function relationships and/or regulation. Conclusions drawn from heterologous expression studies on CRAC channel function, and in particular regulation, should be tempered by these concerns.
6. CRAC channels play essential roles in gonad function and fertility
Knockdown of either STIM-1 or ORAI-1 expression in worms using RNAi causes complete sterility. Video microcopy of anesthesized worms demonstrated that the rates of sheath cell contraction before and during ovulation are decreased by stim-1 or orai-1 RNAi [47;50]. As described earlier, contraction of sheath cells during ovulation is required to pull the spermatheca over the maturing oocyte.
Reduction in the rate and force of sheath cell contraction is expected to reduce fertility rather than result in complete sterility. The main reason for sterility in stim-1(RNAi) and orai-1(RNAi) worms is failure of the distal spermatheca to open thereby preventing oocytes from entering the spermatheca for fertilization.
To further examine the role of STIM-1 in gonad function, we mutated aspartate residues at positions 55 and 57 to alanine (i.e., D55A and D57A). These residues are located in the predicted Ca2+ binding EF-hand domain. Mutation of the analogous amino acids in Drosophila and human STIM1 homologs constitutively activates SOCE [28;30] and ICRAC [29].
Transgenic worms expressing STIM-1(D55A;D57A) are sterile. We did not detect developing embryos or unfertilized oocytes in the uteri of STIM-1(D55A;D57A) worms indicating that the fertility defect is due at least in part to defects in ovulation. Constitutive activation of CRAC channels by the STIM-1 EF-hand mutant likely disrupts cellular Ca2+ homeostasis and signaling events, which in turn disrupts the contractile activity of gonad sheath and spermatheca cells. Figure 1B is a schematic diagram showing the IP3 signaling pathways that regulate sheath cell contraction and opening of the distal spermatheca. The precise role of CRAC channels in this pathway is uncertain, but they could function to refill depleted stores and/or modulate cytoplasmic Ca2+ levels.
7. CRAC channels are not required for oscillatory Ca2+ signaling or ER Ca2+ homeostasis in the worm intestine
As noted above, IP3-dependent oscillatory Ca2+ signaling in intestinal epithelial cells triggers posterior body wall muscle contraction (pBoc) required for defecation. Surprisingly, we found that RNAi knockdown of STIM-1 or ORAI-1 has no effect on pBoc rhythm or the characteristics of intestinal Ca2+ oscillations [47;50]. These results could be explained if RNAi was ineffective in silencing stim-1 and orai-1 expression. However, a number of experimental observations argue strongly against this possibility. First, STIM-1 or ORAI-1 RNAi inhibits mean CRAC channel current in cultured intestinal cells >90% (Figure 6B). Fluorescence levels in worms expressing STIM-1 or ORAI-1 GFP reporters are reduced >90% or suppressed completely by stim-1 and orai-1 RNAi, respectively. Finally, transgenic worms expressing STIM-1(D55A;D57A) exhibit prolonged and arrhythmic pBoc cycles. This defect is fully suppressed by stim-1 or orai-1 RNAi [47;50]. The ability of orai-1 RNAi to suppress the effects the STIM-1 EF hand mutant on pBoc rhythmicity demonstrates that the two genes function together in common pathway.
Endoplasmic reticulum Ca2+ homeostasis is not only important for intracellular Ca2+ signaling, but also for proper protein synthesis and folding. Disruption of ER Ca2+ homeostasis triggers the unfolded protein response (UPR), an intracellular signaling and transcriptional/translational program activated by the accumulation of unfolded proteins in the ER lumen (reviewed by [51;52]). In mammalian cells, inhibition of the sarcoplasmic/ER Ca2+ ATPase (SERCA) with thapsigargin depletes ER Ca2+ stores and triggers the UPR (e.g., [53;54]). We reasoned that if CRAC channels are essential for refilling ER Ca2+ stores during oscillatory Ca2+ signaling, then inhibition of channel activity should induce the UPR in the worm intestine.
To monitor the intestinal UPR, we utilized a transgenic worm strain expressing an hsp-4 transcriptional GFP reporter. hsp-4 encodes a C. elegans homolog of the ER chaperone protein GRP78/BiP and is expressed in the worm intestine and induced by ER stress [55]. Exposure of worms for 6 h to 10 μg/ml of tunicamycin, which induces ER stress, causes a nearly 7-fold increase in intestinal hsp-4::GFP expression [50].
To determine whether store Ca2+ depletion activates the UPR in C. elegans, we knocked down SERCA expression by feeding worms sca-1 double stranded RNA-producing bacteria for 30 h. sca-1 encodes the C. elegans SERCA homolog [56]. sca-1 RNAi causes a nearly 3-fold increase in hsp-4::GFP expression. In contrast, knockdown of STIM-1 expression by RNAi has no effect on hsp-4::GFP expression [50].
Interestingly, knockdown of sca-1 and stim-1 together causes a 30-45% increase hsp-4::GFP expression compared to sca-1 alone. Taken together, these results indicate that C. elegans CRAC channels are not obligate components of all IP3–dependent Ca2+ signaling pathways nor are they essential for ER Ca2+ homeostasis under normal physiological conditions. However, the effects of combined sca-1 and stim-1 RNAi on the UPR suggest that CRAC channels may contribute to the regulation of store Ca2+ levels during experimental manipulations that induce extreme store Ca2+ depletion.
8. Are CRAC channels essential components of all IP3-dependent Ca2+ signaling pathways?
Despite their presence in organisms as diverse as roundworms, fruit flies and humans, and their widespread expression in functionally diverse mammalian cell types [57-59], the physiological roles of CRAC channels are largely unknown. It is widely stated in the literature that CRAC channels and SOCE are essential for generation of IP3-dependent Ca2+ signals and for maintenance of ER Ca2+ levels during Ca2+ signaling events [57-59], but in most cell types, direct evidence supporting this notion is lacking. Interestingly, in SCID patients with a loss-of-function mutation in Orai1 [36], the only non-immunological defects observed are nonprogressive muscle hypotonia and mild psychomotor and mental retardation [60]. This finding is unexpected if CRAC channels play a ubiquitous and essential role in Ca2+ signaling. It is possible that human Orai2 and Orai3 proteins function as SOC channels and compensate for loss-of-function mutations in Orai1. However, C. elegans has only a single Orai1 homolog. Our findings that CRAC channels are not required for oscillatory Ca2+ signaling and ER Ca2+ homeostasis in the worm intestine raise interesting and important questions about store depletion and store-operated Ca2+ channels.
Do ER Ca2+ stores become sufficiently depleted to activate CRAC channels during physiologically relevant Ca2+ signaling events? With the exception of immune cells (reviewed in [61]), CRAC channel and SOCE activation in most cell types has been observed only under conditions of extreme store depletion experimentally induced by SERCA inhibition, supraphysiological IP3 receptor activation, exposure to high concentrations of ionomycin and/or increases in cytoplasmic Ca2+ buffering (e.g., [62-64]). Direct measurements of store Ca2+ levels during physiologically relevant Ca2+ signaling events are lacking. In the one detailed study conducted to date, little or no change in store Ca2+ levels was detected during acetylcholine-induced Ca2+ oscillations in pancreatic acinar cells. Only during stimulation with supraphysiological acetylcholine concentrations was store depletion observed [65].
The relationship between store emptying and CRAC channel activation has been examined indirectly in a number of cell types. Many studies suggest that store depletion is not tightly coupled to SOCE whereas other work suggests a more direct coupling (reviewed in [59]). The apparent absence of direct coupling has been taken as indirect evidence that CRAC channels are regulated by specialized ER stores or ER microdomains (e.g., [59;66-68]). If specialized ER Ca2+ stores exist, then STIM1 homologs may exhibit a heterogenous distribution in the ER membrane. It will thus be important to carefully define the intracellular localization of native STIM1 proteins and determine whether they co-localize with other components of the ER Ca2+ signaling machinery. In addition, while technically demanding, it will be important to measure store Ca2+ levels under conditions that mimic physiologically relevant Ca2+ signaling events.
If CRAC channels are not essential components of all Ca2+ signaling pathways, why are they so widely observed and why have the channel's functional/structural properties been conserved from worms to humans? One possibility is that they may provide cells with a failsafe mechanism for protecting store Ca2+ levels during pathophysiological insults, exposure to cellular stressors and during high rates of protein synthesis. Bacterial toxins [69;70], viral proteins [71;72] ischemia [73] and oxidants [74;75] induce store Ca2+ loss and depletion. Two recent studies have shown that SOCE is activated by ER Ca2+ loss through the translocon [76;77]. Failure to maintain store Ca2+ levels under pathophysiological and stress conditions and during increases in translational activity will disrupt ER protein synthesis, folding and processing, which can cause cell injury and ultimately lead to cell death [51;52]. Consistent with this idea, we observed that stim-1 RNAi does not trigger a UPR in the intestine indicating that CRAC channels are not required for ER Ca2+ homeostasis under normal physiological conditions. However, the intestinal UPR induced by RNAi knockdown of the SERCA encoding gene sca-1 is increased further when STIM-1 expression is knocked down at the same time [50]. Thus, under conditions of extreme store depletion induced by an unphysiological event (i.e., reduced SERCA expression and activity), C. elegans CRAC channels appear to play a role in regulation of store Ca2+ levels.
CRAC channels clearly play critical signaling roles in some cell types. An important role for CRAC channels in immune cell signaling is well established (reviewed by [61]) and they are essential for gonad function and fertility in C. elegans [47;50]. The functional properties of CRAC channels are likely specialized for certain signaling mechanisms. CRAC channels have a very high Ca2+ selectivity and thus will have little effect on membrane potential when they are active and mediating Ca2+ influx. While CRAC channels are not gated directly by voltage, membrane potential will alter Ca2+ flux by changing the electrical driving force for Ca2+. In addition, CRAC channels have been reported to undergo slow voltage dependent changes in macroscopic conductance (reviewed in [59;61]). Thus, variable patterns of CRAC channel mediated Ca2+ influx can be induced by the activity of other plasma membrane ion channels that affect membrane potential. This in turn increases the complexity and information content of Ca2+ signals as well as modulates the rate of store refilling.
Calcium influx through CRAC channels appears to occur at discrete membrane locations where STIM1 proteins accumulate in response to store depletion [35]. Such compartmentalization of Ca2+ entry likely provides a mechanism for specifically regulating downstream cellular and ER signaling molecules that colocalize with STIM1 proteins and CRAC channels. Regulation of CRAC channel activity by store Ca2+ depletion also provides a way to coordinate compartmentalized Ca2+ influx with Ca2+ efflux through IP3 receptors and signaling pathways that control levels of IP3 and associated lipid second messengers.
9. Conclusions and future perspective
The identification of Orai1 and STIM1 proteins has revolutionized our understanding of SOCE. However, numerous important questions remain to be answered. In particular, our studies in C. elegans raise several issues. As noted above, little evidence exists linking CRAC channels to the regulation of physiologically relevant cellular processes. It is therefore essential to define the signaling pathways in which CRAC channels normally function. Direct measurement of the dynamics of ER Ca2+ levels during physiologically relevant Ca2+ signaling events is also needed. CRAC channels are essential for the normal functioning of mammalian immune cells and C. elegans gonad cells. Do Ca2+ stores in these cells become depleted enough to activate CRAC channels because they have a limited volume and/or because rates of ER Ca2+ uptake are slow relative to total store capacity and rates of passive Ca2+ leak and efflux through activated IP3 receptors? It is likely that the functional properties of the ER Ca2+ stores are specifically tailored to the signaling requirements of the cell and the role of CRAC channels in those signaling pathways.
Recent studies suggest that STIM1 regulates other types of channels including TRPC1 [78;79] and the ARC channel [80]. Interestingly, when we generated transgenic worms expressing the STIM-1 EF-hand mutant under the control of the promoter for let-858, a gene that functions in most C. elegans cell types [81], the animals showed numerous serious defects and survived very poorly (Yan, Lamitina and Strange, unpublished observations). This suggests that CRAC channels function in more cell types than revealed by our GFP reporter studies [47;50]. Alternatively, STIM-1 and/or the EF-hand mutant may have functions in addition to regulating the activity of CRAC channels. The forward and reverse genetic tractability of C. elegans may be particularly useful in elucidating the physiological roles and regulation of STIM-1, CRAC channels and SOCE.
Acknowledgments
This work was supported by NIH grants GM74229 and DK51610.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strange K. From genes to integrative physiology: ion channel and transporter biology in Caenorhabditis elegans. Physiol Rev. 2003;83:377–415. doi: 10.1152/physrev.00025.2002. [DOI] [PubMed] [Google Scholar]
- 2.Barr MM. Super models. Physiol Genomics. 2003;13:15–24. doi: 10.1152/physiolgenomics.00075.2002. [DOI] [PubMed] [Google Scholar]
- 3.Estevez AY, Strange K. Genetic and molecular characterization of Ca2+ and IP3 signaling in the nematode Caenorhabditis elegans. In: Putney JW Jr, editor. Calcium Signaling. Boca Raton: CRC Press; 2006. pp. 161–186. [Google Scholar]
- 4.Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- 5.Hubbard EJ, Greenstein D. The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev Dyn. 2000;218:2–22. doi: 10.1002/(SICI)1097-0177(200005)218:1<2::AID-DVDY2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- 7.McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki K, McCarter J, Francis R, Schedl T. emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J Cell Biol. 1996;134:699–714. doi: 10.1083/jcb.134.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin X, Gower NJ, Baylis HA, Strange K. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of smooth muscle-like sheath cells in the nematode Caenorhabditis elegans. Mol Biol Cell. 2004;15:3938–3949. doi: 10.1091/mbc.E04-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clandinin TR, DeModena JA, Sternberg PW. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- 11.Kariya K, Kim BY, Gao X, Sternberg PW, Kataoka T. Phospholipase Cε regulates ovulation in Caenorhabditis elegans. Dev Biol. 2004;274:201–210. doi: 10.1016/j.ydbio.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Bui YK, Sternberg PW. Caenorhabditis elegans inositol 5-phosphatase homolog negatively regulates inositol 1,4,5-triphosphate signaling in ovulation. Mol Biol Cell. 2002;13:1641–1651. doi: 10.1091/mbc.02-01-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu DW, Thomas JH. Regulation of a periodic motor program in C. elegans. J Neurosci. 1994;14:1953–1962. doi: 10.1523/JNEUROSCI.14-04-01953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas JH. Genetic analysis of defecation in Caenorhabditis elegans. Genetics. 1990;124:855–872. doi: 10.1093/genetics/124.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki K, Liu DW, Thomas JH. Genes that control a temperature-compensated ultradian clock in Caenorhabditis elegans. Proc Natl Acad Sci. 1995;92:10317–10321. doi: 10.1073/pnas.92.22.10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- 17.Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C β and γ. J Gen Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teramoto T, Iwasaki K. Intestinal calcium waves coordinate a behavioral motor program in C. elegans. Cell Calcium. 2006;40:319–327. doi: 10.1016/j.ceca.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Christensen M, Strange K. Developmental regulation of a novel outwardly rectifying mechanosensitive anion channel in Caenorhabditis elegans. J Biol Chem. 2001;276:45024–45030. doi: 10.1074/jbc.M107652200. [DOI] [PubMed] [Google Scholar]
- 20.Christensen M, Estevez AY, Yin XM, Fox R, Morrison R, McDonnell M, Gleason C, Miller DM, Strange K. A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron. 2002;33:503–514. doi: 10.1016/s0896-6273(02)00591-3. [DOI] [PubMed] [Google Scholar]
- 21.Estevez AY, Roberts RK, Strange K. Identification of store-independent and store-operated Ca2+ conductances in Caenorhabditis elegans intestinal epithelial cells. J Gen Physiol. 2003;122:207–223. doi: 10.1085/jgp.200308804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol. 2002;539:445–458. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 25.Kozak JA, Cahalan MD. MIC channels are inhibited by internal divalent cations but not ATP. Biophys J. 2003;84:922–927. doi: 10.1016/S0006-3495(03)74909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeromin AV, Roos J, Stauderman KA, Cahalan MD. A store-operated calcium channel in Drosophila S2 cells. J Gen Physiol. 2004;123:167–182. doi: 10.1085/jgp.200308982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ Entry. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 32.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 35.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 37.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 42.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 44.Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium-selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prakriya M, Lewis RS. Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode. J Gen Physiol. 2006;128:373–386. doi: 10.1085/jgp.200609588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorin-Nebel C, Xing J, Yan X, Strange K. CRAC channel activity in C. elegans is mediated by Orai1 and STIM1 homologs and is essential for ovulation and fertility. J Physiol. doi: 10.1113/jphysiol.2006.124883. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (I CRAC) due to local calcium feedback. J Gen Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zweifach A, Lewis RS. Slow calcium-dependent inactivation of depletion-activated calcium current. Store-dependent and -independent mechanisms. J Biol Chem. 1995;270:14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]
- 50.Yan X, Xing J, Lorin-Nebel C, Estevez AY, Nehrke K, Lamitina T, Strange K. Function of a STIM1 homologue in C. elegans: evidence that store-operated Ca2+ entry is not essential for oscillatory Ca2+ signaling and ER Ca2+ homeostasis. J Gen Physiol. 2006;128:443–459. doi: 10.1085/jgp.200609611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 52.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 53.Hong M, Li M, Mao C, Lee AS. Endoplasmic reticulum stress triggers an acute proteasome-dependent degradation of ATF6. J Cell Biochem. 2004;92:723–732. doi: 10.1002/jcb.20118. [DOI] [PubMed] [Google Scholar]
- 54.Durose JB, Tam AB, Niwa M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol Biol Cell. 2006;17:3095–3107. doi: 10.1091/mbc.E06-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 56.Zwaal RR, Van Baelen K, Groenen JT, van Geel A, Rottiers V, Kaletta T, Dode L, Raeymaekers L, Wuytack F, Bogaert T. The sarco-endoplasmic reticulum Ca2+ ATPase is required for development and muscle function in Caenorhabditis elegans. J Biol Chem. 2001;276:43557–43563. doi: 10.1074/jbc.M104693200. [DOI] [PubMed] [Google Scholar]
- 57.Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 58.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 59.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 60.Feske S, Draeger R, Peter HH, Eichmann K, Rao A. The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J Immunol. 2000;165:297–305. doi: 10.4049/jimmunol.165.1.297. [DOI] [PubMed] [Google Scholar]
- 61.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 62.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- 63.Machaca K. Ca2+-calmodulin-dependent protein kinase II potentiates store-operated Ca2+ current. J Biol Chem. 2003;278:33730–33737. doi: 10.1074/jbc.M305023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parekh AB, Fleig A, Penner R. The store-operated calcium current ICRAC: nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 65.Park MK, Petersen OH, Tepikin AV. The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 67.Berridge M. Conformational coupling: a physiological calcium entry mechanism. Sci STKE 2004. 2004:e33. doi: 10.1126/stke.2432004pe33. [DOI] [PubMed] [Google Scholar]
- 68.Penner R, Fleig A. Store-operated calcium entry: a tough nut to CRAC. Sci STKE 2004. 2004:e38. doi: 10.1126/stke.2432004pe38. [DOI] [PubMed] [Google Scholar]
- 69.Bryant AE, Bayer CR, Hayes-Schroer SM, Stevens DL. Activation of platelet gpIIbIIIa by phospholipase C from Clostridium perfringens involves store-operated calcium entry. J Infect Dis. 2003;187:408–417. doi: 10.1086/367964. [DOI] [PubMed] [Google Scholar]
- 70.Saha S, Gupta DD, Chakrabarti MK. Involvement of phospholipase C in Yersinia enterocolitica heat stable enterotoxin (Y-STa) mediated rise in intracellular calcium level in rat intestinal epithelial cells. Toxicon. 2005;45:361–367. doi: 10.1016/j.toxicon.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Tian P, Estes MK, Hu Y, Ball JM, Zeng CQ, Schilling WP. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, Melchers WJ. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lehotsky J, Kaplan P, Babusikova E, Strapkova A, Murin R. Molecular pathways of endoplasmic reticulum dysfunctions: possible cause of cell death in the nervous system. Physiol Res. 2003;52:269–274. [PubMed] [Google Scholar]
- 74.Pariente JA, Camello C, Camello PJ, Salido GM. Release of calcium from mitochondrial and nonmitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J Membr Biol. 2001;179:27–35. doi: 10.1007/s002320010034. [DOI] [PubMed] [Google Scholar]
- 75.Henschke PN, Elliott SJ. Oxidized glutathione decreases luminal Ca2+ content of the endothelial cell ins(1,4,5)P3-sensitive Ca2+ store. Biochem J. 1995;312(Pt 2):485–489. doi: 10.1042/bj3120485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ong HL, Liu X, Sharma A, Hegde RS, Ambudkar IS. Intracellular Ca2+ release via the ER translocon activates store-operated calcium entry. Pflugers Arch. 2006 doi: 10.1007/s00424-006-0163-5. [DOI] [PubMed] [Google Scholar]
- 77.Flourakis M, Van Coppenolle F, Lehen'kyi V, Beck B, Skryma R, Prevarskaya N. Passive calcium leak via translocon is a first step for iPLA2-pathway regulated store operated channels activation. FASEB J. 2006;20:1215–1217. doi: 10.1096/fj.05-5254fje. [DOI] [PubMed] [Google Scholar]
- 78.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 79.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 80.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store-depletion or translocation to the plasma membrane. J Physiol. doi: 10.1113/jphysiol.2006.122432. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelly WG, Xu S, Montgomery MK, Fire A. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics. 1997;146:227–238. doi: 10.1093/genetics/146.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]