Abstract
The liver produces plasma sex hormone–binding globulin (SHBG), which transports sex steroids and regulates their access to tissues. In overweight children and adults, low plasma SHBG levels are a biomarker of the metabolic syndrome and its associated pathologies. Here, we showed in transgenic mice and HepG2 hepatoblastoma cells that monosaccharides (glucose and fructose) reduce human SHBG production by hepatocytes. This occurred via a downregulation of hepatocyte nuclear factor–4α (HNF-4α) and replacement of HNF-4α by the chicken OVA upstream promoter–transcription factor 1 at a cis-element within the human SHBG promoter, coincident with repression of its transcriptional activity. The dose-dependent reduction of HNF-4α levels in HepG2 cells after treatment with glucose or fructose occurred in concert with parallel increases in cellular palmitate levels and could be mimicked by treatment with palmitoyl-CoA. Moreover, inhibition of lipogenesis prevented monosaccharide-induced downregulation of HNF-4α and reduced SHBG expression in HepG2 cells. Thus, monosaccharide-induced lipogenesis reduced hepatic HNF-4α levels, which in turn attenuated SHBG expression. This provides a biological explanation for why SHBG is a sensitive biomarker of the metabolic syndrome and the metabolic disturbances associated with increased fructose consumption.
Introduction
Body mass index is a major determinant of sex hormone–binding globulin (SHBG) concentrations in the blood of men and women (1–3). Low serum SHBG levels in overweight individuals are a biomarker for the metabolic syndrome (4–7) and are predictive of type 2 diabetes (8–10) and cardiovascular disease risk (11, 12). Conversely, high serum SHBG levels in children (13) and women (14) with anorexia nervosa decrease during weight gain, and SHBG measurements represent an index of nutritional status in this type of eating disorder (14). The reason why plasma SHBG levels are invariably low in obese individuals of all ages and sex has been the subject of much debate. There is no reason to suspect that the plasma clearance of SHBG differs in normal-weight and obese individuals, and current evidence favors a mechanism involving reduced SHBG production by hepatocytes in response to a metabolic disturbance associated with excess body mass index and especially visceral adiposity (15, 16). To date, the widely held explanation for this is that elevated insulin in obese and insulin-resistant individuals somehow acts on the liver to decrease hepatic SHBG production (16). Although it has been reported that insulin reduces SHBG production by human HepG2 hepatoblastoma cells (17), a subsequent study indicated that this effect is not specific and may be attributed to a global reduction in protein secretion (18). In view of earlier reports that plasma SHBG levels are negatively correlated with glucose, as well as insulin (19), we have explored the hypothesis that expression of the human SHBG gene in the liver responds to increased exposures to monosaccharides rather than insulin. In addition, because one of the major dilemmas of high-carbohydrate diets in the management of obesity and type 2 diabetes is increased hepatic lipogenesis (20), we have examined whether this contributes to the regulation of SHBG gene expression in the liver.
Results
Human SHBG expression in transgenic mouse livers is decreased by carbohydrates and not by insulin.
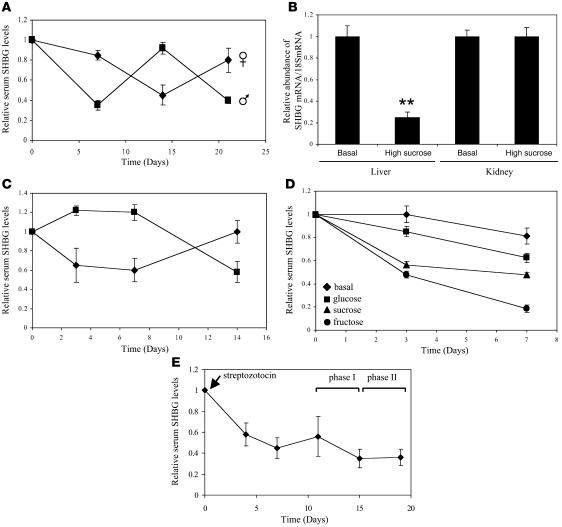
We first manipulated the diets of mice expressing different human SHBG transgenes (21): one line contains the 4.3-kb transcription unit expressed in liver and kidney (22), while the other contains a larger, 11-kb region of human SHBG that is also expressed in male germ cells (23). When male mice expressing an 11-kb human SHBG transgene were fed a high-sucrose diet, human SHBG levels in the blood were reduced by about 50% after 1 week (Figure 1A). Serum SHBG levels in these animals increased to normal levels within 1 week after they were returned to an isocaloric basal diet but decreased again when the animals were fed the high-sucrose diet in a third week of treatment (Figure 1A). When female mice carrying the same transgene were treated first with the basal diet and then fed the high-sucrose diet, a similar reduction in serum SHBG levels was observed, which returned to normal when this group of animals was returned to the basal diet (Figure 1A). In addition, human SHBG mRNA levels were 4-fold lower in the livers of male and female mice fed the high-sucrose diet when compared with the basal diet but were unchanged in the kidney (Figure 1B shows data for male mice only). Similar results were obtained using mice carrying a 4.3-kb human SHBG transgene mutated to disrupt an upstream stimulatory factor (USF) binding site (24) in the proximal promoter (Figure 1C). This USF binding site is adjacent to a hepatocyte nuclear factor–4α (HNF-4α) binding site, and this particular combination of cis-elements has been proposed to constitute a carbohydrate response element in the promoters of other genes (25, 26).
Figure 1. Hepatic production of SHBG in mice expressing human SHBG transgenes (21) is reduced after feeding diets with high monosaccharide content or increasing blood glucose levels by streptozotocin treatment.
(A and B) Mice were fed high-sucrose or isocaloric basal diets for 7 days (3 per group), and the diets were then reversed in two 7-day cycles. Serum SHBG levels are expressed as mean ± SEM relative to pretreatment levels to compensate for between-animal variability (A). At day 21, human SHBG mRNA abundance was determined in relation to 18S RNA (mean ± SEM) in liver and kidney; **P < 0.01 compared with basal diet values (B). (C) Serum SHBG levels in mice expressing a human SHBG transgene lacking a USF-binding site in the promoter (24) were reduced by feeding a high-sucrose diet. Animals (3 per group) were fed a basal diet (squares) or a high-sucrose diet (diamonds) for 7 days, and diets were then reversed for 7 days. Serum SHBG measurements (mean ± SEM) are expressed relative to pretreatment levels. (D) Serum SHBG levels (mean ± SEM) were reduced relative to pretreatment levels in human SHBG transgenic mice (3–4 per group) fed equicaloric diets containing high glucose, sucrose, or fructose, when compared with a basal diet. (E) Human SHBG transgenic mice treated with streptozotocin were maintained on a basal diet for 11 days, followed by a high-sucrose diet (phase I) and the basal diet (phase II). Serum SHBG levels (mean ± SEM) are expressed relative to pretreatment values.
Sucrose, a major dietary disaccharide, is hydrolyzed to glucose and fructose prior to absorption. Therefore, to isolate the potential effects of glucose and fructose, we repeated the experiment using isocaloric diets prepared to contain identical amounts of sucrose, glucose, or fructose as the carbohydrate source. Furthermore, because fructose does not acutely stimulate pancreatic β cell insulin release (27, 28), this experimental paradigm allows us to distinguish between the effects of monosaccharides and insulin on hepatic metabolism. Transgenic mice fed all 3 high-carbohydrate diets for 1 week showed decreased serum SHBG levels, when compared with mice fed the isocaloric basal diet (Figure 1D). Remarkably, the high-fructose diet was the most effective and decreased the SHBG levels by about 80%, while equicaloric diets with glucose and sucrose resulted in 40% and 50% decreases, respectively (Figure 1D). More importantly, these effects occurred within 3 days of feeding fructose or sucrose (Figure 1D).
To further determine how changes in insulin and blood glucose levels influence human SHBG production by the liver, a similar experiment was performed on animals that were first made diabetic by a single injection of streptozotocin (29). These animals were then fed a high-sucrose diet for 5 days (phase I), followed by the basal diet for 4 days (phase II). Failure of pancreatic β cell function was confirmed by an increase in blood glucose levels to greater than 400 mg/dl (normal value, 120 mg/dl) within 4 days of streptozotocin treatment. Serum SHBG levels decreased by 40%–50% within 4–7 days of treatment with streptozotocin, but no further decrease in serum SHBG levels occurred when these mice were fed a high-carbohydrate diet, and no increase occurred when they were returned to the basal diet (Figure 1E).
Monosaccharides reduce SHBG production by HepG2 cells irrespective of the presence of insulin.
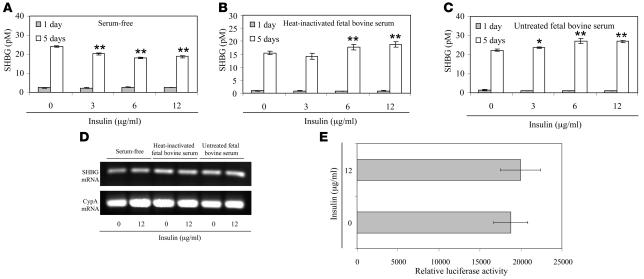
To explore the mechanism(s) responsible for the reduced hepatic production of SHBG in response to monosaccharides observed in vivo, we first reexamined the effects of insulin on SHBG production by HepG2 cells. In the most widely cited report that insulin reduces SHBG production by HepG2 cells (17), the experiments were conducted using serum-free medium without defining the glucose levels. To separate the effects of glucose and insulin, we first examined the effects of 3 different concentrations of insulin on SHBG secretion by HepG2 cells cultured in low-glucose medium, in the absence or presence of fetal FBS or heat-inactivated FBS. As reported previously (17), the amounts of SHBG secreted by HepG2 cells cultured in serum-free medium decrease by about 20% after insulin treatment (Figure 2A). However, since culture of HepG2 cells in serum-free medium results in a global decrease in protein secretion, the observed change is most likely nonspecific, as noted by others (18). By contrast, there were small increases in the amounts of SHBG secreted by insulin-treated HepG2 cells cultured in the presence of heat-inactivated FBS (Figure 2B) or untreated FBS (Figure 2C), but the SHBG mRNA levels did not change in the HepG2 cells cultured under these conditions, even at the highest concentration of insulin used (Figure 2D). To further assess whether insulin influences human SHBG expression, we studied the transcriptional activity of a luciferase reporter gene under the control of the human SHBG promoter (–299/+60 bp relative to the major transcription start site) in HepG2 cells (22) and found no insulin-dependent difference in the reporter gene response (Figure 2E).
Figure 2. Insulin does not influence SHBG production by HepG2 cells.
(A–D) HepG2 cells were cultured in serum-free medium, inactivated FBS, or untreated FBS for 5 days and treated daily with insulin at different concentrations. Accumulation of human SHBG in the medium was measured at timed intervals using an immunofluorometric assay (46) over a 5-day treatment period. Data points are shown as mean ± SD of triplicates; *P < 0.05, **P < 0.01 compared with no insulin treatment (A–C). On day 5 cells were taken for human SHBG mRNA and cyclophilin A mRNA measurements by RT-PCR analyses (D). (E) The effect of insulin (12 μg/ml) on human SHBG promoter activity was analyzed in HepG2 cells in the context of a luciferase reporter gene assay. Data points are mean ± SD of triplicate measurements.
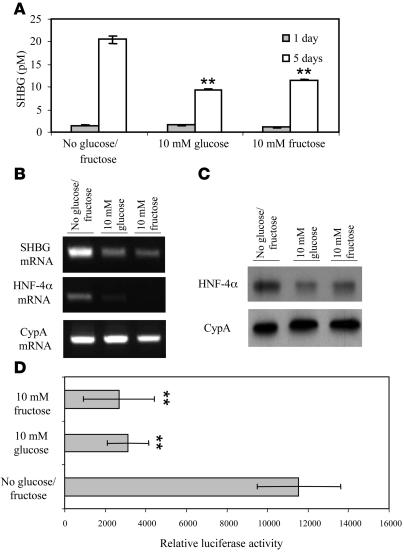
We next studied the effects of monosaccharides on SHBG production by HepG2 cells. When HepG2 cells were cultured in the presence of FBS with 10 mM glucose or fructose for 5 days, SHBG accumulation in the medium was reduced by 50% (Figure 3A) in concert with reduced cellular SHBG mRNA levels (Figure 3B). Because HNF-4α plays a key role in the transcriptional activity of the human SHBG promoter (22), we examined HNF-4α mRNA levels in these cells and found they were substantially reduced after treatment with glucose or fructose (Figure 3B). In addition, a Western blot of cell extracts revealed lower HNF-4α levels after treatment with the monosaccharides (Figure 3C). We also examined human SHBG promoter activity in the context of a luciferase reporter gene in HepG2 cells cultured for 4 days in the presence or absence of 10 mM glucose or fructose and found a 3- to 4-fold reduction in reporter gene expression (Figure 3D).
Figure 3. Monosaccharides decrease human SHBG production by HepG2 cells in concert with reduced cellular HNF-4α levels.
(A) Human SHBG accumulation in the medium of HepG2 cells treated daily with 10 mM of glucose or fructose was substantially reduced. Data points are shown as mean ± SD of triplicates; **P < 0.01 compared with no glucose/fructose supplementation. (B) Human SHBG and HNF-4α mRNA levels determined by RT-PCR in HepG2 cells cultured for 5 days as in A. Cyclophilin A (CypA) mRNA was amplified as in internal control. (C) HNF-4α and cyclophilin A levels measured by Western blotting in HepG2 cells cultured for 5 days as in B. (D) Human SHBG promoter activity was measured in the context of a luciferase reporter gene assay in HepG2 cells cultured for 4 days in the presence or absence of 10 mM glucose or fructose. Data points are shown as mean ± SD of triplicates; **P < 0.01 compared with no glucose/fructose supplementation.
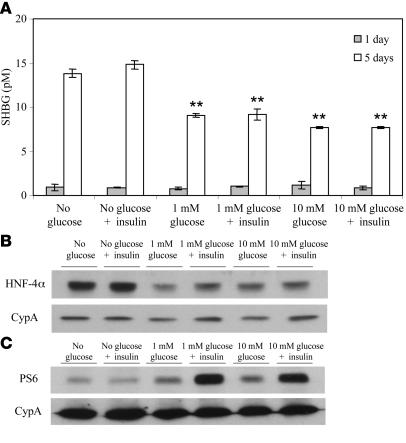
To determine whether insulin influences the downregulation of SHBG gene expression by monosaccharides in HepG2 cells, we measured the SHBG production by cells grown for 5 days in low-glucose medium containing FBS or in the same medium supplemented daily with glucose, insulin, or both (Figure 4A). In this experiment, SHBG levels in the culture medium were reduced by about 50% when cells were grown in the presence of glucose (1 mM or 10 mM), but insulin alone had no effect. Moreover, when insulin was added together with 1 mM or 10 mM glucose, no additional reductions in SHBG concentrations in the medium were observed. Similarly, the levels of HNF-4α in HepG2 cells cultured under the same conditions were uniformly reduced by supplementation with glucose and not influenced by the presence of insulin (Figure 4B). To confirm that the insulin-signaling cascade was functional in the HepG2 cells grown under these conditions, we measured the insulin-induced phosphorylation of the ribosomal protein S6 (30) in the cells and found that it increased only after treatment with insulin in the presence of glucose (Figure 4C) — a finding that casts doubt on whether insulin functions at all in HepG2 cells maintained under conditions in which glucose will be depleted, as in previous reports (17, 18, 31).
Figure 4. Insulin does not alter human SHBG production or HNF-4α protein levels in HepG2 cells cultured in the presence of high glucose.
(A) Human SHBG accumulation in the medium is decreased in HepG2 cells treated daily with 1 mM or 10 mM glucose in the absence or presence of 6 μg insulin/ml for 5 days. Data points are shown as mean ± SD of triplicates; **P < 0.01 compared with no glucose supplementation. (B) Western blots of HNF-4α and cyclophilin A in HepG2 cells treated as in A for 5 days. (C) Western blots of phospho-S6 and cyclophilin A in HepG2 cells treated as in A.
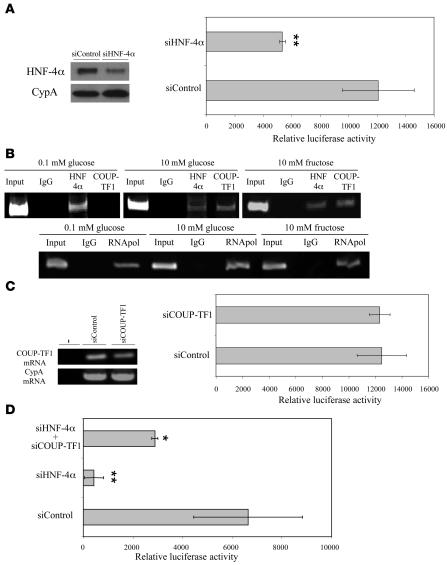
Reduced SHBG production by monosaccharides involves competition between HNF-4α and chicken OVA upstream promoter–transcription factor 1 (COUP-TF1) for binding sites within the SHBG promoter. Overexpression of HNF-4α in HepG2 cells increases human SHBG transcription by binding a cis-element within the human SHBG promoter, and COUP-TF1 modulates this through competitive binding to the same site (22). We therefore first used an siRNA approach to mimic the reduction in HNF-4α levels observed in HepG2 cells after treatment with glucose or fructose and found that this reduced the activity of the human SHBG promoter (Figure 5A). Importantly, this effect was evident in the construct containing less than 300 bp of the promoter (Figure 5A). We then performed a chromatin immunoprecipitation (ChIP) assay using DNA/protein complexes extracted from HepG2 cells cultured in low (0.1 mM) or high (10 mM) glucose or fructose to determine whether HNF-4α and COUP-TF1 bind differentially to sites within the SHBG proximal promoter (22). The results indicate that in the presence of low monosaccharide concentrations, HNF-4α rather than COUP-TF1 bound this region of the SHBG promoter, while binding of COUP-TF1 was more evident when cells were grown in medium containing 10 mM glucose or fructose (Figure 5B). To further demonstrate that interactions between HNF-4α and COUP-TF1 at this site control the human SHBG promoter, we treated HepG2 cells maintained in low glucose-containing medium with an siRNA to reduce COUP-TF1 mRNA levels (Figure 5C). Under these conditions, the HNF-4α/COUP-TF1 binding site in the human SHBG promoter was largely occupied by HNF-4α (Figure 5B), and it was therefore not unexpected that this siCOUP-TF1 treatment did not influence the transcriptional activity of the human SHBG promoter (Figure 5C). As noted above (Figure 5A), when the HepG2 cells were treated under these conditions with an siRNA against HNF-4α mRNA (siHNF-4α) there was a substantial reduction in human SHBG promoter activity (Figure 5D), but coadministration of siCOUP-TF1 together with siHNF-4α abrogated this (Figure 5D).
Figure 5. Competition between HNF-4α and COUP-TF1 at a cis-element within the human SHBG promoter regulates its activity.
(A) HNF-4α levels were reduced in HepG2 cells after transient transfection of an HNF-4α siRNA versus a control siRNA oligonucleotide (left), and siRNA-mediated downregulation of HNF-4α reduced human SHBG promoter activity in a luciferase reporter gene assay (right). Data points are shown as mean ± SD of triplicates; **P < 0.01 compared with cells treated with an siRNA control. (B) ChIP assays of HNF-4α and COUP-TF1 binding to the human SHBG promoter (top). As a control for the ChIP, anti–RNA polymerase (RNApol) antibodies were used with human-specific oligonucleotide primers to PCR amplify the GAPDH promoter (bottom). A nonspecific mouse IgG was used in ChIP reactions to control for nonspecific immunoprecipitation. Positive PCR controls of sheared genomic DNA templates indicated the integrity of the input DNA used in the ChIP reactions. (C) Reduction of COUP-TF1 mRNA in HepG2 cells after siCOUP-TF1 treatment (left) did not influence the activity of the human SHBG promoter under basal conditions (right). Data points are shown as mean ± SD of triplicates. (D) Reduction of human SHBG promoter activity after treatment with siHNF-4α was mitigated by cotreatment with siCOUP-TF1. Data points are shown as mean ± SD of triplicates; *P < 0.05, **P < 0.01 compared with cells treated with an siRNA control.
Monosaccharide-induced lipogenesis acts via HNF-4α to downregulate SHBG in HepG2 cells.
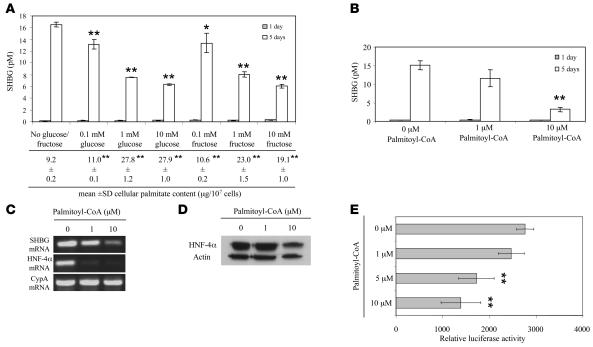
Since fructose enters the glycolytic pathway at dihydroxyacetone phosphate via fructose-6-phosphate, it bypasses key regulatory steps of glycolysis and markedly increases lipogenesis and triglyceride synthesis. This suggested that SHBG gene expression might be coordinately regulated with lipogenic activity, and we set out to test this in HepG2 cells. We first performed an experiment to assess the effects of glucose or fructose supplementation in relation to fatty acid synthesis and changes in SHBG production by HepG2 cells. To do this, we determined the levels of palmitate in HepG2 cells cultured in the absence or presence of 0.1–10 mM glucose or fructose for 5 days and found modest but significant increases in cellular palmitate levels even after treatment with 0.1 mM glucose or fructose that were also associated with small decreases in SHBG production (Figure 6A). However, we observed substantial, 2- to 3-fold increases in cellular palmitate levels after 5 days supplementation with 1–10 mM glucose or fructose, and these were associated with maximal reductions in SHBG production that also occurred after the 1 mM monosaccharide supplementations (Figure 6A).
Figure 6. Increased palmitate levels in HepG2 cells as a result of monosaccharide or palmitoyl-CoA supplementation reduce HNF-4α levels and SHBG production.
(A) Concentrations of human SHBG in the medium from untreated HepG2 cells and HepG2 cells after 5 days supplementation with 0.1–10 mM glucose or fructose. Data points are shown as mean ± SD of triplicates; *P < 0.05, **P < 0.01 compared with no glucose/fructose supplementation. In addition, the cellular content of palmitate was measured in cells after 5-day treatment with monosaccharides as indicated; **P < 0.01 compared with no glucose/fructose supplementation. (B) Concentrations of SHBG in medium from untreated HepG2 cells and HepG2 cells treated for 5 days with 1 μM or 10 μM palmitoyl-CoA. **P < 0.01 compared with no treatment. (C) HNF-4α and SHBG mRNA levels determined by RT-PCR in untreated HepG2 cells and HepG2 cells treated for 5 days with 1 μM or 10 μM palmitoyl-CoA. Cyclophilin A mRNA was amplified as an internal control. (D) Western blots of HNF-4α and actin in extracts of untreated HepG2 cells and HepG2 cells treated for 5 days with 1 μM or 10 μM palmitoyl-CoA. (E) The effects of 1–10 μM palmitoyl-CoA on human SHBG promoter activity was analyzed in HepG2 cells in the context of a luciferase reporter gene. Data points are mean ± SD of triplicate measurements; **P < 0.01 compared with no treatment.
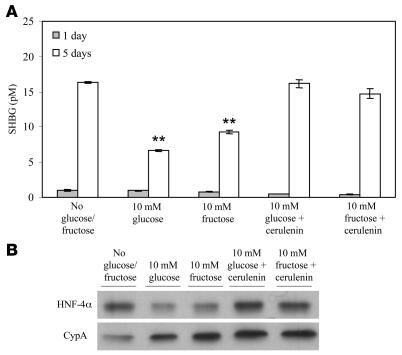
To further demonstrate that increases in palmitate as a result of de novo lipogenesis reduce SHBG in HepG2 cells, we treated HepG2 cells cultured in the presence of 10% delipidated FBS with 1–10 μM palmitoyl-CoA for 5 days. This resulted in a dose-dependent decrease in HepG2 production of SHBG (Figure 6B) that was accompanied by reductions in cellular SHBG mRNA and HNF-4α mRNA (Figure 6C) and immunoreactive HNF-4α (Figure 6D), as well as reductions in the transcriptional activity of the human SHBG promoter (Figure 6E). In addition, we cocultured cells treated with 10 mM glucose or fructose with the fatty acid synthase inhibitor cerulenin (32) and found that this prevented the monosaccharide-dependent reductions in SHBG production (Figure 7A) and cellular HNF-4α levels (Figure 7B).
Figure 7. Monosaccharide-induced reductions of SHBG production by HepG2 cells are blocked by inhibiting fatty acid synthase.
(A) Reduction of human SHBG accumulation in HepG2 medium treated daily with 10 mM glucose or fructose was blocked by cotreatment of the cells with cerulenin (10 μg/ml) over 5 days. Data points are shown as mean ± SD of triplicates; **P < 0.01 compared with no glucose/fructose supplementation. (B) Reduction of HNF-4α levels in HepG2 grown for 5 days in the presence of 10 mM glucose or fructose was also blocked by cerulenin cotreatment.
Discussion
Low plasma SHBG levels are a biomarker of the metabolic syndrome and a harbinger of its associated pathologies. In insulin-resistant individuals, elevated insulin levels have been linked to low plasma SHBG levels, but hyperglycemia is also usually present in conjunction with hypertriglyceridemia. To discriminate the direct effects of insulin and blood sugars on human SHBG gene expression in vivo, we used mice in which human SHBG transgenes are expressed under the control of their own regulatory sequences in an appropriate tissue-specific manner (21, 22). Previous studies have defined the transcription unit responsible for human SHBG gene expression in the liver (21) and mapped key regulatory elements within a proximal promoter sequence (22), which includes a binding site for USF transcription factors (24) that we previously assumed might represent part of a carbohydrate response element (22). However, removal of this USF binding site from a human SHBG transgene has no impact on the rapid reduction of human SHBG levels in the blood of mice after feeding a high-sucrose diet.
Increasing blood glucose levels through feeding high amounts of sucrose (a disaccharide of glucose and fructose) or glucose resulted in decreased human SHBG levels in the blood of mice expressing human SHBG transgenes in their livers, but both treatments increased insulin levels. However, human SHBG levels in the blood were reduced even more effectively when these transgenic mice were fed high concentrations of fructose rather than an equicaloric amount of glucose, and this occurred within just 3 days. Fructose, unlike glucose, fails to acutely stimulate pancreatic β cell insulin release (27), and we therefore suspected that insulin might not play a significant role in reducing the hepatic production of SHBG. Moreover, since the plasma half-life of SHBG is about 38 hours (33), these results imply that the effects of dietary monosaccharides on SHBG gene expression in the liver occur very rapidly.
To further determine whether insulin represses human SHBG gene expression in vivo, we took a second approach that involved using streptozotocin to induce diabetes in the same transgenic mouse model. Remarkably, serum levels of human SHBG declined rapidly as the mice became diabetic and their blood glucose levels rose and were not further reduced upon feeding a high-sucrose diet. Clearly, then, insulin is not directly responsible for repressing human SHBG production, since otherwise its blood levels should have increased as the animals became insulin deficient. Instead, these results are consistent with a rapid repression of hepatic SHBG production in response to increased blood glucose levels, as in our dietary studies. These in vivo studies prompted us to reevaluate whether insulin directly regulates SHBG expression in HepG2 cells. In a comprehensive series of experiments, we demonstrate that the SHBG gene in HepG2 cells responds similarly to the human SHBG transgene in livers of mice; i.e., it is not regulated by insulin but is repressed by monosaccharides.
Several aspects of the experimental design distinguish our work from previous studies. First, previous studies used HepG2 cells grown in serum-free medium for 1 week or more during insulin treatment, without replacing or supplementing the medium in order to allow the accumulation of SHBG to be measured (17, 18, 31). When we replicated these experiments, a modest decline in the accumulation of SHBG in the medium was observed. However, under these conditions the cells are not only serum starved, but the culture medium is depleted of nutrients, and this causes a nonspecific reduction in protein biosynthesis/secretion (18). This conclusion is supported by our observation that insulin fails to reduce steady-state SHBG mRNA levels or the transcriptional activity of the SHBG promoter in HepG2 cells grown in serum-free medium or in medium supplemented with untreated or heat-inactivated FBS. Second, a series of studies with HepG2 cells maintained in 10% FBS to simulate more physiologically relevant conditions provided additional evidence that monosaccharides directly regulate SHBG gene expression in these cells.
Reduced SHBG secretion in response to supplementation with glucose or fructose was accompanied by reductions in SHBG mRNA and the activity of the SHBG promoter in HepG2 cells. Under these conditions, HNF-4α expression was also reduced at the mRNA and protein levels in the cells, and we considered this important, because HNF-4α plays a key role in controlling human SHBG gene expression in the liver (22). This was further illustrated by showing that a similar reduction in SHBG promoter activity occurs in HepG2 cells when the cellular content of HNF-4α is reduced by treatment with siRNA to about the same extent as by monosaccharide treatment.
We have shown previously that SHBG gene transcription in HepG2 cells is under the control of a TATA-less promoter (22). In that report, we demonstrated that HNF-4α binds to a site just upstream from the major transcription start site, where it substitutes for TBP in engaging the transcriptional machinery. We have also demonstrated that competition between HNF-4α and COUP-TF1 occurs at this site, with HNF-4α promoting transcription and COUP-TF1 repressing it (22). We therefore explored whether the reduction of HNF-4α levels in HepG2 cells after exposure to monosaccharides is accompanied by a relative increase in COUP-TF1 binding in this region of human SHBG promoter. The results of a ChIP analysis revealed that this indeed occurs, and further support for this mechanism was obtained in an experiment in which cotreatment with a COUP-TF1 siRNA mitigated the monosaccharide-induced reduction of SHBG promoter activity in HepG2 cells.
Excess dietary carbohydrate is metabolized to fatty acids, with the lipogenic response to fructose being greater than that of glucose when administered in vivo (34). There are several reasons for this. First, the peripheral utilization of glucose is substantially greater than that of fructose, because glucose uptake by adipose and muscle via the GLUT4 transporter increases in response to insulin, while fructose is mainly taken up along with glucose by the GLUT2 transporter in the liver (35). In addition, fructose is a more efficient substrate for lipogenesis than glucose because it bypasses key regulatory steps in glycolysis. Although fructose is likely metabolized in HepG2 cells in much the same way, our data indicate that fructose and glucose are equipotent when administered to these cells with respect to their ability to increase lipogenesis and regulate SHBG gene expression. The most likely explanation for this is that the GLUT1 transporter, which takes up glucose but not fructose (35), is expressed at high levels in HepG2 cells, while expression of the GLUT2 transporter, which takes up both glucose and fructose (35), is relatively low in these cells (data not shown). Thus, despite the additional metabolic steps necessary for glucose to enter lipogenesis, it is likely that HepG2 cells take up glucose more efficiently than fructose and therefore both monosaccharides have equivalent effects on lipogenesis in vitro.
Long-chain fatty acids, including palmitic acid, have been identified as endogenous HNF-4α ligands that may influence its transcriptional activity (36, 37). In our experiments, palmitate levels increased substantially in HepG2 cells treated with glucose and fructose in concert with reductions in the expression of both HNF-4α and SHBG. In addition, inhibition of the regulatory enzyme complex of de novo fatty acid synthesis (38) by cerulenin not only blocked glucose or fructose from reducing SHBG production by HepG2 cells, but also prevented the reduction in cellular HNF-4α levels. These data are also consistent with a reduced loading of HNF-4α onto a cis-element in the human SHBG promoter that is shared with COUP-TF1, and with our finding that cotreatment with COUP-TF1 siRNA mitigates the effects of reduced HNF-4α mRNA levels on SHBG expression. Although decreases in HNF-4α levels in the liver in response to increased de novo lipogenesis provide an explanation for why serum SHBG levels are such a sensitive biomarker of metabolic disturbance, we do not know how increases in lipogenesis reduce HNF-4α expression in HepG2 cells. However, the HNF-4α promoter utilized in these cells also contains a shared binding site for HNF-4α and COUP-TF (39), and it is possible that long-chain fatty acid ligands of HNF-4α influence HNF-4α gene expression at this level.
In conclusion, our findings that monosaccharides regulate human SHBG gene expression by altering hepatic HNF-4α levels provide a mechanism to explain the link between plasma levels of SHBG and the metabolic extremes in obese and anorexic individuals. As far as we know, no one else has noted that monosaccharides influence HNF-4α expression in hepatocytes via their conversion to fatty acids, and this is potentially important because HNF-4α gene variants have been implicated in type 2 diabetes (40). Finally, our finding that hepatic SHBG gene expression is remarkably responsive to fructose implies that plasma SHBG levels represent a useful biomarker of metabolic disturbances associated with excess sugar consumption (28, 41).
Methods
Animals.
Mice expressing human SHBG transgenes have been characterized previously (21). Mice were maintained under standard conditions with food and water provided ad libitum, and the experimental procedures were approved by the Institutional Animal Use Subcommittees of University of Western Ontario and University of British Columbia and conducted in accordance with the Canadian Council on Animal Care.
In vivo experiments.
Transgenic mice containing human SHBG transgenes were maintained on a basal diet containing 53% carbohydrate (PicoLab Mouse Diet 20) and where indicated were fed an isocaloric high-carbohydrate diet (70% sucrose; TD 98029; Harlan-Teklad) or a semisynthetic diet containing casein as the source of protein and in which sucrose, glucose, or fructose were exchanged while maintaining essential nutrient density across all diets (42). Diabetes in mature mice was induced by a single intraperitoneal injection of 200 mg/kg streptozotocin (Sigma-Aldrich) in a 50-mM citrate buffer, pH 4.5 (29). Diabetes in these animals was confirmed by the presence of elevated blood glucose levels (>400 mg/dl) measured by Fast-Take glucose strips (LifeScan Inc.).
Cell culture experiments.
All cell culture reagents were from Life Technologies Inc. HepG2 hepatoblastoma cells (catalog no. HB-8065; ATCC) were routinely maintained in low-glucose DMEM (catalog 11885-084) supplemented with 10% FBS and antibiotics (100 U penicillin/ml and 100 μg streptomycin/ml). For experiments, HepG2 cells were cultured to 30%–50% confluence with low-glucose DMEM alone or in DMEM supplemented with 10% untreated or heat-inactivated FBS, or delipidated FBS (Cocalico Biologicals), prior to the addition of supplements (glucose, fructose, insulin, palmitoyl-CoA, or cerulenin), as indicated. Palmitate levels in HepG2 cells were determined by addition of a known amount of heptadecanoate (17:0) followed by direct methylation (43) and GLC for the separation and quantification of fatty acids (44).
Transient transfections of human SHBG promoter reporter plasmids together with a pCMVlacZ control plasmid (22) were carried out using LipofectAMINE reagent (according to Life Technologies Inc.) or HiPerfect Transfection Reagent (according to QIAGEN). The siRNA experiments were carried out using HiPerfect Transfection Reagent according to QIAGEN, using a control siRNA (catalog 1022076), HNF-4α siRNA (catalog 00161546), or COUP-TF1 siRNA (catalog 00079877). Two days after transfection, the cells were washed twice with PBS and harvested by scraping. After centrifugation, cell pellets were resuspended in 100 μl of 0.25 M Tris-Cl, pH 7.8, and cells were lysed by 3 cycles of freeze-thawing. Appropriate aliquots of cell extracts were used for measurements of luciferase and β-galactosidase activity (22). To correct for transfection efficiency, light units from the luciferase assay were divided by the OD reading from the β-galactosidase assay.
Human SHBG measurements.
To determine the human SHBG concentrations in the serum of transgenic mice, we used a saturation binding capacity assay with [3H]5α-dihydrotestosterone as the labeled ligand (45). Human SHBG accumulation in culture medium from HepG2 cells was measured using a time-resolved immunofluorometric assay (46).
RNA analysis.
Total RNA was extracted from HepG2 cells using TRIzol reagent (Invitrogen). RT was performed at 42°C for 50 minutes using 3 μg of total RNA and 200 U of Superscript II together with an oligo-dT primer and reagents provided by Invitrogen. An aliquot of the RT product was amplified in a 35-μl reaction using PCR SuperMix (Invitrogen) with appropriate oligonucleotide primer pairs corresponding to human HNF-4α (forward 5′-GCTCCTCCTTCTGCTGCTGC-3′ and reverse 5′-GGAAGAGCTTGAGACAGGCC-3′), SHBG (forward 5′-GTTGCTACTACTGCGTCACAC-3′ and reverse 5′-GCCATCTCCCATCATCCAGCCG-3′), COUP-TF1 (forward 5′-AGTGCCTCAAAGTGGGCATGAGG-3′ and reverse 5′-AGCGCCTTGAGCTTCTCCACC-3′), and cyclophilin A (forward 5′-ATGGTCAACCCCACCGTG-3′ and reverse 5′-TGCAATCCAGCTAGGCATG-3′) mRNA sequences. The PCR was performed for 40 cycles at 94°C for 15 seconds, 57–65°C for 30 seconds, and 72°C for 1 minute, and PCR products were resolved by electrophoresis in a 1% agarose gel.
Western blot analysis.
Soluble protein was extracted from HepG2 cells by homogenization in 50 mM Tris-HCl, pH 7.9, 300 mM KCl, 1.5 mM MgCl2, 0.01% Nonidet P-40, 20% glycerol supplemented with protease inhibitors (Roche Diagnostics) at 4°C, and used for Western blotting with antibodies against human HNF-4α (C-19; catalog sc-6556; Santa Cruz Biotechnology Inc.), human cyclophilin A (SA-296; BIOMOL Int.), human actin (C-11; catalog sc-1615; Santa Cruz Biotechnology Inc.), and human phospho-S6 ribosomal protein (catalog 2211; Cell Signaling Technology Inc.). Specific antibody-antigen complexes were identified using an HRP-labeled goat anti-rabbit IgG or rabbit anti-goat IgG and chemiluminescent substrates (Pierce) by exposure to x-ray film.
ChIP assays.
We performed ChIP assays with a ChIP-IT kit (Active Motif Inc.) following the manufacturer’s instructions. Briefly, HepG2 cells were cross-linked with 1% formaldehyde for 10 minutes at room temperature. After washing and treatment with glycine Stop-Fix solution, the cells were resuspended in lysis buffer and incubated for 30 minutes on ice. The cells were homogenized, and nuclei were resuspended in shearing buffer and subjected to optimized ultrasonic disruption conditions to yield 100- to 400-bp DNA fragments. The chromatin was precleared with protein G beads and incubated (overnight at 4°C) with 1 μg of each of the following specific antibodies: negative control mouse IgG and positive control anti-RNA polymerase (provided in the ChIP-IT kit), anti–COUP-TF1 (N-19; catalog sc-6575; Santa Cruz Biotechnology Inc.), and anti–HNF-4α (C-19; catalog sc-6556; Santa Cruz Biotechnology Inc.). Protein G beads were then added to the antibody/chromatin incubation mixtures and incubated for 1.5 hours at 4°C. After extensive washings, the immunoprecipitated DNA was removed from the beads in an elution buffer. To reverse cross-links and remove RNA, 5 M NaCl and RNase were added to the samples and incubated for 4 hours at 65°C. The samples were then treated with proteinase K for 2 hours at 42°C, and the DNA was purified using gel exclusion columns. The purified DNA was subjected to PCR amplification (1 cycle of 95°C for 2 minutes, 40 cycles of 94°C for 30 seconds, 64°C for 30 seconds, and 72°C for 30 seconds) using specific forward (5′-GATCCCCAGAGGGGTGATAGC-3′) and reverse (5′-GGGTAAAGGAAACAGGGGCAC-3′) primers designed to amplify a 153-bp region in the human SHBG promoter (22). As a control, PCR amplification (1 cycle of 95°C for 2 minutes, 35 cycles of 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 45 seconds) of the human GAPDH promoter was performed using forward (5′-TACTAGCGGTTTTACGGGCG-3′) and reverse (5′-TCGAACAGGAGGAGCAGAGAGAGCGA-3′) primers. The PCR products were resolved by electrophoresis in a 6% acrylamide gel and visualized after ethidium bromide staining.
Statistics.
Data were analyzed using 1-way ANOVA, and P values of < 0.05 were considered significant.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (to G.L. Hammond) and a Michael Smith Foundation for Health (MSFHR) Research Unit Award in Nutrition Research (to S.M. Innis and G.L. Hammond). G.L. Hammond holds a Tier I Canada Research Chair in Reproductive Health. S.M. Innis was supported by an MSFHR Distinguished Scholar Award and holds a Child and Family Research Institute Senior Scholarship. The authors thank Roger Dyer and Caroline Underhill for technical assistance.
Footnotes
Nonstandard abbreviations used: ChIP, chromatin immunoprecipitation; COUP-TF1, chicken OVA upstream promoter–transcription factor 1; HNF-4α, hepatocyte nuclear factor–4α; SHBG, sex hormone–binding globulin; USF, upstream stimulatory factor.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:3979–3987 (2007). doi:10.1172/JCI32249
References
- 1.De Moor P., Joossens J.V. An inverse relation between body weight and the activity of the steroid binding-globulin in human plasma. Steroidologia. 1970;1:129–136. [PubMed] [Google Scholar]
- 2.Kopelman P.G., Pilkington T.R., White N., Jeffcoate S.L. Abnormal sex steroid secretion and binding in massively obese women. Clin. Endocrinol. (Oxf.) 1980;12:363–369. doi: 10.1111/j.1365-2265.1980.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 3.Davidson B.J., et al. Free estradiol in postmenopausal women with and without endometrial cancer. J. Clin. Endocrinol. Metab. 1981;52:404–408. doi: 10.1210/jcem-52-3-404. [DOI] [PubMed] [Google Scholar]
- 4.Laaksonen D.E., et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 5.Kalme T., et al. Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J. Clin. Endocrinol. Metab. 2005;90:1550–1556. doi: 10.1210/jc.2004-0762. [DOI] [PubMed] [Google Scholar]
- 6.Tchernof A., Toth M.J., Poehlman E.T. Sex hormone-binding globulin levels in middle-aged premenopausal women. Associations with visceral obesity and metabolic profile. Diabetes Care. 1999;22:1875–1881. doi: 10.2337/diacare.22.11.1875. [DOI] [PubMed] [Google Scholar]
- 7.Heald A.H., et al. Low sex hormone binding globulin is a potential marker for the metabolic syndrome in different ethnic groups. Exp. Clin. Endocrinol. Diabetes. 2005;113:522–528. doi: 10.1055/s-2005-865807. [DOI] [PubMed] [Google Scholar]
- 8.Haffner S.M., Valdez R.A., Morales P.A., Hazuda H.P., Stern M.P. Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. J. Clin. Endocrinol. Metab. 1993;77:56–60. doi: 10.1210/jcem.77.1.8325960. [DOI] [PubMed] [Google Scholar]
- 9.Lindstedt G., et al. Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM. 12-year follow-up of population study of women in Gothenburg, Sweden. Diabetes. 1991;40:123–128. doi: 10.2337/diab.40.1.123. [DOI] [PubMed] [Google Scholar]
- 10.Sutton-Tyrrell K., et al. Sex-hormone-binding Globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 11.Haffner S.M., Katz M.S., Stern M.P., Dunn J.F. Association of decreased sex hormone binding globulin and cardiovascular risk factors. Arteriosclerosis. 1989;9:136–143. doi: 10.1161/01.atv.9.1.136. [DOI] [PubMed] [Google Scholar]
- 12.Lapidus L., Lindstedt G., Lundberg P.A., Bengtsson C., Gredmark T. Concentrations of sex-hormone binding globulin and corticosteroid binding globulin in serum in relation to cardiovascular risk factors and to 12-year incidence of cardiovascular disease and overall mortality in postmenopausal women. Clin. Chem. 1986;32:146–152. [PubMed] [Google Scholar]
- 13.Pascal N., et al. Serum concentrations of sex hormone binding globulin are elevated in kwashiorkor and anorexia nervosa but not in marasmus. Am. J. Clin. Nutr. 2002;76:239–244. doi: 10.1093/ajcn/76.1.239. [DOI] [PubMed] [Google Scholar]
- 14.Barbe P., Bennet A., Stebenet M., Perret B., Louvet J.P. Sex-hormone binding globulin and protein-energy malnutrition indexes as indicators of nutritional status in women with anorexia nervosa. Am. J. Clin. Nutr. 1993;57:319–322. doi: 10.1093/ajcn/57.3.319. [DOI] [PubMed] [Google Scholar]
- 15.Haffner S.M. Sex hormone-binding protein, hyperinsulinemia, insulin resistance and noninsulin-dependent diabetes. Horm. Res. 1996;45:233–237. doi: 10.1159/000184794. [DOI] [PubMed] [Google Scholar]
- 16.Pugeat M., Cousin P., Baret C., Lejeune H., Forest M.G. Sex hormone-binding globulin during puberty in normal and hyperandrogenic girls. J. Pediatr. Endocrinol. Metab. 2000;13:1277–1279. [PubMed] [Google Scholar]
- 17.Plymate S.R., Matej L.A., Jones R.E., Friedl K.E. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J. Clin. Endocrinol. Metab. 1988;67:460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 18.Crave J.C., Lejeune H., Brébant C., Baret C., Pugeat M. Differential effects of insulin and insulin-like growth factor-1 on the production of plasma steroid binding globulins by human hepatoblastoma-derived (Hep G2) cells. J. Clin. Endocrinol. Metab. 1995;80:1283–1289. doi: 10.1210/jcem.80.4.7536204. [DOI] [PubMed] [Google Scholar]
- 19.Haffner S.M., Katz M.S., Stern M.P., Dunn J.F. The relationship of sex hormones to hyperinsulinemia and hyperglycemia. Metabolism. 1988;37:683–688. doi: 10.1016/0026-0495(88)90091-1. [DOI] [PubMed] [Google Scholar]
- 20.Wilson P.W., D’Agostino R.B., Parise H., Sullivan L., Meigs J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 21.Jänne M., Deol H.K., Power S.G., Yee S.P., Hammond G.L. Human sex hormone-binding globulin gene expression in transgenic mice. Mol. Endocrinol. 1998;12:123–136. doi: 10.1210/mend.12.1.0050. [DOI] [PubMed] [Google Scholar]
- 22.Jänne M., Hammond G.L. Hepatocyte nuclear factor-4 controls transcription from a TATA-less human sex hormone-binding globulin gene promoter. J. Biol. Chem. 1998;273:34105–34114. doi: 10.1074/jbc.273.51.34105. [DOI] [PubMed] [Google Scholar]
- 23.Selva D.M., Hogeveen K.N., Seguchi K., Tekpetey F., Hammond G.L. A human sex hormone-binding globulin isoform accumulates in the acrosome during spermatogenesis. J. Biol. Chem. 2002;277:45291–45298. doi: 10.1074/jbc.M205903200. [DOI] [PubMed] [Google Scholar]
- 24.Selva D.M., Hogeveen K.N., Hammond G.L. Repression of the human sex hormone-binding globulin gene in Sertoli cells by upstream stimulatory transcription factors. J. Biol. Chem. 2005;280:4462–4468. doi: 10.1074/jbc.M409616200. [DOI] [PubMed] [Google Scholar]
- 25.Diaz Guerra M.J., et al. Functional characterization of the L-type pyruvate kinase gene glucose response complex. Mol. Cell. Biol. 1993;13:7725–7733. doi: 10.1128/mcb.13.12.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaulont S., Kahn A. Transcriptional control of metabolic regulation genes by carbohydrates. FASEB J. 1994;8:28–35. doi: 10.1096/fasebj.8.1.8299888. [DOI] [PubMed] [Google Scholar]
- 27.Curry D.L. Effects of mannose and fructose on the synthesis and secretion of insulin. Pancreas. 1989;4:2–9. doi: 10.1097/00006676-198902000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Elliott S.S., Keim N.L., Stern J.S., Teff K., Havel P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 29.Ito M., Kondo Y., Nakatani A., Naruse A. New model of progressive non-insulin-dependent diabetes mellitus in mice induced by streptozotocin. Biol. Pharm. Bull. 1999;22:988–989. doi: 10.1248/bpb.22.988. [DOI] [PubMed] [Google Scholar]
- 30.Weng Q.P., et al. Regulation of the p70 S6 kinase by phosphorylation in vivo. J. Biol. Chem. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- 31.Loukovaara M., Carson M., Adlercreutz H. Regulation of production and secretion of sex hormone binding globulin in HepG2 cell cultures by hormones and growth factors. J. Clin. Endocrinol. Metab. 1995;80:160–164. doi: 10.1210/jcem.80.1.7829605. [DOI] [PubMed] [Google Scholar]
- 32.Menendez J.A., et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cousin P., Déchaud H., Grenot C., Lejeune H., Pugeat M. Human variant sex hormone-binding globulin (SHBG) with an additional carbohydrate chain has a reduced clearance rate in rabbit. J. Clin. Endocrinol. Metab. 1998;83:235–240. doi: 10.1210/jcem.83.1.4515. [DOI] [PubMed] [Google Scholar]
- 34.Teff K.L., et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 35.Thorens B. Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am. J. Physiol. 1996;270:G541–G553. doi: 10.1152/ajpgi.1996.270.4.G541. [DOI] [PubMed] [Google Scholar]
- 36.Hertz R., Magenheim J., Berman I., Bar-Tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4α. Nature. 1998;392:512–516. doi: 10.1038/33185. [DOI] [PubMed] [Google Scholar]
- 37.Dhe-Paganon S., Duda K., Iwamoto M., Chi Y.I., Shoelson S.E. Crystal structure of the HNF4 alpha ligand binding domain in complex with endogenous fatty acid ligand. J. Biol. Chem. 2002;277:37973–37976. doi: 10.1074/jbc.C200420200. [DOI] [PubMed] [Google Scholar]
- 38.Rumberger J.M., Wu T., Hering M.A., Marshall S. Role of hexosamine biosynthesis in glucose-mediated up-regulation of lipogenic enzyme mRNA levels. J. Biol. Chem. 2003;278:28547–28552. doi: 10.1074/jbc.M302793200. [DOI] [PubMed] [Google Scholar]
- 39.Hatzis P., Talianidis I. Regulatory mechanisms controlling human hepatocyte nuclear factor 4α gene expression. Mol. Cell. Biol. 2001;21:7320–7330. doi: 10.1128/MCB.21.21.7320-7330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamagata K., et al. Mutations in the hepatocyte nuclear factor-4α gene in maturity-onset diabetes of the young (MODY1). Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 41.Bray G.A., Nielsen S.J., Popkin B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 42.Innis S.M., Rioux F.M., Auestad N., Ackman R.G. Marine and freshwater fish oil varying in arachidonic, eicosapentaenoic and docosahexaenoic acids differ in their effects on organ lipids and fatty acids in growing rats. J. Nutr. 1995;125:2286–2293. doi: 10.1093/jn/125.9.2286. [DOI] [PubMed] [Google Scholar]
- 43.Friesen R., Innis S.M. Trans fatty acids in human milk in Canada declined with the introduction of trans fat food labeling. J. Nutr. 2006;136:2558–2561. doi: 10.1093/jn/136.10.2558. [DOI] [PubMed] [Google Scholar]
- 44.Innis S.M., Dyer R.A. Brain astrocyte synthesis of docosahexaenoic acid from n-3 fatty acids is limited at the elongation of docosapentaenoic acid. J. Lipid Res. 2002;43:1529–1536. doi: 10.1194/jlr.m200120-jlr200. [DOI] [PubMed] [Google Scholar]
- 45.Hammond G.L., Lähteenmäki P.L. A versatile method for the determination of serum cortisol binding globulin and sex hormone binding globulin binding capacities. Clin. Chim. Acta. 1983;132:101–110. doi: 10.1016/0009-8981(83)90237-1. [DOI] [PubMed] [Google Scholar]
- 46.Selva D.M., et al. Human sperm sex hormone-binding globulin isoform: characterization and measurement by time-resolved fluorescence immunoassay. J. Clin. Endocrinol. Metab. 2005;90:6275–6282. doi: 10.1210/jc.2005-1192. [DOI] [PubMed] [Google Scholar]