Abstract
A sex disparity in airway responsiveness to cholinergic stimulation has been observed in laboratory mice in that males are considerably more responsive than females, but the basis for this difference is unclear. In this report, we demonstrate that male sex hormones promote murine airway responsiveness to cholinergic stimulation via vagus nerve-mediated reflex mechanisms. In tissue bath preparations, no sex-based differences were observed in the contractile responses of isolated tracheal and bronchial ring segments to carbachol, indicating that the mechanism(s) responsible for the in vivo sex difference is (are) absent ex vivo. Bilateral cervical vagotomy was found to abolish in vivo airway responsiveness to methacholine in male mice, whereas it did not alter the responses of females, suggesting a regulatory role for male sex hormones in promoting reflex airway constriction. To test this possibility, we next studied mice with altered circulating male sex hormone levels. Castrated male mice displayed airway responsiveness equivalent to that observed in intact females, whereas administration of exogenous testosterone to castrated males restored responsiveness, albeit not to the level observed in intact males. Administration of exogenous testosterone to intact female mice similarly enhanced responsiveness. Importantly, the promotive effects of exogenous testosterone in castrated male and intact female mice were absent when bilateral vagotomy was performed. Together, these data indicate that male sex hormones promote cholinergic airway responsiveness via a vagally mediated reflex mechanism that may be important in the regulation of airway tone in the normal and diseased lung.
Keywords: respiratory mechanics, methacholine, androgens
Murine Models are Increasingly used to study pathogenic mechanisms and potential therapies for a variety of human lung diseases. This is due in part to the short breeding time required to generate sufficient numbers of animals for study, the well-understood murine immune system, the relative ease with which genomic manipulations can be made, and the advent of sensitive and reliable methods to measure lung function in small animals. Including lung function parameters in the assessment of experimental lung disease models provides important insight into physiological outcomes that cannot always be predicted based on biochemical and morphological assessments. Furthermore, and perhaps most importantly, doing so more closely relates these models to the human conditions they are intended to emulate. As such, it is somewhat surprising that whereas factors including genetic background and obesity are recognized to influence lung function, respiratory mechanics and airway responsiveness in mice (12, 15, 22, 26, 27), the potential influence of sex in murine studies of lung function and in murine models of lung disease is often overlooked.
Limited examples are available, but sex differences in certain aspects of murine lung function have been documented. In particular, total lung capacity and enhanced pause (Penh) responses to methacholine aerosol have been reported to differ in naïve male and female mice (19, 22, 24). Importantly, we recently observed a considerable sex difference in murine airway responsiveness to cholinergic stimulation, as assessed by invasive measurement of respiratory mechanics, wherein the airways of naïve male C57BL/6 and BALB/c mice were found to be considerably more sensitive to methacholine aerosol than were those of naïve females (4). Furthermore, the minimal airway responsiveness of female mice to methacholine is not likely due to effects mediated by female sex hormones, as alteration of circulating levels via ovariectomy or exogenous administration of estradiol fails to alter responsiveness (5). At present, however, the potential for male sex hormones to influence airway responsiveness is unknown.
To address this issue, the present study utilized in vivo and ex vivo techniques to evaluate the effects of male sex hormones on airway responsiveness to cholinergic stimulation. Our data indicate that male sex hormones promote airway responsiveness in male and female mice and that this phenomenon is dependent on vagally mediated parasympathetic reflex effects on airway smooth muscle contractility. These results suggest an important role for male sex hormones in the regulation of airway tone and have important implications concerning the design and interpretation of murine studies of lung disease for which functional parameters constitute significant endpoints.
MATERIALS AND METHODS
Animals and treatments
All studies were conducted in accordance with principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the National Institute of Environmental Health Sciences. Adult male and female C57BL/6 mice (8- to 12-wk-old; The Jackson Laboratory, Bar Harbor, ME) were used. When performed, surgical castration of male mice occurred 3 wk before experimentation. Similarly, subcutaneous implantation of pellets containing 5-α-dihydrotestosterone (15 mg, 60-day release; DHT; Innovative Research of America, Sarasota, FL) or placebo into castrated male mice or intact female mice occurred 3 wk before experimentation. The DHT pellets have previously been shown to exert androgenic activity in mice 3–4 wk postimplantation (16). When performed, bilateral cervical vagotomy occurred immediately before analysis of lung function and airway responsiveness. Briefly, the vagus nerves on each side of the trachea/esophagus were identified under a dissecting microscope, carefully dissected from the carotid arteries and surrounding connective tissue, and severed immediately before tracheostomy and attachment to the mechanical ventilator as described below. On animals not undergoing bilateral vagotomy, sham procedures were performed by identifying and isolating the vagus nerves but not proceeding with the severance.
Analysis of contractile responses in isolated tracheal and bronchial ring segments
Naïve male and female mice were killed with an overdose of sodium pentobarbital (80 mg/kg ip), and the lungs and trachea were quickly removed en bloc and placed in Krebs bicarbonate solution (pH 7.40) containing (in mM) NaCl (117.9), KCl (4.69), CaCl2·2H2O (2.5), KH2PO4 (1.18), D-glucose (10.0), MgSO4 (0.57), and NaHCO3 (27.37). Superficial fat and connective tissue were carefully cleaned from the trachea and mainstem bronchi, and ring segments 3–4 mm in length were prepared. For each mouse studied, two tracheal rings (1 “top” and 1 “bottom”) and two bronchial rings (1 from the left bronchus and 1 from the right bronchus) were used, and the responses of these pairs were averaged to calculate values for each animal; no differences between the responses of top and bottom tracheal rings or between the responses of left and right bronchial rings were observed. Two triangular stainless steel hooks were carefully fed through the lumens, and the ring segments were then placed in individual double-jacketed glass organ baths (2 ml volume; Radnoti Glass Technology, Monrovia, CA) containing Krebs solution (pH 7.40, 37°C) continuously bubbled with 95% O2 and 5% CO2. The lower hooks were anchored to the bottom of the baths, and the upper hooks were attached to pressure transducers connected to a PowerLab data acquisition and analysis system (ADInstruments, Colorado Springs, CO).
Ring segments were allowed to equilibrate for 45–60 min, during which time the bath solution was changed every 10–15 min. Also during this time, the resting tension of tracheal ring segments was gradually increased from 0 to 0.5 g and that of bronchial ring segments from 0 to 0.25 g. On establishment of stable resting tensions, responsiveness of ring segments to cumulatively administered carbachol (10−8–10−4.5 M; Sigma, St. Louis, MO) was assessed. Concentrations were not increased until the response to the previous concentration had stabilized, typically 3–4 min after administration. At the conclusion of each experiment, ring segments were blotted dry and weighed. Force generation was calculated as milligrams of tension developed per milligrams of tissue weight and expressed as the percent change from resting tension values.
Analysis of lung function and airway responsiveness
Invasive analysis of lung function was performed with the FlexiVent mechanical ventilator system (SCIREQ, Montreal, Québec, Canada) as described previously (4). Briefly, mice were anesthetized with urethane (1.5 g/kg ip), and once surgical anesthesia was established, a tracheostomy was performed, and a 19-gauge stainless-steel cannula was inserted into the trachea. When performed, bilateral vagotomy occurred immediately before tracheostomy. Animals were then paralyzed with pancuronium bromide (0.8 mg/kg ip) and placed on a 37°C heating pad, and the cannula was connected to the computer-controlled ventilator. Ventilation was maintained at a rate of 150 breaths/min and a tidal volume of 7.5 ml/kg with a positive end-expiratory pressure of 3 cmH2O. Heart rate was monitored with a portable monitor (CardioMonitor; BAS Vetronics, West Lafayette, IN) to ensure proper anesthetic depth. Baseline values for each mouse were obtained by applying a 2-s perturbation at a frequency of 2.5 Hz followed by an 8-s pseudorandom perturbation consisting of waveforms of mutually prime frequencies (0.5–19.6 Hz) a total of 3 times at 30-s intervals; these maneuvers generated data that were fit to the single compartment and constant phase models of respiratory mechanics, respectively. The averages of these measurements for each mouse served as its baseline values. Following acquisition of baseline data, airway responsiveness to aerosolized methacholine (0–25 mg/ml PBS, delivered by ultrasonic nebulizer) was assessed. Aerosols were delivered for 10 s without altering the ventilatory pattern, after which the 2- and 8-s perturbations were applied consecutively every 30 s for 5 min. Peak responses during each 5-min period were determined, and only values with a coefficient of determination of 0.95 or greater were used (based on this criterion, ~1% of single compartment model data points and 10% of constant phase model data points for each animal were excluded). In particular, changes in total respiratory system resistance (R), Newtonian resistance (Rn; indicative of central airways responsiveness), and tissue damping (G; indicative of peripheral airways responsiveness) were monitored. Brief occlusion of the expiratory tube was performed before taking baseline measurements and before each aerosol administration to prevent atelectasis and reset the volume history.
Immunoblotting
Whole-lung lysates were prepared from frozen lung tissues using 100 mM Tris base buffer (pH 8.5). Immunoblotting for the M2 muscarinic acetylcholine receptor (M2AchR) was performed on lysates using a rabbit polyclonal antibody (sc-1504; Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:100, followed by a horseradish peroxidase-conjugated bovine anti-rabbit polyclonal antibody (sc-2374, Santa Cruz Biotechnology) at a dilution of 1:1,000. Bound antibodies were detected and visualized with an enhanced chemiluminescent detection system (Pierce, Rockford, IL). To account for potential variation in housekeeping gene expression and gel loading, blots were subsequently probed for GAPDH using a goat anti-human polyclonal antibody (sc-20357, Santa Cruz Biotechnology) that also recognizes murine GAPDH. Relative band intensities, expressed as arbitrary units of M2AchR to GAPDH, were determined by densitometry with a ChemiImager 5500 system (Alpha Innotech, San Leandro, CA).
Statistical analyses
All data are presented as group means ± SE. Ring tension data were fit to a Hill model for analysis using nonlinear mixed effects models. The nonlinear models were fit using SAS statistical software (Cary, NC). Maximum contractile responses and concentrations of carbachol resulting in half-maximum contractions (EC50 values) were calculated to determine and compare the sensitivity of ring segments from male and female mice to carbachol. In vivo methacholine response data were analyzed by two-way ANOVA followed by Bonferroni posttests at each concentration of methacholine using GraphPad Prism software (GraphPad Software, San Diego, CA) with equal variance assumed for all analyses. Quantitative immunoblotting data were analyzed by an unpaired Student’s t-test. In all instances, statistical significance was defined as P < 0.05.
RESULTS
Carbachol-induced contraction of isolated tracheal and bronchial ring segments is not sex-dependent
We have previously observed the airways of naïve male mice to be considerably more responsive to aerosolized methacholine than those of naïve female mice (4). To examine whether this disparity is related to innate differences in airway smooth muscle contractile properties, the responses to cholinergic stimulation of tracheal and bronchial ring segments isolated from naïve male and female mice were examined. Tracheal and bronchial rings from male and female mice responded similarly to stimulation with carbachol with no significant differences observed between the sexes (Fig. 1, A and B). Specifically, EC50 values for male and female tracheal rings were 170 ± 31 and 140 ± 16 nM, respectively, while values for bronchial rings were 170 ± 62 and 170 ± 63 nM. Maximum contractile responses also did not differ between the sexes in either tracheal or bronchial rings (P > 0.05 for both). Importantly, the average weights of the tissues studied did not differ significantly between sexes (0.82 ± 0.06 and 0.78 ± 0.05 mg for male and female tracheal rings, respectively, and 0.75 ± 0.06 and 0.63 ± 0.05 mg for bronchial rings), nor did the average initial resting tensions (602 ± 43 and 576 ± 31 mg/mg tissue for male and female tracheal rings, respectively, and 436 ± 46 and 474 ± 36 mg/mg tissue for bronchial rings). Thus differential airway smooth muscle contractile responses to direct cholinergic stimulation do not appear to underlie the in vivo sex difference in airway responsiveness.
Fig. 1.
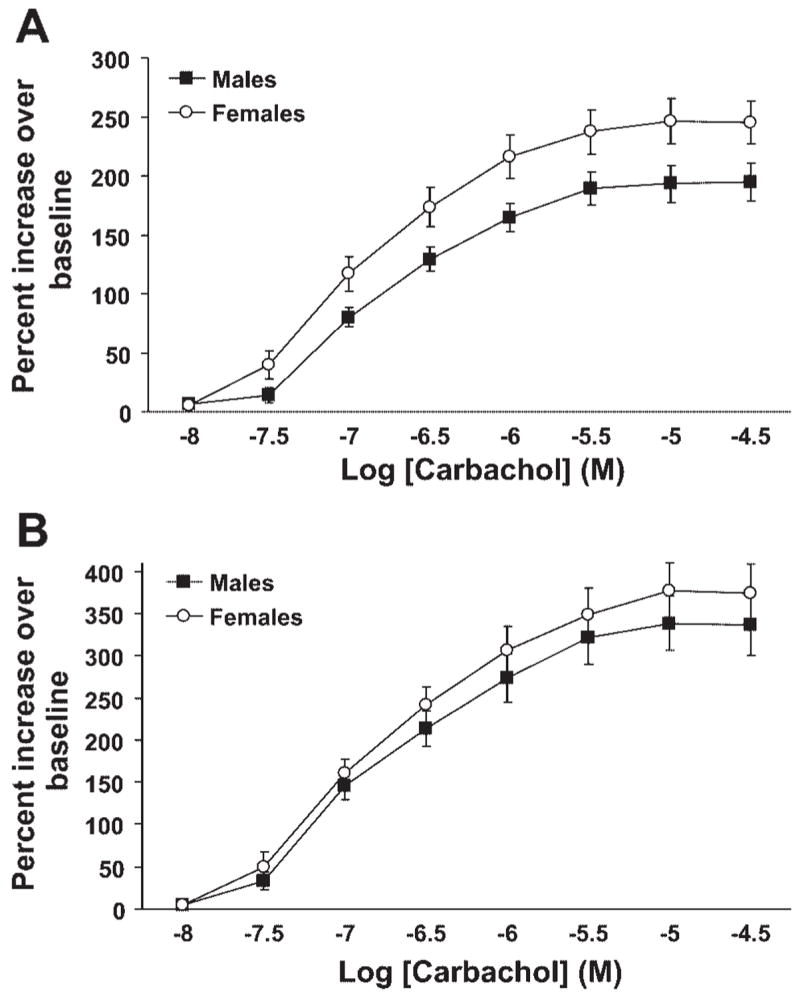
Carbachol-induced contractile responses in tracheal and bronchial ring segments isolated from naïve male and female mice. A: concentration-response curves for tracheal ring segments (n = 11 males and 12 females). B: concentration-response curves for bronchial ring segments (n = 9 per group).
Bilateral vagotomy abolishes in vivo airway responsiveness to methacholine in male mice
As cholinergic stimulation has been shown to cause reflex bronchoconstriction via vagus nerve-mediated mechanisms in rats, sheep, and dogs (2, 30, 32), we predicted that similar reflex mechanisms might exist in mice and may underlie the lack of sex differences observed in isolated airway segments studied ex vivo. Thus the effect of bilateral vagotomy on methacholine-induced airway responses was assessed in vivo. Intact male mice were significantly more responsive to methacholine aerosol than were intact female mice (Fig. 2, A–C), consistent with our previous observations (4). Surgical severing of the vagus nerves at the level of the trachea eliminated airway responsiveness to methacholine in male mice but did not affect the responses of female mice (Fig. 2, A–C). This effect was observed for all three parameters of responsiveness measured (R, Rn, and G), thereby suggesting a regulatory role for male sex hormones in promoting methacholine-induced reflex constriction of central and peripheral airways of intact male mice. Importantly, changes in respiratory system elastance paralleled those observed for R, Rn, and G, whereas baseline values of R, Rn and G did not differ between males and females and were not affected by vagotomy.
Fig. 2.
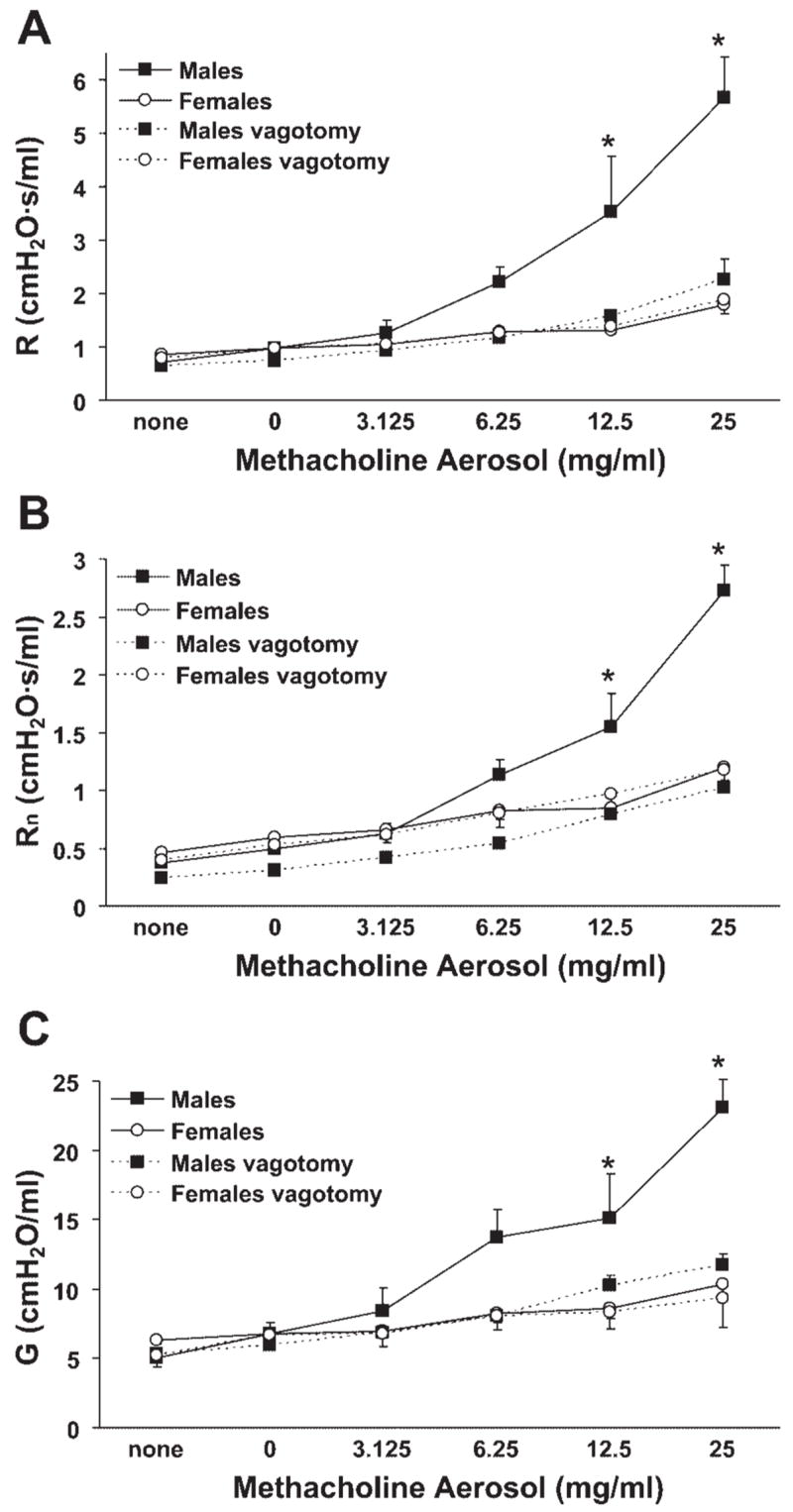
Methacholine-induced airway responsiveness in male mice, measured as changes in total respiratory system resistance (R), Newtonian resistance (Rn), and tissue damping (G), was greater than in female mice and was abolished by bilateral vagotomy. A: total respiratory system resistance. B: Newtonian resistance. C: tissue damping. *P < 0.05 vs. all other groups (n = 7–8 per group).
Male sex hormones promote in vivo airway responsiveness to methacholine that is mediated by vagal reflex mechanisms
Sex hormone-mediated effects represented the most obvious potential source of the sex difference in airway responsiveness observed in vivo. As we have previously observed a lack of alteration of airway responsiveness in ovariectomized and in estradiol-treated female mice relative to naïve female mice (5), we focused our attention on the influence of male sex hormones. Airway responsiveness in castrated male mice was comparable to that observed in intact females, suggesting that male sex hormones were promoting the responses to methacholine (Fig. 3, A–C). Confirming this inference was the effect of DHT administration to castrated male mice and to intact female mice; in both cases, airway responsiveness to methacholine was increased (Fig. 3, A–C). It should be noted that the responsiveness resulting from DHT administration in castrated male and intact female mice was not equivalent to that observed in intact male mice (compare data presented in Fig. 3 to those in Fig. 2), indicating the likely involvement of other gonadal and/or developmental factors in the responsiveness of intact male mice to methacholine. Greater responsiveness in castrated male and intact female mice administered DHT may have been observed if a higher dose of DHT was used, although we intentionally used a dose that has previously demonstrated androgenic activity in mice (16). Importantly, however, the responses of castrated male and intact female mice administered DHT were eliminated by bilateral vagotomy (Fig. 4). Furthermore, respiratory system elastance was affected in a similar manner as R, Rn, and G, and baseline values of R, Rn, and G did not differ among the various treatment groups. Collectively, these findings indicate a vagally mediated promotive effect of male sex hormones on in vivo airway responsiveness to cholinergic stimulation.
Fig. 3.
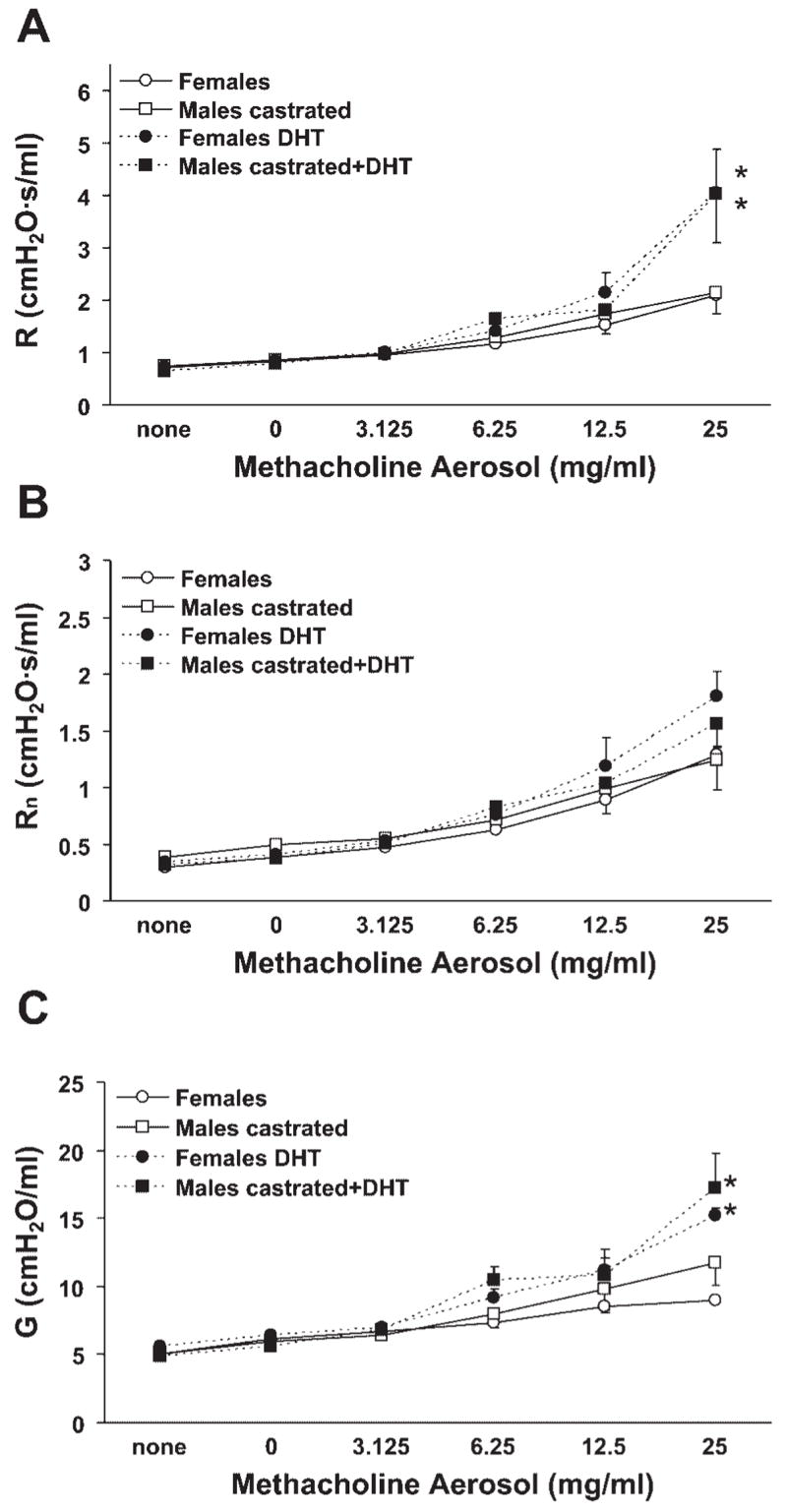
Methacholine-induced airway responsiveness was minimal in intact female and castrated male mice and was increased by 5-α-dihydrotestosterone (DHT) administration. A: total respiratory system resistance. B: Newtonian resistance. C: tissue damping. *P < 0.05 vs. corresponding groups not administered DHT (n = 4–6 per group).
Fig. 4.
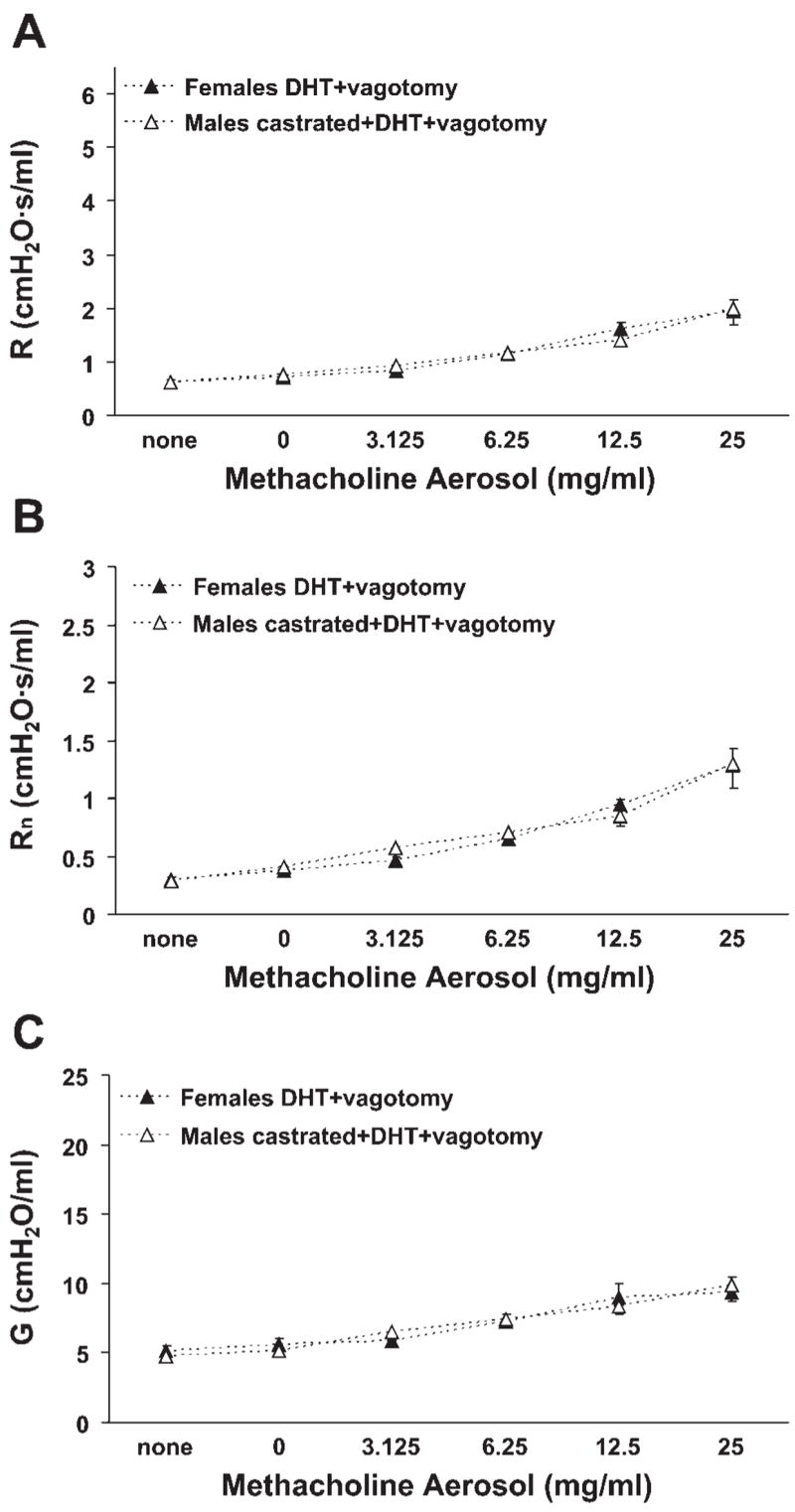
Bilateral vagotomy resulted in minimal methacholine-induced airway responsiveness in intact female and castrated male mice administered DHT. A: total respiratory system resistance. B: Newtonian resistance. C: tissue damping. n = 5 per group.
Pulmonary M2AchR protein expression is equivalent in intact male and female mice
The M2AchR is responsible for negative feedback regulation of acetylcholine release in the neuromuscular junction (9), and mice genetically deficient in this receptor display enhanced airway reactivity to cholinergic and vagal stimulation (8). Hence, pulmonary M2AchR protein levels were determined in naïve male and female mice to determine if differences in M2AchR expression might underlie the in vivo sex differences in airway responsiveness. Similar M2AchR protein levels (expressed as a ratio of M2AchR-to-GAPDH) were observed in males and females (Fig. 5), suggesting that this was not the case.
Fig. 5.
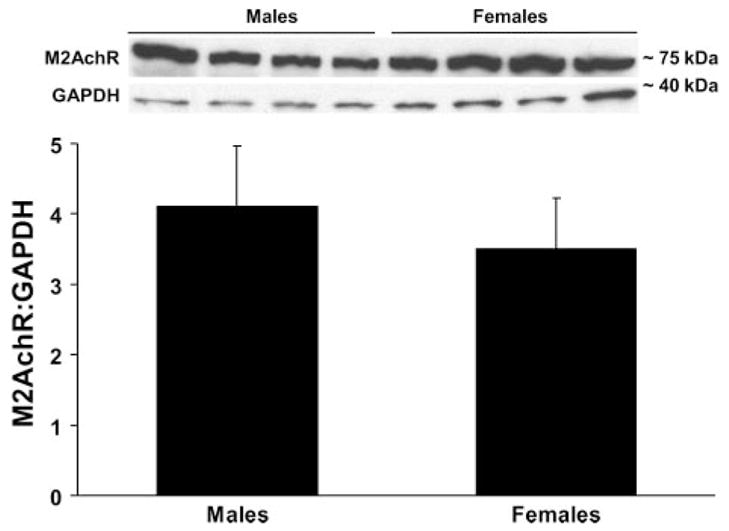
Pulmonary M2 muscarinic acetylcholine receptor (M2AchR) protein levels did not differ in intact male and female mice (n = 4 per group).
DISCUSSION
Given the widespread use of mice as a model to study the pathogenesis of lung diseases and associated functional alterations, it is important to recognize factors that might influence experimental endpoints of interest. In this respect, the roles of sex and sex hormones are receiving increasing attention, particularly with regard to their effects on lung function. The present study was thus undertaken to examine the basis for the disparity in airway responsiveness to cholinergic stimulation that exists between naïve adult male and female mice (4). Results indicate that the exaggerated airway responsiveness of male mice relative to that of female mice appears to be due to promotive effects of male sex hormones that are dependent on a reflex pathway mediated by the vagus nerves.
Differences in pulmonary anatomy exist between male and female mice, with females typically having proportionally smaller lungs and larger conducting airways than males (22) and possessing smaller and more numerous alveoli (18). Despite these dissimilarities, sex-dependent variation in baseline mechanical properties of the murine lung appears to be minimal (22, 24). On the basis of our previous observation of a sex difference in airway responsiveness to methacholine aerosol, we sought to determine if there is an inherent difference in the abilities of male and female airway smooth muscle to respond to direct cholinergic stimulation. Minimal sex differences in the contractility of isolated tracheal and bronchial ring segments to direct cholinergic stimulation were observed, however, suggesting that this is not the case. Numerous other studies have documented a lack of correlation between in vivo and ex vivo human airway responsiveness to cholinergic stimulation (1, 23, 28), suggesting that the mechanism(s) responsible for in vivo responsiveness is (are) not necessarily present ex vivo. This was likely the case in the present study as well.
Parasympathetic innervation of the respiratory system is recognized as contributing significantly to the maintenance of airway tone in normal and diseased settings, and a significant role for parasympathetic reflex pathways in the bronchoconstrictive effects of numerous compounds (e.g., histamine, serotonin, and acetylcholine) has been demonstrated in a variety of species (reviewed in Ref. 3). Specifically, bilateral vagotomy or cooling of the vagus nerves significantly reduces bronchoconstriction induced by cholinergic agonists in rats, sheep, and dogs (2, 30, 32). However, we are unaware of reported effects of vagus nerve interruption on responsiveness of mice to cholinergic stimulation, and in particular to inhaled cholinergic agonists. We observed that bilateral vagotomy abolished the responsiveness of intact male mice to aerosolized methacholine, whereas it did not alter the responsiveness of intact female mice, suggesting that airway responsiveness to cholinergic stimulation in male mice is mediated almost entirely by vagal reflex pathways. The same may be true in female mice but may merely be undetectable in our assessments due to the relatively nominal innate responsiveness of females to begin with. The observation that castration of male mice eliminated airway responsiveness to a similar extent as did bilateral vagotomy suggested a potential association between male sex hormone effects and the vagal contribution to airway reactivity. This was confirmed when DHT administration was found to increase responsiveness of castrated male and intact female mice and was further corroborated when vagotomy was shown to nullify this effect. Of note, testosterone has also been shown to promote baroreceptor-mediated experimental bradycardia (7), suggestive of a general promotive effect of male sex hormones on parasympathetic vagal activity. Our observations imply a novel role for male sex hormones in promoting airway responsiveness to cholinergic stimulation and underscore the importance of accounting for sex in the design and analysis of murine studies of lung function and disease.
To begin to address the underlying mechanistic basis for male sex hormone-mediated promotion of responsiveness to cholinergic stimulation, we determined pulmonary M2AchR expression levels in naïve male and female mice. The M2AchR mediates negative feedback inhibition of acetylcholine release from parasympathetic nerve terminals in the airways (9), and its inhibition or absence promotes airway responsiveness to vagal stimulation (8, 31). Furthermore, we have observed that the airways of female estrogen receptor-α-deficient mice are male-like (i.e., more sensitive than those of wild-type females to methacholine aerosol) and that this phenotype is associated with decreased M2AchR expression and function (5). No difference was observed in the amount of M2AchR protein in the lungs of naïve male and female mice, however, indicating that this mechanism was not likely contributing to the sex difference in airway reactivity observed here.
In addition to direct effects on specific receptors, it is thought that in most instances chemical mediator-induced bronchoconstriction results from indirect or subsequent effects on multiple receptors and pathways that mediate reflex responses including rapidly adapting stretch receptors, slowly adapting stretch receptors, and pulmonary and bronchial C fibers (reviewed in Ref. 6). Furthermore, afferent nerves are subject to varying degrees of neuromodulation by many mediators and autocoids such that the electrical excitability of the nerves can be significantly altered (29). The integration of pulmonary vagal afferent nerve inputs in the nucleus tractus solitarius provides another level of complexity in the processing of afferent information and in the subsequent regulation of efferent output to the lungs. Although we favor an enhanced excitatory (cholinergic) component in intact male mice as underlying the observed sex difference in airway responsiveness, an alternative explanation is that excitatory (cholinergic) and inhibitory (adrenergic and/or nonadrenergic) reflexes effectively cancel one another in female but not in male mice, implying an impaired or less effective inhibitory reflex in male mice. Parasympathetic nerve-mediated relaxations have been attributed in part to nitric oxide and/or S-nitrosothiols (10, 11, 20), and there is published evidence for sex differences in S-nitrosothiol metabolism in mice (14), supporting the possibility of sex differences in this mechanism as underlying our observations. Whether male sex hormones exert regulatory effects on these and/or other processes involved in the control of airway tone and responsiveness to cholinergic stimulation remains to be determined and represents an important area of future investigation.
A recent report indicates a similar sex difference in airway responsiveness to methacholine in adult guinea pigs to that which we have observed in adult mice, namely an increased sensitivity of males compared with females (21). In contrast to our observations in adult mice, however, healthy adult female humans are generally more sensitive to methacholine-induced airway constriction (measured as the decline in forced expiratory volume in 1 s, or FEV1) than are healthy adult male humans (13, 17). Whereas female mouse airways are relatively larger than those of males, the opposite is true in humans, and these anatomical differences may contribute to the apparent species difference in cholinergic airway responsiveness between sexes. In humans, it has been demonstrated that the sex disparity in responsiveness can be accounted for by taking the relative differences in lung and airway size into account (25). In our study, mice were ventilated based on body weight (and by correlation, lung size) such that tidal volume per unit body weight was equivalent for all mice examined. It is therefore difficult to directly compare our murine airway responsiveness data to those generated from human studies, and other potentially confounding factors must also be recognized. These include but are not limited to environmental factors (exposure to second hand smoke, ozone, etc.), which may affect human but not murine studies, and the innate methodological differences in human and murine lung function assessment protocols. Regardless, it is interesting to note that greater airway responsiveness appears to be associated with smaller caliber airways in both species.
In conclusion, we have demonstrated that male sex hormones promote murine airway responsiveness to cholinergic stimulation via a vagally mediated reflex pathway. This effect of male sex hormones represents the basis for the increased sensitivity of male vs. female mice to aerosolized methacholine and should be carefully considered when designing and interpreting murine studies of lung function that include cholinergic responsiveness as an experimental endpoint. Delineating the underlying basis for this effect of male sex hormones may reveal novel mechanisms underlying functional abnormalities associated with a variety of lung disease states.
Acknowledgments
We are grateful to Drs. Donald Cook and Dianne Walters for helpful comments during preparation of this manuscript. This research was conducted in part at the National Institute of Environmental Health Sciences Inhalation Facility under contract to Alion Science and Technology.
GRANTS
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. J. W. Card is the recipient of a Senior Research Training Fellowship from the American Lung Association of North Carolina.
References
- 1.Armour CL, Black JL, Berend N, Woolcock AJ. The relationship between bronchial hyperresponsiveness to methacholine and airway smooth muscle structure and reactivity. Respir Physiol. 1984;58:223–233. doi: 10.1016/0034-5687(84)90150-6. [DOI] [PubMed] [Google Scholar]
- 2.Badier M, Soler M, Mallea M, Delpierre S, Orehek J. Cholinergic responsiveness of respiratory and vascular tissues in two different rat strains. J Appl Physiol. 1988;64:323–328. doi: 10.1152/jappl.1988.64.1.323. [DOI] [PubMed] [Google Scholar]
- 3.Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol. 2006;101:971–985. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- 4.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177:621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, Graves JP, Walker VR, Flake GP, Voltz JW, Zhu D, Jacobs ER, Dakhama A, Larsen GL, Loader JE, Gelfand EW, Germolec DR, Korach KS, Zeldin DC. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha deficient mice. Am J Respir Crit Care Med. 2007;175:126–135. doi: 10.1164/rccm.200509-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8:291–301. doi: 10.1046/j.1440-1843.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Mas MM, Afify EA, Mohy El-Din MM, Omar AG, Sharabi FM. Testosterone facilitates the baroreceptor control of reflex bradycardia: role of cardiac sympathetic and parasympathetic components. J Cardiovasc Pharmacol. 2001;38:754–763. doi: 10.1097/00005344-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JT, Vincent SG, Gomeza J, Yamada M, Wess J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J. 2004;18:711–713. doi: 10.1096/fj.03-0648fje. [DOI] [PubMed] [Google Scholar]
- 9.Fryer AD, Jacoby DB. Muscarinic receptors and control of airway smooth muscle. Am J Respir Crit Care Med. 1998;158:S154–S160. doi: 10.1164/ajrccm.158.supplement_2.13tac120. [DOI] [PubMed] [Google Scholar]
- 10.Hasaneen NA, Foda HD, Said SI. Nitric oxide and vasoactive intestinal peptide as co-transmitters of airway smooth-muscle relaxation: analysis in neuronal nitric oxide synthase knockout mice. Chest. 2003;124:1067–1072. doi: 10.1378/chest.124.3.1067. [DOI] [PubMed] [Google Scholar]
- 11.Kakuyama M, Ahluwalia A, Rodrigo J, Vallance P. Cholinergic contraction is altered in nNOS knockouts. Cooperative modulation of neural bronchoconstriction by nNOS and COX. Am J Respir Crit Care Med. 1999;160:2072–2078. doi: 10.1164/ajrccm.160.6.9808105. [DOI] [PubMed] [Google Scholar]
- 12.Levitt RC, Mitzner W. Expression of airway hyperreactivity to acetylcholine as a simple autosomal recessive trait in mice. FASEB J. 1988;2:2605–2608. doi: 10.1096/fasebj.2.10.3384240. [DOI] [PubMed] [Google Scholar]
- 13.Leynaert B, Bousquet J, Henry C, Liard R, Neukirch F. Is bronchial hyperresponsiveness more frequent in women than in men? A population-based study. Am J Respir Crit Care Med. 1997;156:1413–1420. doi: 10.1164/ajrccm.156.5.9701060. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 15.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L856–L865. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Graves J, Bradbury JA, Zhao Y, Swope DL, King L, Qu W, Clark J, Myers P, Walker V, Lindzey J, Korach KS, Zeldin DC. Regulation of mouse renal CYP2J5 expression by sex hormones. Mol Pharmacol. 2004;65:730–743. doi: 10.1124/mol.65.3.730. [DOI] [PubMed] [Google Scholar]
- 17.Manfreda J, Sears MR, Becklake MR, Chan-Yeung M, Dimich-Ward H, Siersted HC, Ernst P, Sweet L, Van Til L, Bowie DM, Anthonisen NR. Geographic and gender variability in the prevalence of bronchial responsiveness in Canada. Chest. 2004;125:1657–1664. doi: 10.1378/chest.125.5.1657. [DOI] [PubMed] [Google Scholar]
- 18.Massaro GD, Mortola JP, Massaro D. Sexual dimorphism in the architecture of the lung’s gas-exchange region. Proc Natl Acad Sci USA. 1995;92:1105–1107. doi: 10.1073/pnas.92.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 2005;35:1496–1503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 20.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regal JF, Regal RR, Meehan JL, Mohrman ME. Primary prevention of asthma: age and sex influence sensitivity to allergen-induced airway inflammation and contribute to asthma heterogeneity in Guinea pigs. Int Arch Allergy Immunol. 2006;141:241–256. doi: 10.1159/000095294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinhard C, Eder G, Fuchs H, Ziesenis A, Heyder J, Schulz H. Inbred strain variation in lung function. Mamm Genome. 2002;13:429–437. doi: 10.1007/s00335-002-3005-6. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JA, Raeburn D, Rodger IW, Thomson NC. Comparison of in vivo airway responsiveness and in vitro smooth muscle sensitivity to methacholine in man. Thorax. 1984;39:837–843. doi: 10.1136/thx.39.11.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz H, Johner C, Eder G, Ziesenis A, Reitmeier P, Heyder J, Balling R. Respiratory mechanics in mice: strain and sex specific differences. Acta Physiol Scand. 2002;174:367–375. doi: 10.1046/j.1365-201x.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz J, Schindler C, Zemp E, Perruchoud AP, Zellweger JP, Wuthrich B, Leuenberger P, Ackermann-Liebrich U. Predictors of methacholine responsiveness in a general population. Chest. 2002;122:812–820. doi: 10.1378/chest.122.3.812. [DOI] [PubMed] [Google Scholar]
- 26.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95:938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
- 27.Tankersley CG, Rabold R, Mitzner W. Differential lung mechanics are genetically determined in inbred murine strains. J Appl Physiol. 1999;86:1764–1769. doi: 10.1152/jappl.1999.86.6.1764. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SM, Pare PD, Armour CL, Hogg JC, Schellenberg RR. Airway reactivity in chronic obstructive pulmonary disease. Failure of in vivo methacholine responsiveness to correlate with cholinergic, adrenergic, or nonadrenergic responses in vitro. Am Rev Respir Dis. 1985;132:30–35. doi: 10.1164/arrd.1985.132.1.30. [DOI] [PubMed] [Google Scholar]
- 29.Taylor-Clark T, Undem BJ. Transduction mechanisms in airway sensory nerves. J Appl Physiol. 2006;101:950–959. doi: 10.1152/japplphysiol.00222.2006. [DOI] [PubMed] [Google Scholar]
- 30.Wagner EM, Jacoby DB. Methacholine causes reflex bronchoconstriction. J Appl Physiol. 1999;86:294–297. doi: 10.1152/jappl.1999.86.1.294. [DOI] [PubMed] [Google Scholar]
- 31.Watson N, Barnes PJ, Maclagan J. Actions of methoctramine, a muscarinic M2 receptor antagonist, on muscarinic and nicotinic cholinoceptors in guinea-pig airways in vivo and in vitro. Br J Pharmacol. 1992;105:107–112. doi: 10.1111/j.1476-5381.1992.tb14219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanaura S, Goto K, Kitagawa H, Hosokawa T, Misawa M, Kobayashi N, Hayakawa H. Role of vagus nerves in bronchoconstriction induced by chemical mediators. Nippon Yakurigaku Zasshi. 1982;79:571–579. [PubMed] [Google Scholar]


