Abstract
We have analyzed the localization of dendritic cells (DCs) in non-lesional gray matter (NLGM) in comparison to non-lesional white matter (NLWM) and acute or chronic active multiple sclerosis (MS) lesions. Immunohistochemistry was performed on cryostat sections for DCs markers (CD209, CD205, CD83) and other markers for inflammatory cells (CD68, CD8, CD4, CD3, CCR7, CCR5). We found cells expressing CD209 and containing myelin basic protein in both perivascular and parenchymal areas of NLGM. Our findings showing the expression of CD209+ cells in NLGM parenchymal areas are surprising relative to the previous literature which reported the presence of CD209+ DCs only in MS plaque perivascular areas. Although less numerous than CD209+ cells, NLGM cells expressing mature DCs marker CD205 were consistently detected in perivascular cuffs of most lesions. In double labeling experiments, some but not all of the CD209+ cells also expressed CD68 and CCR5. We also found CD209+ cells in close contact with CD3+ lymphocytes suggesting that DCs might contribute to the local activation of pathogenic T cells in the NLGM. Since injury to the NLGM is one of the key factors associated with disability accumulation, targeting DCs may represent a possible new therapeutic approach in MS to prevent disease progression.
Keywords: Dendritic Cells, Non-Lesional Gray Matter, T lymphocytes, Macrophages, Multiple sclerosis
INTRODUCTION
Multiple sclerosis (MS) is the prototype for central nervous system (CNS) demyelinating diseases in humans. MS is considered an autoimmune disease in which myelin and the myelin-producing oligodendrocytes (OLGs) are the targets of immune attack (Cudrici et al., 2006; Hauser and Oksenberg, 2006; Steinman, 2001). The essential requirements for initiating CNS inflammation characteristic of MS are the expression of encephalitogenic antigens, generation of chemotactic signals in the CNS, expression and up-regulation of adhesion molecules on endothelial cells, and activation of antigen-specific CD4+ T cells (Hauser and Oksenberg, 2006; Steinman, 2001). CD4 T cells are primed in the periphery and then enter the CNS. In the perivascular space they encounter myelin antigen expressed by local antigen-presenting cells, microglia, and DCs (Greter et al., 2005; McMahon et al., 2005). The reactivated CD4 T cells then invade the parenchyma of the CNS and release proinflammatory cytokines and activate microglia (Heppner et al., 2005). In addition, CD8 cytotoxic T lymphocytes are associated with axon damages and tend to also be recruited in the CNS parenchyma in MS (Neumann et al., 2002).
DCs are antigen-presenting cells critical in the initiation of adaptive immunity and triggering of autoimmunity (Reis e Sousa, 2006). Myeloid-lineage dendritic cells, immune cells originating in the bone marrow, reside as immature cells in nonlymphoid organs and fluid phase playing a role in antigen capture (Bailey et al., 2006; Greter et al., 2005). DCs are present in all tissue including CNS where they reside in the meninges, choroid plexus (Matyszak and Perry, 1996; McMenamin, 1999) and in the cerebrospinal fluid (Pashenkov et al., 2001). In pathological states DCs can be found in the brain (Fischer and Reichmann, 2001; Kostulas et al., 2002; Ma-Krupa et al., 2004; Serafini et al., 2000). DCs have been found to be crucial in the initiation of experimental autoimmune encephalomyelitis (EAE), an animal model for MS (Greter et al., 2005). In MS, DCs have been shown to infiltrate perivascular cuffs from MS plaques (Greter et al., 2005; Plumb et al., 2003; Serafini et al., 2006). DCs may both activate T cells in secondary lymphoid tissue and have a pathological role in re-activating primed T cells, which have infiltrated the brain tissue, enabling these T cells to damage myelin sheaths or the axons themselves (Greter et al., 2005). DCs in brain tissue may directly attack OLGs and neurons by production of nitric oxide or inflammatory cytokines (Reis e Sousa, 2006).
Post-mortem data have shown perivascular infiltration in the NLWM (Allen and McKeown, 1979; Trapp et al., 1998) and in some MS cortical lesions (Peterson et al., 2001) but little is known on the presence of inflammatory infiltrates in NLGM. Recent MRI data demonstrated abnormalities in most of NLWM (Filippi et al., 1995) and NLGM (Valsasina et al., 2005). No systematic studies were performed to localize the DCs in the adjacent normal gray and white matter or to establish the extent of immature or mature DCs that are retained in these areas. In this study we analyzed using immunohistochemistry and specific antibodies, the localization of DCs in NLGM in relationship with that of corresponding MS plaques and NLWM. Our data showing myelin contained DCs present in both perivascular and parenchymal deposits in all studied areas in relation with T lymphocytes suggest that MS is a generalized CNS disease.
MATERIALS AND METHODS
Brain Tissue
Frozen brain tissue specimens were obtained at autopsy from seven patients with a definite diagnosis of MS from the Human Brain and Spinal Fluid Resource Center, Veterans Affairs West Los Angeles Health Care Center. Active lesions contained abundant infiltrates consisting of T cells and macrophages with detectable myelin degradation products. Inflammation was restricted to the lesion margins in chronic active lesions. Regions of NLWM and NLGM that lacked macroscopic or histological evidence of demyelination were also used. The samples were derived from patients between the ages of 30 and 62 with a mean of 50. Five healthy control brain samples were obtained from the Cooperative Human Tissue Network, Charlottesville, VA.
Antibodies
Mouse anti-human CD209 was from R&D Systems Inc. (Minneapolis, MN), mouse monoclonal anti-human CD205 was from eBioscience (San Diego, CA) and the mouse anti-human CD123 was from Biolegend (San Diego, CA). The rabbit anti-human CD3 antibody and monoclonal antibody to human macrophages (anti-CD68) were from Dako (Carpanteria, CA). The monoclonal anti-CD3 and anti-CD4 were from NovoCastra (Newcastle upon Tyne, U.K.) and rabbit anti-myelin basic protein (MBP) was from Sternberger Monoclonals (Lutherville, MD). The CCR7 antibody was from R&D Systems and CCR5 antibody was from Calbiochem (San Diego, CA). The mouse monoclonal anti-CD8 was from Santa Cruz Biotech (Santa Cruz, CA) and the anti-human CD83 and anti-CD4 were from BD Bioscience (San Jose, CA). The horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG and goat anti-rabbit IgG were from Jackson ImmunoResearch (West Grove, PA) and the HRP-conjugated goat anti-mouse antibody was from Santa Cruz Biotech.
Immunohistochemistry
Brain immunohistochemical staining was performed as previously described (Rus et al., 2005) using monoclonal and polyclonal antibodies listed in Table 1. The air-dried cryostat sections (4-6μm) were fixed for 10 min in acetone containing 0.3% H2O2 to remove endogenous peroxidase. Sections were incubated with mouse monoclonal antibodies against CD209 (clone 120507), or CD205 (clone MG38) diluted 1/50, 2h at room temperature. The sections were washed 3 times for 3 min with PBS pH 7.4, and then incubated for 1h at room temperature with rabbit anti-mouse HRP-conjugated IgG or HRP-conjugated goat anti-mouse antibody. The specific reaction was developed using NovaRED (Vector Labs, Burlingame, CA). A similar indirect immunoperoxidase technique was used for CD3, CD4, CD8, CD68, and CD83 staining. For staining of OLG/myelin using mouse monoclonal MAB 328 (Chemicon, Temacula, CA) the cryostat sections incubated overnight at 4°C with the MAB 328 (1:10,000) in 0.1% Tween-PBS as previously described (Niculescu et al., 2004). The sections were washed several times in PBS, reacted with anti-mouse biotinylated secondary antibody (1:250) in PBS, and then with avidin-biotin-peroxidase complex (Vector Labs). After washing, sections were reacted using NovaRED.
Table 1.
Antibodies used for characterization of DCs and inflammatory infiltrates.
| Markers | Cellular Specificity | Known
Function |
Clones | Source |
|---|---|---|---|---|
| CD3-ε | T Cells | Signaling component of T cell receptor complex | Poly-Clonal, rabbit | Santa Cruz Biotechnnology |
| CD4 | Helper/inducer T Cell, monocytes, macrophages | Transmembrane glycoprotein receptor for the human immunodeficiency virus | RPA-T4 | BD Pharmingen |
| CD8-α | Cytotoxic T lymphocytes | Facilitate antigen recognition by the TCR and strengthen avidity TCR-antigen interaction | D-9 | Santa Cruz Biotechnolgy |
| CD68 | Macrophages/monocytes, microglia, DCs | Phagocytosis of tissue macrophages and binding of tissue specific leptin or selectin for homing of macrophage subsets | EBM11 | DAKO |
| CCR5 | Immature DCs, memory T-cells, monocytes/macrophages | Receptor for RANTES, MIP-1α and β, coreceptor for HIV | Poly-clonal, rabbit | Calbiochem |
| CCR7 | Naïve and central memory T cells, mature DCs | Receptor for MIP-3 β | 150503 | R&D Systems |
| CD83 | Co-stimulatory molecule on DCs, activated B and T cells | Role in antigen presentation and/or lymphocyte activation | HB15e | BD Pharmingen |
| CD205 | High expression on mature DCs. Low expression on immature DCs, leukocytes | Endocytic receptor for antigens; involved in MHC class II antigen presentation leading to T cell activation, | MG38 | eBioscience |
| CD209/DC-SIGN | High expression on monocytes derived immature DCs, low expression on mature DCs | C-type lectin receptor that binds to ICAM-3 leading to DC-T cell interaction | 120507 | R&D Systems |
DC-SIGN=dendritic cells specific intercellular adhesion molecule-3 grabbing nonintegrin
Double staining for CD209 with CD68, MBP, CCR5, and CD3
For double staining, cryosections were initially processed for CD209 immunostaining, as described above, and the reaction developed with NovaRed. Then sections were treated with 0.3% H2O2 to remove peroxidase, in excess, and incubated with the rabbit anti-CD68 diluted 1/50 for 2h at room temperature. Slides were washed several times in PBS, reacted with HRP-conjugated goat anti- rabbit antibody (Jackson Immunoresearch Labs), and then sections were reacted with diaminobenzidine (Pierce, Rockford, IL). A similar technique was used for the double staining of CD209 with MBP, CCR5 or CD3. Double stained cells were quantified by light microscopy using a 40X objective. Control sections were prepared by immunostaining without the primary antibody, or using control isotype IgG instead of the primary antibody.
RESULTS
Inflammatory infiltrates in NLGM
We performed immunohistochemical analysis on 20 areas of grossly involved white matter plaques and areas of grossly uninvolved tissue from seven MS patients and five healthy controls. The inflammatory reaction was severe in active lesions; however, an inflammatory infiltrate was also found in NLWM and NLGM (Table 2). The inflammation was diffused and consisted of mononuclear cells positive for CD68 in perivascular cuffs as well as in parenchymal areas (Fig. 1A). There was also diffuse infiltration in these regions of CD3+ cells (Fig. 1B). Most of CD3+ cells were present in perivascular area, but in some cases a mild parenchymal infiltrate was found. Both CD8 (Fig. 1C) and CD4 positive cells (Fig. 1D) were found in perivascular areas. The presence of inflammatory infiltrate was associated with microglial activation, as shown by the increased staining in parenchymal CD68+ cells and the formation of clusters of microglial cells (data not shown). These data indicate that inflammatory infiltrate is not confined to the active MS lesions but is also present to a lesser degree in the NLWM and NLGM. In control normal brains no CD3, CD4, CD8 or CD68 positive cells were found. We also examined the expression of CCR5 and CCR7. Serial sections through areas with positive CD3+ cells revealed positive CCR5 staining on some of the infiltrating cells (Fig. 1E). Some of the CCR5 positive cells have microglial morphology (Fig. 1E insert). CCR7 was rarely detected on perivascular cells (Fig. 1F) but was routinely detected on PBMC cytospins as a positive control (data not shown). These inflammatory CCR7- T cells are therefore activated effector memory T cells (Rus et al., 2005). Instead CCR7 predominantly stained parenchymal cells with a morphology resembling activated microglia in NLGM and NLWM (Fig. 1F, insert) as described previously for MS plaques (Kivisakk et al., 2004).
Table 2.
Expression of inflammatory infiltrates in MS lesions and controls
| Case no. (Age/Sex) | CD3 | CD4 | CD8 | CD68 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lesion (no.) | Lesion type | Perivascular | Parenchymal | Perivascular | Parenchymal | Perivascular | Parenchymal | Perivascular | Parenchymal | |
| 1 (38/F) | Frontal plaques (3) | Acute | + | + | + | + | + | + | +++ | +++ |
| NLWM | ++ | + | ++ | + | + | + | ++ | ++ | ||
| NLGM | + | - | ++ | + | + | - | ++ | - | ||
| 2 (61/F) | Occipital plaques (3) | Ch. active | + | + | - | - | + | - | ++ | ++ |
| NLWM | ++ | + | ++ | + | + | - | ++ | +++ | ||
| NLGM | +++ | ++ | + | - | + | + | ++ | + | ||
| 3 (62/M) | Parietal plaques (3) | Ch. active | ++ | + | ND | ND | - | - | +++ | ++ |
| NLWM | ++ | + | ND | ND | + | - | ++ | +++ | ||
| NLGM | ++ | + | + | + | ++ | - | ++ | + | ||
| 4 (51/F) | Frontal plaques (3) | Acute | ++ | + | ND | ND | + | - | +++ | +++ |
| NLWM | ++ | + | + | + | - | - | +++ | ++ | ||
| NLGM | ND | ND | + | - | ND | ND | ++ | + | ||
| 5 (60/M) | Occipital plaques (3) | Acute | +++ | + | ND | ND | + | - | ++ | + |
| NLWM | + | + | ++ | - | ++ | + | +++ | ++ | ||
| NLGM | + | + | + | - | - | - | ++ | + | ||
| 6 (51,F) | Occipital plaques (3) | Acute | + | - | + | - | + | - | ++ | ++ |
| NLWM | ++ | - | + | - | ++ | - | ++ | +++ | ||
| NLGM | ++ | + | ++ | - | + | - | ++ | ++ | ||
| 7 (30, F) | Frontal plaques (3) | Acute | ++ | + | + | - | + | - | +++ | ++ |
| NLGM | + | - | + | - | ++ | - | ++ | + | ||
| 8-12 | Normal (4) | Control WM | - | - | - | - | - | - | - | - |
| Control GM | - | - | - | - | - | - | - | - | ||
F, female; M, male; NLWM, non-lesion white matter; NLGM, non-lesion gray matter. -, negative; +, slightly positive; ++, positive; +++, highly positive; ND, not determined; Ch. active, chronic active
Figure 1. Expression of inflammatory infiltrate in NLGM in MS brain.
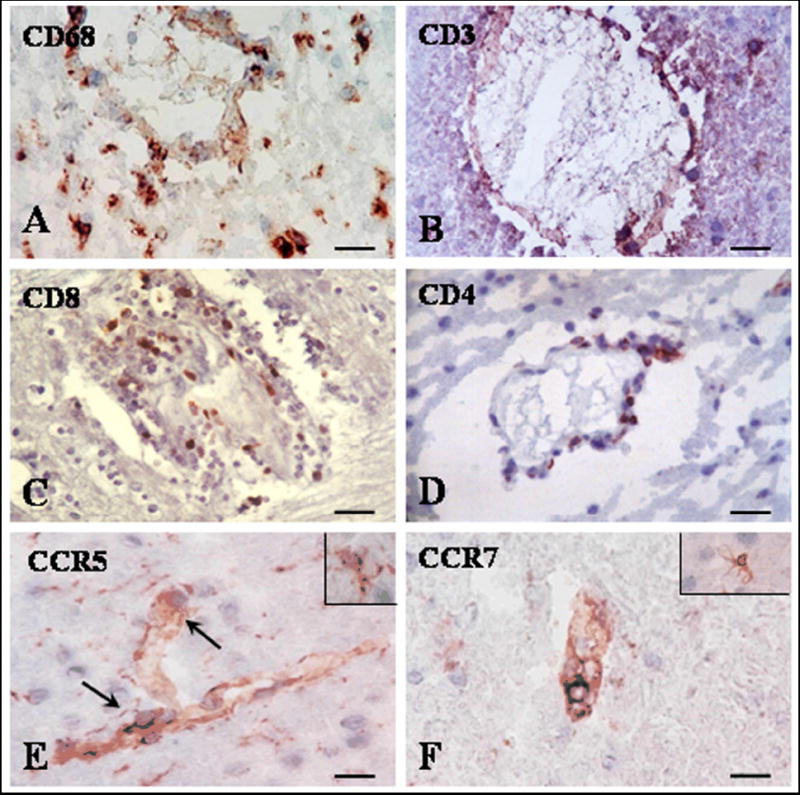
Cryostat sections were stained for CD68, CD3, CD8, CD4, CCR5 and CCR7 by indirect immunoperoxidase. Perivascular and parenchymal infiltrates were commonly found for CD68 (A). Most of CD3, CD4 and CD8+ cells were present in perivascular area (B-D). CCR5 (E) was present on infiltrative perivascular cells and also on glial cells (see insert I n E). Rare CCR7 perivascular positive cells were found (F); glial cells also expressed CCR7 (F, insert). A-F, Bar= 20 μM.
Localization of dendritic cells in NLGM and controls
We tested for the presence of DCs using the same tissue blocks that were previously used to stain for the presence of inflammatory cells. To identify immature DCs, we used immunohistochemistry and a monoclonal antibody against CD209 (DC-SIGN), a C-type lectin receptor (Soilleux et al., 2002). CD209 positive cells were present in all MS brain examined. We found CD209 staining on cells in both perivascular and parenchymal locations (Fig. 2, Table 3). Blood vessels surrounded by CD209 positive cells were found in NLGM, NLWM and MS plaques areas (Fig. 2, Table 3). The parenchymal deposits were rather extensive in some of the NLGM areas (Fig. 2D). The pattern of staining was often both cytoplasmic and on the cell surface membrane. No CD209 immunoreactivity was detected on vascular endothelia. In control brain CD209 positive cells were rarely seen in few perivascular area (Fig. 2E), choroid plexus (Fig. 2F) and meninges (data not shown).
Figure 2. Expression of CD209 positive dendritic cells in NLGM.
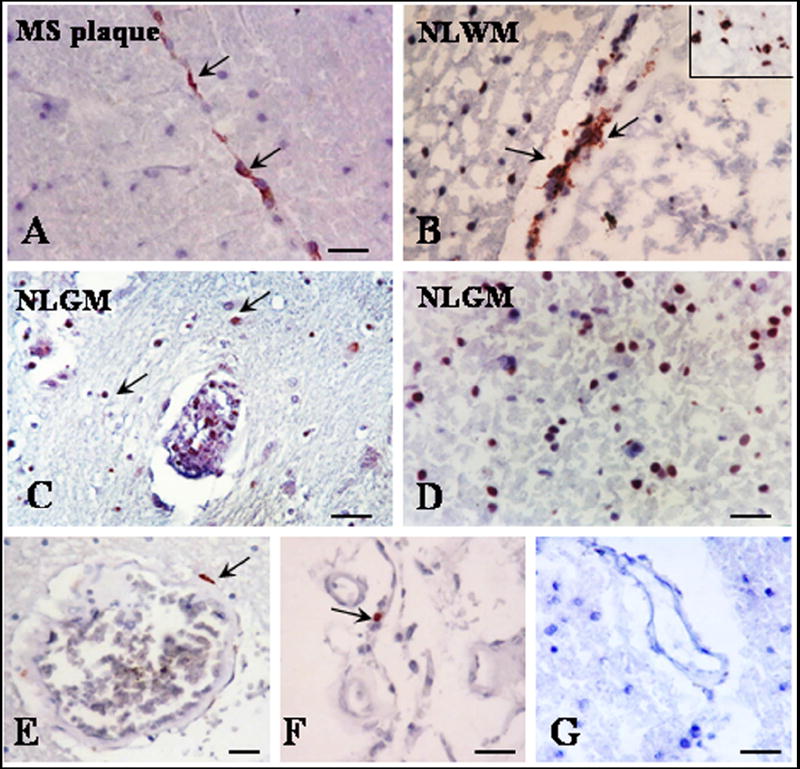
Cryostat sections were stained for CD209 by indirect immunoperoxidase. Perivascular CD209 positive DCs were commonly found in MS plaque (A), NLWM (B) and NLGM (C) (arrows). Parenchymal CD209 deposits were also seen in NLWM (B, insert) and NLGM (D). Rare perivascular CD209 positive cells were seen in control brains white matter (E) and choroid plexus (F). No staining was seen when secondary antibodies were used with non-specific or no primary antibody (G). A-G, Bar= 20 μM. MS plaque= multiple sclerosis plaque, NLGM= non-lesional gray matter, NLWM= non-lesional white matter.
Table 3.
Expression of DCs in MS lesions and control
| Case no. (Age/Sex) | CD83 | CD205 | CD209 | |||||
|---|---|---|---|---|---|---|---|---|
| Lesion (no.) | Lesion type | Perivascular | Parenchymal | Perivascular | Parenchymal | Perivascular | Parenchymal | |
| 1 (38/F) | Frontal plaques (3) | Acute | + | - | ++ | ++ | ++ | ++ |
| NLWM | + | - | + | - | ++ | - | ||
| NLGM | - | - | + | - | ++ | - | ||
| 2 (61/F) | Occipital plaques (3) | Ch. Active | + | - | - | - | ++ | +++ |
| NLWM | + | - | ++ | ++ | ++ | ++ | ||
| NLGM | + | - | ++ | - | ++ | +++ | ||
| 3 (62/M) | Parietal plaques (3) | Ch. Active | - | - | + | - | ++ | + |
| NLWM | + | - | + | - | ++ | + | ||
| NLGM | + | - | + | - | ++ | +++ | ||
| 4 (51/F) | Frontal plaques (3) | Acute | + | - | + | - | ++ | - |
| NLWM | - | - | + | - | ++ | ++ | ||
| NLGM | - | - | ND | ND | ++ | ++ | ||
| 5 (60/M) | Occipital plaques (3) | Acute | ++ | - | + | - | ++ | + |
| NLWM | + | - | + | - | +++ | ++ | ||
| NL GM | + | - | + | + | ++ | + | ||
| 6 (51,F) | Occipital plaques (3) | Acute | + | - | + | - | ++ | - |
| NLWM | + | - | + | + | ++ | - | ||
| NLGM | + | - | - | - | +++ | ++ | ||
| 7 (30, F) | Frontal plaques (2) | Acute | + | - | + | - | + | - |
| NLGM | ++ | - | + | - | +++ | ++ | ||
| 8-12 | Normal (4) | Control WM | - | - | - | - | - | - |
| Control GM | - | - | - | - | + | - | ||
F, female; M, male; NLWM, non-lesion white matter; NLGM, non-lesion gray matter. -, negative; +, slightly positive; ++, positive; +++, highly positive; ND, not determined; Ch. active, chronic active
Further, we investigated if DCs in MS brain also express CD205; CD205 is highly expressed by mature DCs, belong to the macrophage mannose receptor family of C-type lectin endocytic receptors and is involved in antigen uptake and processing (Kato et al., 2006). CD205 is also expressed by CD8+ DCs that are believed to play a role in cross priming (Kato et al., 2006). We found CD205 positive cells mainly in perivascular cuffs of NLGM, NLWM and MS plaques (Fig. 3). A smaller number of CD205 positive cells were found in the perivascular cuffs when compared with CD209 immunoreactivity for the same areas. Rare CD205 immunoreactivity was also detected in the parenchyma of four out of nineteen lesions studied (Table 3).
Figure 3. Expression of CD205 and CD83 positive dendritic cells in NLGM.
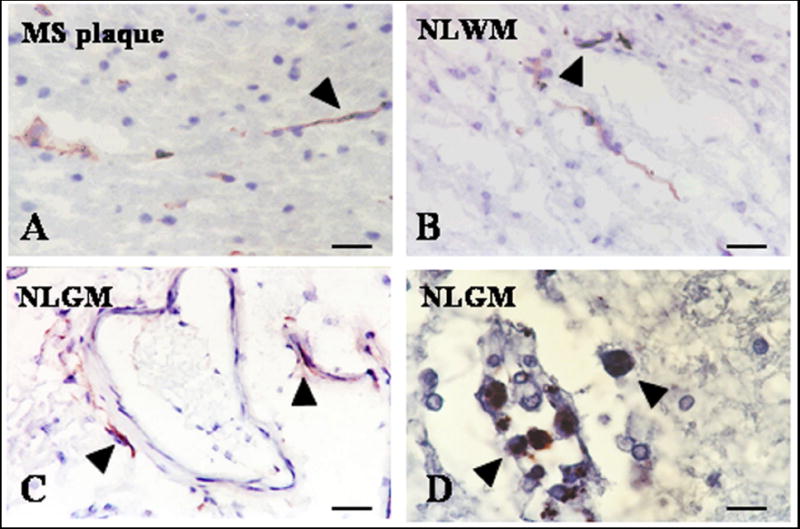
Cryostat sections were stained for CD205 and CD83 by indirect immunoperoxidase. Fewer periventricular CD205 (A-C, arrowheads) and CD83 positive DCs (D-arrowheads) were found in all examined areas when compared with those that were CD209 positive. A-D, Bar= 20 μM; MS plaque= multiple sclerosis plaque, NLGM= non-lesional gray matter, NLWM= non-lesional white matter.
The presence of mature DCs in the MS brain was also investigated using an anti-CD83 antibody. CD83 is a costimulatory molecule present on DCs and was used as a marker for mature DCs on tissue sections (Plumb et al., 2003). We found CD83 positive cells in the perivascular cuffs of most of NLGM, NLWM and MS plaques areas (Table 3). Not all perivascular areas in the same plaques expressed CD83. However in sixteen out of twenty sections examined small numbers of CD83 positive cells were present in some perivascular cuffs (Fig. 3D). When compared with CD209 staining on the same sections, fewer CD83 positive cells were identified that were localized only in perivascular cuffs. It is important to mention that CD209 staining was abundant in parenchymal areas whereas no staining for CD83 was found in these areas. CD83 staining was absent in normal control brains.
We also immunostained for CD123, a maker for plasmacytoid DCs. No CD123 positive cells were detected in MS plaques, NLWM or NLGM (data not shown).
Colocalization of CD209 positive cells with CD68, MBP and CCR5
On serial sections, the pattern of CD209 was found to be comparable to that of CD68 staining. To further prove that CD209 positive cells express CD68, double staining studies were performed. We found that some of the CD209 cells also expressed CD68 suggesting a monocyte/microglial origin of these cells (Fig. 4A). Because of their role in antigen processing, we investigated if CD209 positive cells were also involved in myelin uptake in NLGM. Double staining for CD209 and MBP showed the presence of double positive cells at the edges of acute, chronic active lesions and NLGM (Fig. 4B). CD209/MBP positive cells were also found in in perivascular and parenchymal areas. These data suggest that myelin uptake by immature DCs occurs also in the NLGM of MS brains. CD209 was also found to colocalize with the chemokine receptor CCR5 (Fig. 4C) suggesting a role for this receptor in DCs recruitment.
Figure 4. Co-localization of dendritic cells with CD68, MBP, CCR5, and CD3 in NLGM.
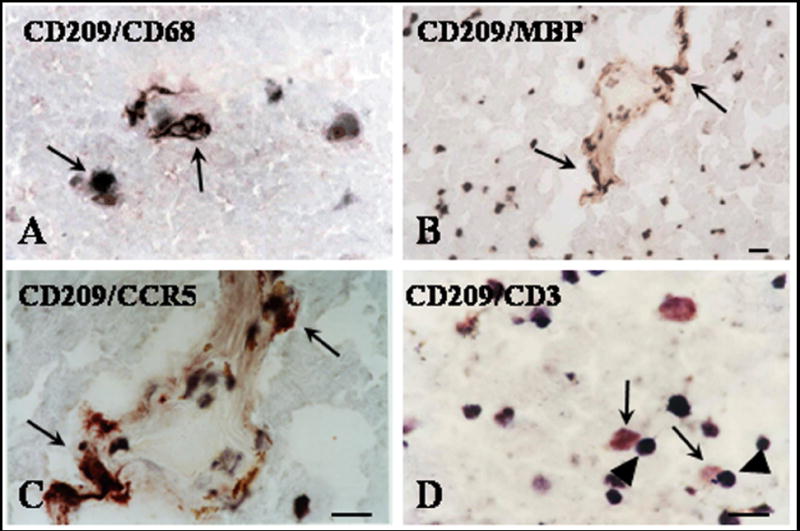
Cryostat sections were stained by double labeling for CD209 and CD68 (A), MBP (B), CCR5 (C), or CD3 (D) as described in the text. Many of CD209 positive DCs (red deposits) also stained positive for CD68 (dark brown deposits-A), MBP (dark brown deposits-B) or CCR5 (dark brown deposits-C). CD209 positive DCs (red deposits-arrows) were found in close contact with CD3 cells (dark-brown-arrowheads) (D). A, C, D Bar= 20 μM; B Bar= 10 μM. MBP=myelin basic protein.
Proximity of CD209 with CD3 cells
In many cases neighboring cells with the morphology of lymphocytes were seen to be closely juxtaposed to CD209 positive cells. We used double staining for CD209 and CD3 to confirm that these cells situated in close proximity with CD209 cells are T cells. Indeed many of the cells situated close to CD 209 were CD3 positive (Fig. 4D). Since CD209 is a DC receptor that binds to ICAM-3 expressed predominantly on naïve T cells, it is possible that these cells in close contact with DCs represent naïve T cells.
DISCUSSION
In this manuscript we examined the expression DCs and inflammatory infiltrates in NLGM areas and show, for the first time that immature and mature myeloid DCs are present in NLGM and NLWM areas. We also found that DCs are present in these areas in association with rather abundant inflammatory infiltrates. Most of DCs exhibit an immature phenotype known to play an important role in immune surveillance (Bailey et al., 2006; Reis e Sousa, 2006). Previous studies have shown the presence of immature DCs in areas of MS plaque with most of these cells localized in perivascular cuffs (Greter et al., 2005; Plumb et al., 2003; Serafini et al., 2006). We extended these studies by showing that CD209+ DCs are present both in perivascular cuffs and in parenchymal areas in all samples studied. We also found that the inflammatory infiltrates in the NLGM and NLWM are less abundant when compared to the corresponding MS plaques. The same is true for immature DCs with the exception of some parenchymal areas in NLGM that were found to contain larger amounts of CD209+ positive cells (Fig. 2D, Table 3). Fewer perivascular DCs expressing CD205 and CD83 makers for the mature cells were found compared to the CD209+ DCs. Since CD205 is also expressed by CD8+ DCs it is possible that these perivascular CD205+ cells play a role in CD8+ T cells priming (Kato et al., 2006).
Our data showing the presence of DCs and the inflammatory infiltrates in NLGM is not surprising in view of the recent brain MRI data using non-conventional MRI techniques such as the magnetization transfer ratio (MTR) (Agosta et al., 2006). In addition, post-mortem data have shown perivascular infiltration, myelin thinning and axonal loss in the NLWM (Allen and McKeown, 1979; Trapp et al., 1998), diffuse demyelination (Kutzelnigg et al., 2005), axonal transaction (Rus et al., 2005) and apoptotic loss of neurons (Peterson et al., 2001) in cortical MS lesions. These features, especially irreversible demyelination and neuroaxonal damage, are likely to be major contributors to the MTR decrease seen in NLGM of MS brains (Agosta et al., 2006). Moreover it is important to mention that NLGM damage was found to be one of the key factors associated with disability accumulation (Agosta et al., 2006).
It was suggested that intracerebral DCs are most likely derived from precursors recruited from circulation and differentiated in CNS or from resting microglia, which acquire DC phenotype (Platten and Steinman, 2005; Reichmann et al., 2002; Ulvestad et al., 1994). We found that CD209+ DCs in non-lesional adjacent tissue and MS plaques express CCR5. It is well known that myeloid DCs from MS patients respond chemotactically to CCR5 ligands, CCL5 and CCL3, which are expressed in MS lesions (Pashenkov et al., 2002). In addition, astrocytes were shown to produce chemokines that are able to attract immature DCs but not mature DCs (Ambrosini et al., 2005). Thus, expression of CCR5 may contribute to recruitment of myeloid DCs to MS brain by stimulating transendothelial migration (Lin et al., 1998; Zozulya et al., 2007). CCL3 together with matrix metalloproteinases seem to play a critical role in migration of DCs across the endothelial monolayers (Zozulya et al., 2007). CCL3 could also stimulate DCs and endothelial cells production of membrane-bound and soluble Flt3 ligand (Solanilla et al., 2000). Interestingly, inhibition of Flt3 ligand signaling induces apoptosis in human DCs and targeted inhibition of Flt3 significantly improves the course of experimental autoimmune encephalomyelitis, an experimental model for MS (Whartenby et al., 2005). In the NLGM we also found CCR7 and CCR5 expression on microglia. CCR7 expressed by naïve T cells and DCs is necessary for these cells to enter lymph nodes (Pahuja et al., 2006). Challenge with innate antigen and protein antigens induces CCR7 expression by microglia in vitro and in vivo suggesting that microglia develops into a DC like antigen-presenting cell with migratory potential (Dijkstra et al., 2006; McMahon et al., 2006). Together these data suggest that DCs may arise from both circulating precursors and glial cells.
Immature DCs from NLGM were found to be in close contact with T cells indicating that these antigen-presenting cells might contribute to local T cells activation. Similar data showing interaction between immature DCs with T cells have been reported in MS plaques (Mullen et al., 2006) in which DCs were found to express co-stimulatory molecule CD86 and to interact with CD8 cells (Mullen et al., 2006; Serafini et al., 2006). Immature DCs in NLGM were also found by us to engulf myelin antigens in agreement with their function in antigen processing and presentation (Reis e Sousa, 2006). Recently, myeloid DCs presenting endogenous myelin peptides were found to drive the differentiation of CD4+ Th17 cells during EAE (Bailey et al., 2007). Th17 cells represent a distinct lineage of CD4+ T cells shown to mediate tissue pathology in autoimmune diseases including EAE (Gutcher and Becher, 2007). Together, these findings suggest that antigen presenting myeloid DCs play an important role in T cells activation, proliferation and induction of tissue damage.
Our data support the proposed critical role of myeloid DCs in the initiation and perpetuation of CNS inflammation (Greter et al., 2005). Furthermore, our findings indicate that inflammatory events like those in the MS plaques, where DCs mature in the perivascular space leading to the expansion of T cells, also occur in NLGM and NLWM. Both glatiramer acetate and interferon β were able to suppress DC function in relapsing remitting MS (Hussien et al., 2001; Sanna et al., 2006; Schreiner et al., 2004) suggesting that DC-targeted therapies could be beneficial in autoimmune demyelination. Thus, targeting DCs and DC-T cell interactions represents a possible new therapeutic approach in MS. Such an early therapeutic intervention might lead to less injury to the NLGM, one of the key factors associated with MS disability accumulation.
Acknowledgments
This work was supported by US Public Health Grant NS 42011 (HR), NS041435 (PAC) and by National Multiple Sclerosis Society PP0997 (SIVJ), the Veteran’s Administration Maryland Health Care System, MS Center of Excellence, Baltimore MD (HR and SIVJ).
MS brain tissue was obtained from the Human Brain and Spinal Fluid Resource Center, VA West Los Angeles Health Care Center, Los Angeles, CA, which is sponsored by the NINDS/NIH, NMSS, and Dept. of Veterans Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosta F, Rovaris M, Pagani E, Sormani MP, Comi G, Filippi M. Magnetization transfer MRI metrics predict the accumulation of disability 8 years later in patients with multiple sclerosis. Brain. 2006;129:2620–2627. doi: 10.1093/brain/awl208. [DOI] [PubMed] [Google Scholar]
- Allen IV, McKeown SR. A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci. 1979;41:81–91. doi: 10.1016/0022-510x(79)90142-4. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64:706–715. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Carpentier PA, McMahon EJ, Begolka WS, Miller SD. Innate and adaptive immune responses of the central nervous system. Crit Rev Immunol. 2006;26:149–188. doi: 10.1615/critrevimmunol.v26.i2.40. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Cudrici C, Niculescu T, Niculescu F, Shin ML, Rus H. Oligodendrocyte cell death in pathogenesis of multiple sclerosis: Protection of oligodendrocytes from apoptosis by complement. J Rehabil Res Dev. 2006;43:123–132. doi: 10.1682/jrrd.2004.08.0111. [DOI] [PubMed] [Google Scholar]
- Dijkstra IM, de Haas AH, Brouwer N, Boddeke HW, Biber K. Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo. Glia. 2006;54:861–872. doi: 10.1002/glia.20426. [DOI] [PubMed] [Google Scholar]
- Filippi M, Campi A, Dousset V, Baratti C, Martinelli V, Canal N, Scotti G, Comi G. A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology. 1995;45:478–482. doi: 10.1212/wnl.45.3.478. [DOI] [PubMed] [Google Scholar]
- Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- Hussien Y, Sanna A, Soderstrom M, Link H, Huang YM. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J Neuroimmunol. 2001;121:102–110. doi: 10.1016/s0165-5728(01)00432-5. [DOI] [PubMed] [Google Scholar]
- Kato M, McDonald KJ, Khan S, Ross IL, Vuckovic S, Chen K, Munster D, MacDonald KP, Hart DN. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol. 2006;18:857–869. doi: 10.1093/intimm/dxl022. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, Tucky B, Wujek J, Ravid R, Staugaitis SM, Lassmann H, Ransohoff RM. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol. 2004;55:627–638. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- Kostulas N, Li HL, Xiao BG, Huang YM, Kostulas V, Link H. Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke. 2002;33:1129–1134. doi: 10.1161/hs0402.105379. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Lin CL, Suri RM, Rahdon RA, Austyn JM, Roake JA. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur J Immunol. 1998;28:4114–4122. doi: 10.1002/(SICI)1521-4141(199812)28:12<4114::AID-IMMU4114>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyszak MK, Perry VH. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience. 1996;74:599–608. doi: 10.1016/0306-4522(96)00160-1. [DOI] [PubMed] [Google Scholar]
- McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- McMahon EJ, Bailey SL, Miller SD. CNS dendritic cells: critical participants in CNS inflammation? Neurochem Int. 2006;49:195–203. doi: 10.1016/j.neuint.2006.04.004. [DOI] [PubMed] [Google Scholar]
- McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- Mullen KM, Rozycka M, Rus H, Hu L, Cudrici C, Zafranskaia E, Pennington MW, Johns DC, Judge SI, Calabresi PA. Potassium channels Kv1.3 and Kv1.5 are expressed on blood-derived dendritic cells in the central nervous system. Ann Neurol. 2006;60:118–127. doi: 10.1002/ana.20884. [DOI] [PubMed] [Google Scholar]
- Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- Niculescu T, Weerth S, Niculescu F, Cudrici C, Rus V, Raine CS, Shin ML, Rus H. Effects of complement C5 on apoptosis in experimental autoimmune encephalomyelitis. J Immunol. 2004;172:5702–5706. doi: 10.4049/jimmunol.172.9.5702. [DOI] [PubMed] [Google Scholar]
- Pahuja A, Maki RA, Hevezi PA, Chen A, Verge GM, Lechner SM, Roth RB, Zlotnik A, Alleva DG. Experimental autoimmune encephalomyelitis develops in CC chemokine receptor 7-deficient mice with altered T-cell responses. Scand J Immunol. 2006;64:361–369. doi: 10.1111/j.1365-3083.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Teleshova N, Kouwenhoven M, Kostulas V, Huang YM, Soderstrom M, Link H. Elevated expression of CCR5 by myeloid (CD11c+) blood dendritic cells in multiple sclerosis and acute optic neuritis. Clin Exp Immunol. 2002;127:519–526. doi: 10.1046/j.1365-2249.2002.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- Platten M, Steinman L. Multiple sclerosis: trapped in deadly glue. Nat Med. 2005;11:252–253. doi: 10.1038/nm0305-252. [DOI] [PubMed] [Google Scholar]
- Plumb J, Armstrong MA, Duddy M, Mirakhur M, McQuaid S. CD83-positive dendritic cells are present in occasional perivascular cuffs in multiple sclerosis lesions. Mult Scler. 2003;9:142–147. doi: 10.1191/1352458503ms890oa. [DOI] [PubMed] [Google Scholar]
- Reichmann G, Schroeter M, Jander S, Fischer HG. Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. J Neuroimmunol. 2002;129:125–132. doi: 10.1016/s0165-5728(02)00184-4. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- Rus H, Pardo CA, Hu L, Darrah E, Cudrici C, Niculescu T, Niculescu F, Mullen KM, Allie R, Guo L, Wulff H, Beeton C, Judge SI, Kerr DA, Knaus HG, Chandy KG, Calabresi PA. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci U S A. 2005;102:11094–11099. doi: 10.1073/pnas.0501770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna A, Fois ML, Arru G, Huang YM, Link H, Pugliatti M, Rosati G, Sotgiu S. Glatiramer acetate reduces lymphocyte proliferation and enhances IL-5 and IL-13 production through modulation of monocyte-derived dendritic cells in multiple sclerosis. Clinical & Experimental Immunology. 2006;143:357–362. doi: 10.1111/j.1365-2249.2006.02997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155:172–182. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Serafini B, Columba-Cabezas S, Di Rosa F, Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol. 2000;157:1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi GL, Aloisi F. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol. 2006;65:124–141. doi: 10.1097/01.jnen.0000199572.96472.1c. [DOI] [PubMed] [Google Scholar]
- Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, Coleman N, Lee B. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- Solanilla A, Grosset C, Lemercier C, Dupouy M, Mahon FX, Schweitzer K, Reiffers J, Weksler B, Ripoche J. Expression of Flt3-ligand by the endothelial cell. Leukemia. 2000;14:153–162. doi: 10.1038/sj.leu.2401635. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Ulvestad E, Williams K, Bjerkvig R, Tiekotter K, Antel J, Matre R. Human microglial cells have phenotypic and functional characteristics in common with both macrophages and dendritic antigen-presenting cells. J Leukoc Biol. 1994;56:732–740. doi: 10.1002/jlb.56.6.732. [DOI] [PubMed] [Google Scholar]
- Valsasina P, Benedetti B, Rovaris M, Sormani MP, Comi G, Filippi M. Evidence for progressive gray matter loss in patients with relapsing-remitting MS. Neurology. 2005;65:1126–1128. doi: 10.1212/01.wnl.0000178982.53965.70. [DOI] [PubMed] [Google Scholar]
- Whartenby KA, Calabresi PA, McCadden E, Nguyen B, Kardian D, Wang T, Mosse C, Pardoll DM, Small D. Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:16741–16746. doi: 10.1073/pnas.0506088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulya AL, Reinke E, Baiu DC, Karman J, Sandor M, Fabry Z. Dendritic Cell Transmigration through Brain Microvessel Endothelium Is Regulated by MIP-1{alpha} Chemokine and Matrix Metalloproteinases. J Immunol. 2007;178:520–529. doi: 10.4049/jimmunol.178.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]


