Abstract
CGRP receptor activation has been implicated in peripheral and central sensitization. The role of spinal CGRP receptors in supraspinal pain processing and higher integrated pain behavior is not known. Here we studied the effect of spinal inhibition of CGRP1 receptors on supraspinally organized vocalizations and activity of amygdala neurons. Our previous studies showed that pain-related audible and ultrasonic vocalizations are modulated by the central nucleus of the amygdala (CeA).
Vocalizations in the audible and ultrasonic range and hindlimb withdrawal thresholds were measured in awake adult rats before and 5-6h after induction of arthritis by intraarticular injections of kaolin and carrageenan into one knee. Extracellular single-unit recordings were made from neurons in the latero-capsular division of the CeA (CeLC) in anesthetized rats before and after arthritis induction. CGRP1 receptor antagonists were applied to the lumbar spinal cord intrathecally (5μl/min) 6h postinduction of arthritis.
Spinal administration of peptide (CGRP8-37, 1μM) and non-peptide (BIBN4096BS, 1μM) CGRP1 receptor antagonists significantly inhibited the increased responses of CeLC neurons to mechanical stimulation of the arthritic knee but had no effect under normal conditions. In arthritic rats, the antagonists also inhibited the audible and ultrasonic components of vocalizations evoked by noxious stimuli and increased the threshold of hindlimb withdrawal reflexes. The antagonists had no effect on vocalizations and spinal reflexes in normal rats
These data suggest that spinal CGRP1 receptors are not only important for spinal pain mechanisms but also contribute significantly to the transmission of nociceptive information to the amygdala and to higher integrated behavior.
Keywords: Calcitonin gene-related peptide, amygdala, arthritic pain, spino-parabrachio-amygdaloid pain pathway, audible vocalizations, ultrasonic vocalizations, nociception, central sensitization, electrophysiology, BIBN4096BS
1. Introduction
The amygdala has emerged as an important brain center involved in pain processing and pain modulation (Neugebauer et al., 2004), in addition to its roles in emotional learning and memory and affective disorders such as anxiety and depression (Davidson 2002; Davis 1998; LeDoux 2000; Maren 2005; Phelps and Ledoux 2005). The amygdala includes several anatomically and functionally distinct nuclei. The latero-capsular division of the central nucleus (CeLC) is thought to be a key player in the affective processing of nociceptive information (Neugebauer et al., 2004). The CeLC receives highly integrated polymodal information with affective content from the lateral and basolateral amygdala as well as relatively unprocessed nociceptive inputs from the spinal cord and brainstem (for references see Braz et al., 2005; Gauriau and Bernard 2002; Neugebauer et al., 2004).
Nociceptive information from the spinal cord reaches the CeLC directly, not involving the thalamus, through the spino-parabrachio-amygdaloid and spino-amygdaloid pathways (Cliffer et al., 1991; Gauriau and Bernard 2002; Neugebauer et al., 2004). Importantly, there is good evidence to suggest that these pathways are anatomically and pharmacologically distinct. The spino-parabrachio-amygdaloid pathway arises from lamina I neurons which receive peptidergic afferent input whereas the spino-amygdaloid projections originate from lamina V neurons which are thought to receive afferent input from non-peptidergic afferents through lamina II neurons (Braz et al., 2005; Gauriau and Bernard 2004). The majority of spinal lamina I neurons which project to supraspinal sites, particularly the parabrachial area, express neurokinin 1 receptors; are innervated by substance P containing afferents; respond to noxious stimuli and contribute to pain behavior (Honor et al., 1999; Mantyh et al., 1997; Spike et al., 2003; Todd et al., 2002; Willis and Coggeshall, 2004). Primary afferents that contain calcitonin gene-related peptide (CGRP) also terminate in lamina I (Carlton et al., 1988), which contains numerous CGRP binding sites (Coggeshall and Carlton 1997; Cottrell et al., 2005).
CGRP is a 37 amino acid peptide that activates adenylyl cyclase and protein kinase A through G-protein-coupled receptors, including the CGRP1 receptor for which selective antagonists are available (Doods et al., 2000; Poyner 1996; Van Rossum et al., 1997; Wimalawansa 1996). The involvement of CGRP in peripheral and spinal pain mechanisms is well established (Cridland and Henry 1988; Galeazza et al., 1995; Neugebauer et al., 1996; Ruda et al., 2000; Schaible 1996; Sun et al., 2003; 2004a). We showed recently that CGRP also plays an important role in nociceptive transmission from the parabrachial area to the amygdala (Han et al., 2005b). The CeLC is delineated by its abundance of CGRP-immunoreactive terminals of fibers from the lateral parabrachial area (de Lacalle and Saper 2000; Harrigan et al., 1994; Kruger et al., 1988; Schwaber et al., 1988), which is part of the spino-parabrachio-amygdaloid pathway. These terminals innervate CeLC neurons that project to brainstem areas such as the periaqueductal gray (Harrigan et al., 1994; Schwaber et al., 1988). CGRP appears to be an important signaling molecule along the peptidergic nociceptive pathway from primary afferents and lamina I neurons to the parabrachial area and amygdala.
2. Methods
Male Sprague Dawley rats (250-350 g) were housed in a temperature controlled room and maintained on a 12 h day/night cycle. Water and food were available ad libitum. Electrophysiological and behavioral data were obtained from untreated normal rats and rats with monoarthritis in the knee (5-6 h after induction). All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch (UTMB) and conform to the guidelines of the International Association for the Study of Pain (IASP) and of the National Institutes of Health (NIH).
2.1 Arthritis pain model
An experimental mono-arthritis was induced in the left knee joint of adult rats as described in detail previously (Han et al., 2005b; Neugebauer et al., 2003; Neugebauer and Li 2003). A kaolin suspension (4%, 80-100 μl) was injected into the joint cavity through the patellar ligament with a syringe (1 ml) and needle (25 GA, 5/8 inch). After repetitive flexions and extensions of the knee for 15 min, a carrageenan solution (2%, 80-100 μl) was injected into the knee joint cavity, and the leg was flexed and extended for another 5 min. This treatment paradigm reliably leads to inflammation of the knee within 1-3 h, which reaches a maximum plateau at 5-6 h, and persists for days (Han et al., 2005b; Neugebauer et al., 2003; Neugebauer and Li 2003). Electrophysiological and behavioral measurements of arthritis pain-related changes were made at the 5-6 h time point.
2.2 Electrophysiology: extracellular single-unit recording
2.2.1 Animal preparation and anesthesia
Adult rats (250-350 g; Sprague Dawley) were anesthetized with pentobarbital sodium (50 mg/kg, i.p.) as described previously (Han et al., 2005b; Li and Neugebauer 2004b; Neugebauer and Li 2003). A cannula was inserted into the trachea for artificial respiration and to measure end-tidal CO2 levels. A catheter in the jugular vein allowed continuous administration of anesthetic (see below) and fluid support (3-4 ml/kg/h Ringer's lactate solution, i.v.). The carotid artery was catheterized for blood pressure monitoring. Depth of anesthesia was assessed as follows: by regularly testing the corneal blink, hindpaw withdrawal, and tail-pinch reflexes; continuously monitoring the end-tidal CO2 levels (kept at 4.0 ± 0.2 %), arterial blood pressure (kept at 135 ± 5 mmHg), heart rate, and electrocardiogram pattern; and checking for abnormal breathing patterns. Core body temperature was maintained at 37°C by means of a homeothermic blanket system. Animals were mounted in a stereotaxic frame (David Kopf Instruments), paralyzed with pancuronium (induction, 0.3-0.5 mg, i.v.; maintenance, 0.3 mg/h, i.v.), and artificially ventilated (3-3.5 ml; 55-65 strokes/min). Constant levels of anesthesia were maintained by continuous intravenous infusion of pentobarbital (15 mg/kg/h). A small unilateral craniotomy was performed at the sutura fronto-parietalis level for the recording of CeLC neurons and at the sutura occipito-parietalis for insertion of the stimulation electrode in the pontine parabrachial area (PB) as described previously (Han et al., 2005b; Li and Neugebauer 2004b; Neugebauer and Li 2002; Neugebauer and Li 2003).
2.2.2 Recording and identification of amygdala neurons
Extracellular recordings were made from single neurons in the latero-capsular division of the central nucleus of the amygdala (CeLC) with glass-insulated carbon filament electrodes (3-5 MΩ) as described previously (Han et al., 2005b; Li and Neugebauer 2004b; Neugebauer and Li 2003), using the following stereotaxic coordinates (Paxinos and Watson, 1998): 2.0-2.2 mm caudal to bregma; 3.8-4.2 mm lateral to midline; depth of 7-9 mm. The recorded signals were amplified, displayed on analog and digital storage oscilloscopes, fed into a window discriminator, digitized by an interface (CED 1401+; Cambridge Electronics Design, Cambridge, UK), and recorded on a computer (Dell Pentium 4). Spike 2 software (version 3; Cambridge Electronics Design) was used for on-line and off-line analysis of single-unit activity. An individual CeLC neuron was identified by the configuration, shape, and height of the recorded action potentials (spikes) in response to innocuous and noxious mechanical stimulation (compression) of the knee as described in detail previously (Han et al., 2005b; Li and Neugebauer 2004b; Neugebauer and Li 2003).
CeLC neurons were also activated orthodromically by electrical stimulation (square-wave pulses, 50-500μA, 150μs) in the lateral pontine parabrachial area, where the monosynaptic connections of the spino-ponto-amygdaloid pain pathway to the CeLC originate (Gauriau and Bernard 2004). The stereotaxic coordinates of the monopolar stimulation electrode were: 1–2 mm rostral to the lambda and 2.2 mm lateral to midline at the depth of 7.3 mm. Monosynaptic orthodromic activation was assumed if the evoked action potentials occurred at a fixed latency after the electrical stimulus and if they “followed” low frequency (20 Hz) but not high frequency (>50 Hz) stimulation as described in detail before (Neugebauer and Li 2002, 2003; see Fig. 1E). The inability to follow high frequency stimulation (> 50 Hz) rules out antidromic activation (see Neugebauer et al., 1999).
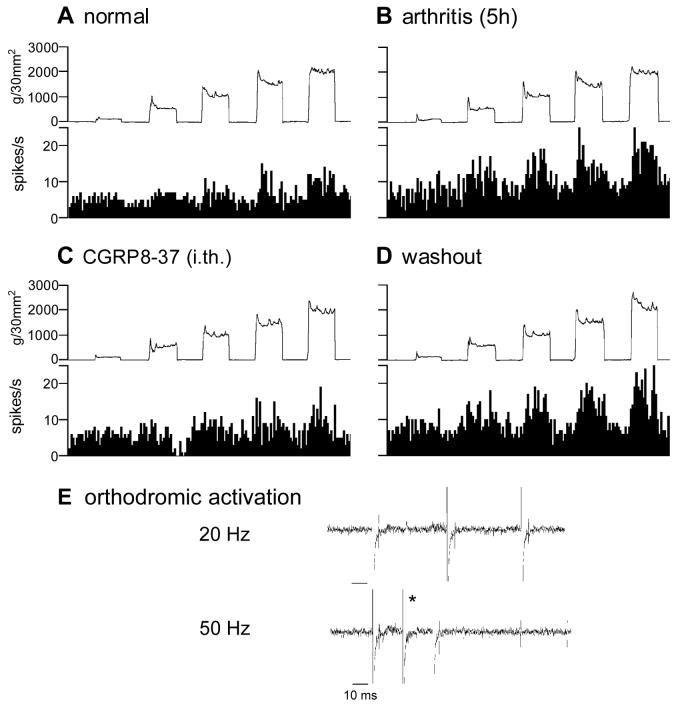
Figure 1. Spinal administration of CGRP8-37 inhibits the enhanced responses of an amygdala neuron in the arthritis pain state.
Extracellular single-unit recordings of the responses (spikes/s) of one neuron in the latero-capsular part of the central nucleus of the amygdala (CeLC) to brief (15 s) graded mechanical stimulation of the knee joint before (A) and 5 h after induction of the knee joint arthritis (B). Intrathecal application of the CGRP1 receptor antagonist CGRP8-37 (1 μM, 15 min; dissolved in ACSF) inhibited the evoked responses and background activity of the neuron (C). This effect was reversed during washout with ACSF (D). Bin width of peristimulus time histograms is 1 s. Top traces in A-D show the actual stimulus intensities (force); they are online recordings of the calibrated output of the force transducer on the forceps used to compress the knee with graded intensities (see 2.2.3). (E) Electrical stimulation in the pontine parabrachial nucleus activated the neuron orthodromically, but not antidromically (see 2.2.2 and Neugebauer and Li 2002). The asterisk indicates where an evoked action potential would be expected if the spike followed high-frequency stimulation.
2.2.3 Mechanical Stimulation
Mechanical stimuli of innocuous (100 and 500 g/30 mm2) and noxious (1500 and 2000 g/30 mm2) intensity were applied to the knee and other parts of the receptive field (e.g., ankle) by means of a forceps equipped with a force transducer, whose calibrated output was amplified and displayed in grams on a liquid crystal display screen (Han et al., 2005b; Li and Neugebauer 2004b; Neugebauer and Li 2003). The output signal was also fed into the CED interface and recorded on the computer for on-line and off-line analysis. Stimulus intensities of 100-500 g/30 mm2 applied to the knee and other deep tissue are considered innocuous because they do not evoke hindlimb withdrawal reflexes in awake rats and are not felt to be painful when tested on the experimenters. Pressure stimuli >1500 g/30 mm2 are noxious because they evoke hindlimb withdrawal reflexes in awake rats and are distinctly painful when applied to the experimenters (Han et al., 2005b; Li and Neugebauer 2004b; Neugebauer and Li 2003). Stimulus-response functions were obtained with the use of a wide range of innocuous and noxious stimuli (100-2000 g/30 mm2).
2.2.4 Classification of neurons
In this study, neurons were selected that had a receptive field in the knee and responded more strongly to noxious than innocuous stimuli, because these so-called multireceptive (MR) neurons have been shown in our previous studies to become sensitized consistently in the arthritis pain model (Han et al., 2005b; Li and Neugebauer 2004b; Neugebauer and Li 2003). Size and thresholds of the receptive fields in deep tissue and skin were mapped using graded mechanical stimuli of innocuous and noxious intensities (see 2.2.3). Mechanical stimuli were considered to activate deep tissue (joints, muscles) if the stimulation of overlying skin evoked no or a clearly distinct response. Cutaneous input was distinguished from deep tissue input by selective stimulation of skin folds gently raised from the underlying deep tissue. The focus of this study was on the processing of nociceptive information from the deep tissue. Size and threshold of the total receptive field, background activity, and responses to innocuous and noxious stimuli (see above) were recorded before and for >6h after induction of arthritis in the knee. Innocuous and noxious stimuli (15 s duration each) were applied three times in a control period of at least 2 h before arthritis induction and then every hour after induction of arthritis.
2.2.5 Drugs and drug application
Calcitonin gene-related peptide fragment 8-37 (CGRP8-37), a selective peptide CGRP1 receptor antagonist (Poyner 1996; Van Rossum et al., 1997; Wimalawansa 1996), was purchased from Bachem, Torrance, CA. BIBN4096BS, a selective non-peptide CGRP1 receptor antagonist (Doods et al., 2000), was kindly supplied by Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany. Known concentrations of CGRP1 receptor antagonists were administered intrathecally under normal conditions (no arthritis) or 5-6 h after induction of arthritis. Several hours before the electrophysiological recordings, a lumbar puncture was performed using a spinal needle (18 GA) that entered the subarachnoid space at the level of the cauda equina (L5/6 vertebrae) (Storkson et al., 1996). After removing the needle, a catheter (PE10 tubing; o.d. 0.61 mm) connected with PE50 tubing to an infusion pump (Harvard Apparatus, Holliston, MA) was inserted through the guide cannula. The catheter was positioned with its tip pointing rostrally at the spinal segments L3/4 and sutured to the skin. In control experiments, the catheter was inserted through the atlanto-occipital membrane to position its tip on the cervical enlargement (C4). Artificial cerebrospinal fluid (ACSF) was pumped through the catheter throughout the experiment to maintain stable conditions in the tissue. ACSF contained (in mM): NaCl 117, KCl 4.7, NaH2PO4 1.2, CaCl2 2.5, MgCl2 1.2, NaHCO3 25, and glucose 11. ACSF was oxygenated and equilibrated to pH 7.4 with a mixture of 95% O2/5% CO2. Drugs were dissolved in ACSF on the day of the experiment and administered intrathecally at a rate of 2 μl/min for 15-20 min to establish equilibrium in the tissue.
Background activity and responses to innocuous and noxious stimulation of the knee (see 2.2.3) were measured every 5 min during drug application. Based on initial observations showing that drug effects reached a plateau after 10 min, the 15 min time point was selected for the full set of tests (including stimulus-response functions, see 2.2.3) and for the comparison of drug effects.
2.3 Behavior: vocalizations and hindlimb withdrawal reflexes
2.3.1 Experimental protocol and intrathecal drug application
On day 1, an externally accessible catheter was implanted intrathecally as in the electrophysiology experiments (see 2.2.5). During anesthesia with sodium pentobarbital (50 mg/kg. i.p.), a lumbar puncture was performed and a catheter (PE10 tubing; o.d. 0.61 mm) connected with PE50 tubing to an infusion pump (Harvard Apparatus, Holliston, MA) was carefully implanted, its tip aiming rostrally at the spinal segments L3/4. In the afternoon of day 2, baseline (pre-arthritis) vocalizations and spinal withdrawal reflexes were measured in normal rats. The behavioral tests were repeated in the afternoon of day 3 in the same animals 5-6 h after arthritis induction in one knee (see 2.1). CGRP1 receptor antagonists (see 2.2.5) were then administered intrathecally through the catheter, and behavior was measured at 15 min of continued drug administration and again at 30 min of washout with ACSF (see 2.2.5). Drugs were dissolved in ACSF on the day of the experiment and administered intrathecally at a rate of 2 μl/min.
2.3.2 Audible and ultrasonic vocalizations
Vocalizations were recorded and analyzed as described in detail previously (Han and Neugebauer 2005). The experimental setup [Han JS, Neugebauer V (2005) U.S. Patent Application 98006/28 and Provisional Patent 60\664089] included a custom-designed recording chamber, a condenser microphone (audible range, 20 Hz to 16 kHz) connected to a preamplifier, and an ultrasound detector (25 ± 4 kHz), filter and amplifier (UltraVox four-channel system; Noldus Information Technology, Leesburg, VA). Vocalizations in the audible and ultrasonic ranges were recorded simultaneously but with different microphones (condenser microphone and bat detector, respectively) connected to separate channels of the amplifier. Data acquisition software (UltraVox 2.0; Noldus Information Technology) was adjusted to monitor the occurrence of vocalizations within user-defined frequencies and recorded the number and duration of digitized events (audible and ultrasonic vocalizations) on a computer (Dell Pentium 4) as described previously (Han et al., 2005a; Han and Neugebauer 2005). This computerized recording system was set to suppress nonrelevant audible sounds and to ignore ultrasounds outside the defined frequency range (25 ± 4 kHz). Animals were placed in the recording chamber for acclimation 1 h before the vocalization measurements and for habituation (1 h on 2 days). The recording chamber ensured the stable positioning of the animal at a fixed distance from the sound detectors and allowed the reproducible stimulation of the knee joint. The chamber contained openings for the hindlimbs to allow the application of innocuous (100 g/30 mm2) and noxious (2000 g/30 mm2) mechanical stimuli with a calibrated forceps as in the electrophysiology studies (see 2.2.3).
2.3.3 Hindlimb withdrawal reflexes
The threshold of spinally organized withdrawal reflexes in response to stimulation of the knee was determined subsequently to the vocalization measurements. Mechanical stimuli of increasing intensity (steps of 50 g/30 mm2) were manually applied to the knee joint by means of a calibrated forceps equipped with a force transducer, whose calibrated output was amplified and displayed in grams on a liquid crystal display (LCD) screen as in the electrophysiological experiments (see 2.2.3 and 2.3.2). Withdrawal threshold was defined as the minimum stimulus intensity (force in g; displayed on the LCD screen) that evoked a withdrawal reflex of the hindlimb. The test was repeated three times and the values were averaged to calculate the threshold (force in g).
2.4 Euthanasia
At the end of the experiment the animals was sacrificed by decapitation using a guillotine (Harvard Apparatus Decapitator). This method of sacrifice is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and approved by the Institutional Animal Care and Use Committee (IACUC). Rats used in the electrophysiology experiments were still under general anesthesia with pentobarbital sodium when being decapitated. Rats used for behavioral tests were anesthetized briefly with methohexital sodium (Brevital®, 40-60 mg/kg, i.p.) for euthanasia by decapitation.
2.5 Verification of recording and drug administration sites
The localization of the tip of the intrathecal catheter was verified at the end of the experiment by visual inspection after a small laminectomy. For the histologic verification of the recording sites of CeLC neurons a direct current (250 μA for 3 min) was injected through the carbon filament recording electrode at the end of the electrophysiological experiment. The brain was removed and submerged in 10% formalin and potassium ferrocyanide. Tissues were stored in 20% sucrose before they were frozen sectioned at 50 μm. Sections were stained with Neutral Red, mounted on gel-coated slides and cover-slipped. The boundaries of the different amygdala nuclei were easily identified under the microscope (Han et al., 2005b; Li and Neugebauer 2004b; Neugebauer and Li 2003). Lesion/recording sites were plotted on standard diagrams (from Paxinos and Watson, 1998) (see Fig. 9).
Figure 9.
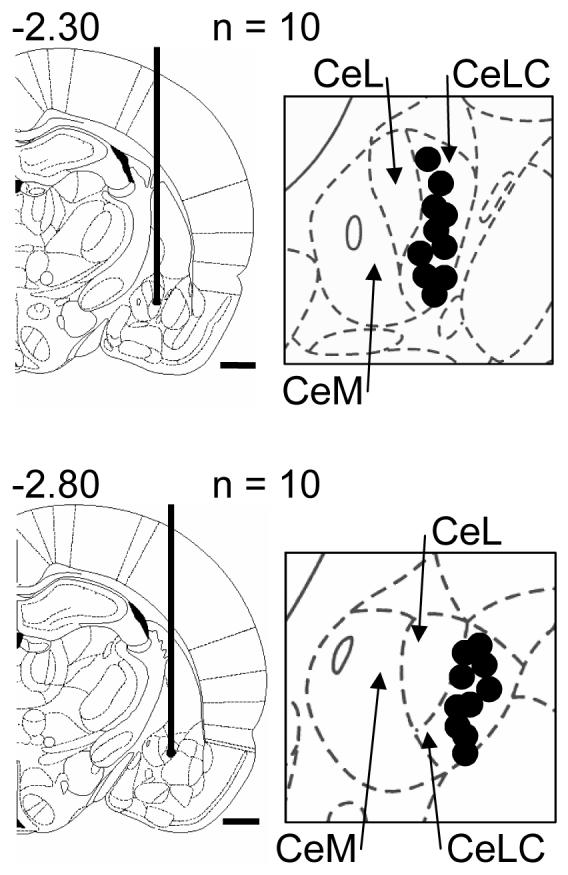
Histologically verified sites (black dots) of the recording electrodes in the CeLC (see 2.5) in the electrophysiological experiments. The boundaries of the different amygdala nuclei are easily identified under the microscope. Diagrams adapted from (Paxinos and Watson, 1998) show coronal sections through the right hemisphere at different levels posterior to bregma (−2.30 and −2.80). Next to each diagram is shown in detail the CeA and its subdivisions, the medial (CeM), lateral (CeL) and latero-capsular (CeLC) part. Calibration bars for diagrams are 1 mm.
2.5 Data analysis and statistics
All averaged values are given as the mean ± SEM. Statistical significance was accepted at the level P < 0.05. GraphPad Prism 3.0 software (GraphPad Software, San Diego, CA) was used for all statistical analysis except when noted.
Extracellularly recorded single-unit activity (action potentials) was analyzed off-line from peristimulus rate histograms using Spike2 software (version 3; CED). Evoked responses were expressed as spikes (action potentials) per second (Hz) by subtracting from the total activity during the stimulus (15 s) any background activity (Hz) in the 15 s preceding the stimulus. Responses of the same neuron before and during drug administration were compared using a paired t-test.
Duration of audible and ultrasonic vocalizations was normalized to pre-arthritis (normal) conditions. The duration was defined as the arithmetic sum (total amount) of the durations of individual vocalization events that occurred during or after a brief single stimulus (see 2.2.3) within a 1 min time interval. A paired t-test was used to compare behavioral outcomes (vocalizations and withdrawal thresholds) in the same animal before and during drug administration.
3. Results
Our previous studies showed that neurons in the latero-capsular part of the central nucleus of the amygdala (CeLC) develop synaptic plasticity and increased responsiveness in a model of arthritic pain (Neugebauer et al., 2004) through a mechanism that involves CGRP1 receptor activation in the amygdala (Han et al., 2005b). However, the contribution of spinal CGRP receptors to the transmission of nociceptive information to the amygdala is not known. Here, we determined the effects of CGRP1 receptor antagonists in the spinal cord on activity in amygdala neurons and on supraspinally and spinally organized pain behaviors.
3.1 Blockade of spinal CGRP1 receptors inhibits the responses of amygdala (CeLC) neurons in the arthritis pain model
A total of 20 neurons were recorded in the CeLC (see Fig. 9). As in our previous studies (Neugebauer and Li 2002, 2003) these neurons were activated orthodromically from the lateral pontine parabrachial area (PB, see Fig. 1E), where the monosynaptic connections of the spino-ponto-amygdaloid pain pathway to the CeLC originate (Gauriau and Bernard 2004). The CeLC neurons were multireceptive (MR), because they responded more strongly to noxious than to innocuous stimuli (see 2.2.4). We showed previously that all MR neurons become sensitized to afferent input in the arthritis pain model, whereas neurons that respond only to noxious stimuli (nociceptive-specific neurons) do not (Han et al., 2005b; Li and Neugebauer 2004b; Neugebauer and Li 2003). Therefore neurons that are activated by innocuous as well as noxious stimuli in arthritis represent sensitized MR neurons. As in our previous studies (Li and Neugebauer 2004a; 2004b; Neugebauer and Li 2002; Neugebauer and Li 2003) all neurons had large bilateral receptive fields in the deep tissue of the forelimbs, trunk and hindlimbs, including knee, ankle and paw; 8 of these neurons also had bilateral receptive fields in the skin of the back, hip and thigh. Mechanical thresholds were in the innocuous range between 100 and 500 g/30mm2. In this study 5 neurons were recorded continuously before and after arthritis induction whereas another 5 neurons were tested only under normal conditions and 10 neurons were recorded only in the arthritis pain state (>5 h postinduction). Evoked responses and background activity of the 5 neurons recorded during development of the arthritis increased significantly (P < 0.001-0.05, paired t-test) 5-6h postinduction of arthritis (innocuous, from 5.4 ± 1.0 Hz to 9.1 ± 1.5 Hz; noxious, 10.1 ± 1.2 Hz to 16.1 ± 1.5 Hz; background, 4.8 ± 0.6 Hz to 7.4 ± 0.7 Hz).
3.1.1 Effect of a peptide antagonist (CGRP8-37)
Intrathecal application of CGRP8-37 (1 μM, 15 min) to the lumbar spinal cord inhibits the increased responses of CeLC neurons 5-6 h postinduction of arthritis. Figure 1 shows an individual example. The neuron responded more strongly to noxious (1500 and 2000 g/30 mm2) than innocuous (100 and 500 g/30 mm2) stimulation of the knee (Fig. 1A). Continuous recordings of this neuron show that the responses (spikes/s) to graded stimuli increased after arthritis induction (Fig. 1B; 5 h postinduction). Intrathecal administration of a CGRP1 receptor antagonist (CGRP8-37; 1 μM, 15 min) strongly reduced the increased responses (Fig. 1C). This inhibitory effect was reversible upon washout with ACSF application for 30 min (Fig. 1D). CGRP8-37 also inhibited the increased background of this neuron. The neuron was activated orthodromically, but not antidromically, by electrical stimulation in the PB, because it followed a 20 Hz but not a high frequency 50 Hz stimulus (Fig. 1E). The spike also had a variable latency.
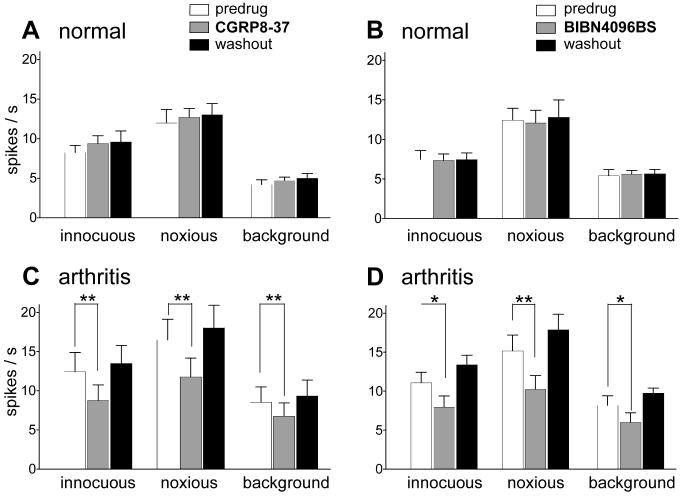
The inhibitory effects of spinally administered CGRP8-37 in the arthritis pain state (5-6 h postinduction) are summarized for the sample of neurons (n = 5) in Figure 2. Analysis of the raw data (Fig. 2C) shows that the responses to normally innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) stimuli and background activity decreased during intrathecal administration of CGRP8-37 (1 μM, 15 min). The drug effects were significant (P < 0.01-0.001; paired t-test; n = 5) and reversible upon washout with ACSF (30 min). CGRP8-37 had no significant effect on the evoked responses and background activity of CeLC neurons recorded under normal conditions (no arthritis, n = 5; Fig. 2A).
Figure 2. Inhibitory effects of spinally administered CGRP antagonists on the responses of amygdala neurons in the arthritis pain state but not under normal conditions.
(A) and (B) Intrathecally (i.th.) administered CGRP8-37 (1 μM, 15 min, n = 5 neurons; A) and BIBN4096BS (1 μM, 15 min, n = 5 neurons; B) had not effect on the evoked responses and background activity of CeA neurons under normal conditions (no arthritis). (C) and (D) Intrathecal (i.th.) administration of CGRP8-37 (1 μM, 15 min, n = 5 neurons; C) and BIBN4096BS (1 μM, 15 min, n = 5 neurons; D) inhibited the activity of CeLC neurons 5-6 h postinduction of arthritis in the knee. Background activity and responses to normally innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) stimulation of the arthritic knee were recorded before and during (15 min) drug administration and 30 min after washout in ACSF (see examples in Fig. 1 and 3). (A-D) Original data (spikes/s) were averaged across the sample of neurons. * P < 0.05, ** P < 0.01, paired t-test.
As a control for the potential spread of drugs to rostral sites, CGRP8-37 was administered intrathecally onto the cervical enlargement 5-6 h postinduction of arthritis. Cervical application of CGRP8-37 (1 μM, 15 min) had no significant effect on the evoked and background activity of amygdala neurons in the CeLC (n = 4; Fig. 3A).
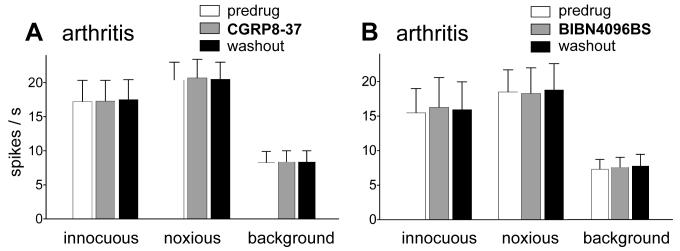
Figure 3. Placement control. Drug administration onto the cervical enlargement had no effect on the responses of amygdala neurons in arthritis.
As a control for the potential spread of drugs to rostral sites, CGRP8-37 (A) and BIBN4096BS (B) were administered intrathecally onto the cervical enlargement 5-6 h postinduction of arthritis. Cervical application of CGRP8-37 (1 μM, 15 min, n = 4) or BIBN4096BS (1 μM, 15 min, n = 4) had no significant effect on the evoked and background activity of amygdala (CeLC) neurons. Background activity and responses to normally innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) stimulation of the arthritic knee were recorded before and during (15 min) drug administration and 30 min after washout in ACSF.
3.1.2 Effect of a non-peptide antagonist (BIBN4096BS)
Intrathecal application of BIBN4096BS (1 μM, 15 min) onto the lumbar spinal cord inhibits the responses of CeLC neurons in the arthritis pain model. Figure 4 shows an individual example. The neuron responded to a wide range of stimulus intensities (100 – 2000 g/30 mm2) 5 h postinduction of arthritis (Fig. 4A), which is consistent with its classification as a sensitized MR neuron (see 2.2.4 and 3.1). Intrathecal administration of a CGRP1 receptor antagonist (BIBN4096BS; 1 μM, 15 min) strongly reduced the increased responses (Fig. 4B). This inhibitory effect was reversible upon washout with ACSF application for 30 min (Fig. 4C).
Figure 4. Spinal administration of BIBN4096BS inhibits the enhanced responses of an amygdala neuron in the arthritis pain state.
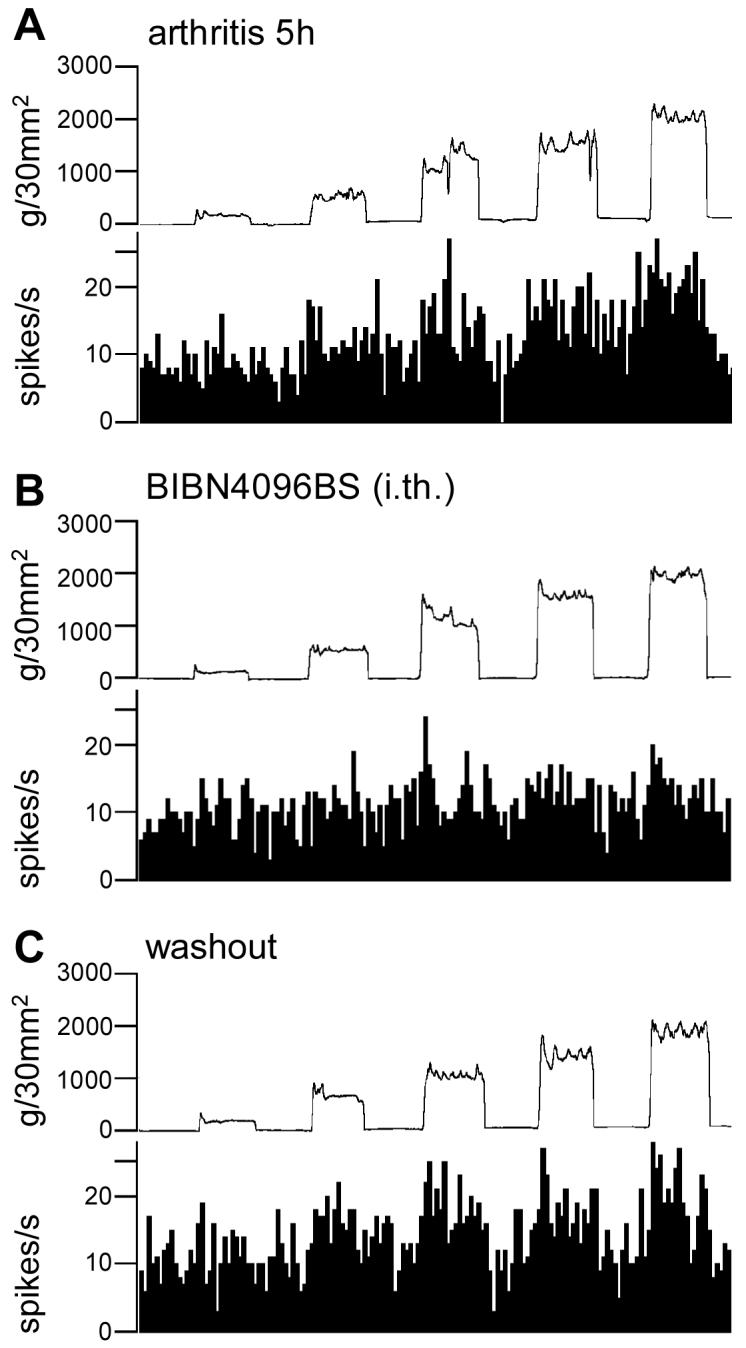
Extracellular single-unit recordings of the responses (spikes/s) of one CeLC neuron to brief (15 s) graded mechanical stimulation of the knee joint 5 h postinduction of arthritis in the knee (A). Intrathecal application of the CGRP1 receptor antagonist BIBN4096BS (1 μM, 15 min; dissolved in ACSF) inhibited the evoked responses of the neuron (B). This effect was reversed during 30 min washout with ACSF (C). Bin width of peristimulus time histograms is 1 s. Top traces in A-C show the actual stimulus intensities (force) (see Fig. 1 and 2.2.3).
Figure 2 summarizes the inhibitory effects of spinally administered BIBN4096BS on amygdala neurons (n = 5) in the arthritis pain model. Analysis of the raw data (Fig. 2D) shows that the responses to normally innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) stimulation of the arthritic knee and background activity decreased during intrathecal administration of BIBN4096BS to the lumbar cord (1 μM, 15 min). The drug effects were significant (P < 0.05-0.001, paired t-test; n = 5) and reversible upon washout with ACSF (30 min). BIBN4096BS had no significant effect on the evoked responses and background activity of CeLC neurons recorded under normal conditions (no arthritis, n = 5; Fig. 2B).
As a control for the potential spread of drugs to rostral sites, BIBN4096BS was administered intrathecally onto the cervical enlargement 5-6 h postinduction of arthritis. Cervical application of BIBN4096BS (1 μM, 15 min) had no significant effect on the evoked and background activity of amygdala neurons in the CeLC (n = 4; Fig. 3B).
These data suggest that spinal CGRP1 receptors contribute to the transmission of nociceptive information to the amygdala in the arthritis pain model.
3.2 Blockade of spinal CGRP1 receptors inhibits increased vocalizations and spinal reflex responses in the arthritis pain model
The behavioral consequences of pain-related changes in the CeLC include increased vocalizations in the audible and ultrasonic ranges and increased spinal reflexes (Han et al., 2005a; Han and Neugebauer 2005), which can be inhibited by blockade of CGRP1 receptors in the amygdala (Han et al., 2005b). Here we determined the effects of intrathecal treatment with CGRP1 receptor antagonists on the increased vocalization responses and spinal reflexes of arthritic animals.
3.2.1 Vocalizations
Vocalizations to innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) stimuli were measured in the same animals (n = 13) before arthritis induction (baseline control), 5-6 h after induction of arthritis, and during intrathecal drug application in arthritis. Rats did not vocalize spontaneously in a control period of 5-10 min before stimulation.
3.2.1.1 Effect of CGRP8-37
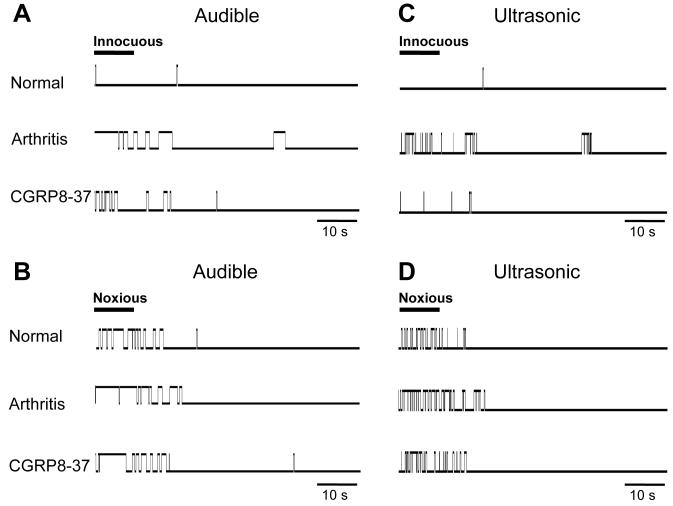
Figure 5 shows original recordings to illustrate the protocol and data acquisition. A computerized analysis system (Han et al., 2005a; Han and Neugebauer 2005) was used to record individual vocalization events (shown as upward deflections from baseline). Vocalizations in the audible (20 Hz to 16 kHz; A and B) and ultrasonic (25 ± 4 kHz; C and D) ranges were measured during and after brief innocuous (A and C) and noxious (B and D) stimulation of the knee. Recordings were made in the same animal before arthritis (normal), 5-6 h after arthritis induction and during intrathecal administration of CGRP8-37 (1 μM, 15 min). Consistent with our previous studies (Han et al., 2005a; Han et al., 2005b; Han and Neugebauer 2005) the duration and number (rate) of audible and ultrasonic vocalizations increased in the arthritis pain model. Under normal conditions, only noxious, but not innocuous, stimuli evoked a clear vocalization response, whereas the same animal vocalized to (normally) innocuous and noxious stimuli after arthritis induction, reflecting a state of allodynia and hyperalgesia, respectively. Intrathecal application of CGRP8-37 strongly inhibited the increased vocalizations.
Figure 5. Increased audible and ultrasonic components of vocalizations of an arthritic animal are inhibited by spinal administration of CGRP8-37.
Original recording records of vocalization in the audible (< 16 kHz; A, B) and ultrasonic (25 ± 4 kHz; C, D) ranges in one animal using an adjustable recording chamber and computerized analysis system as described before (Han et al., 2005a). Vocalizations were measured in response to innocuous (500 g/30 mm2; A, C) and noxious (2000 g/30 mm2; B, D) stimulation of the knee (10 s) for a period of 1 min starting with the onset of the stimulus. Stimulus duration is indicated by the horizontal bar in A-D. Each upward deflection from baseline indicates a vocalization response (“event”) and its duration. Under normal conditions, noxious, but not innocuous, stimulation evoked a vocalization response. 5-6 h postinduction of arthritis, a normally innocuous stimulus now evoked vocalizations and vocalizations to the noxious stimulus increased. Intrathecal application of CGRP8-37 (1 μM; 15 min) inhibited the vocalizations of the arthritic animal. Arthritis and drug application affected both vocalizations during stimulation and vocalizations that continued after stimulation (“vocalization afterdischarges”). No vocalizations were detected in a control period of 5-10 min before stimulation.
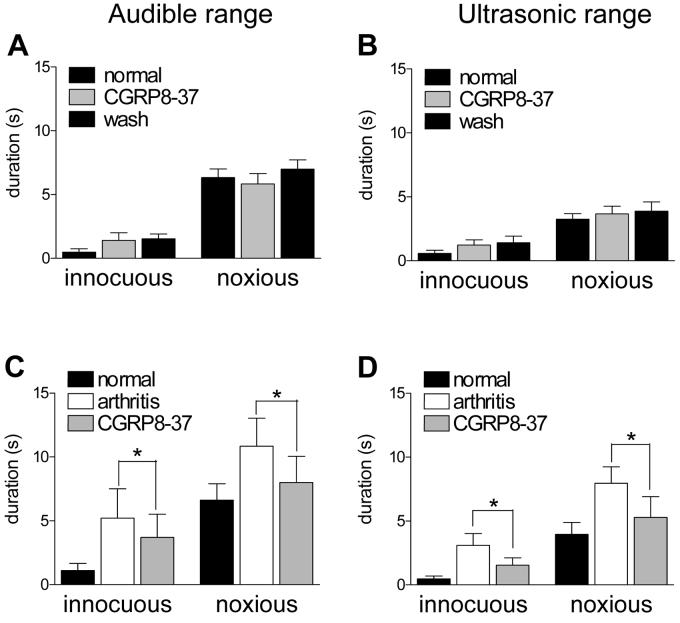
The effects of CGRP8-37 in the whole group of normal animals (n = 4) and arthritic animals (n = 5) are summarized in Figure 6. Intrathecal application of CGRP8-37 (1 μM, 15 min) had no effect on vocalizations in normal animals (A, B) but significantly (P < 0.05, paired t-test; n = 5) attenuated the duration of vocalizations of arthritic animals (C, D) when administered 5-6h postinduction of arthritis. No apparently different effects were found on vocalizations during stimulation and vocalization afterdischarges (see Fig. 5). Therefore, the total duration (sum of individual vocalization events) is shown. Predrug vocalizations were measured during administration of ACSF through the catheter, thus serving as vehicle controls.
Figure 6. Spinal administration of CGRP8-37 inhibits increased vocalizations of arthritic animals.
Vocalizations were recorded in awake animals as described before (Han et al., 2005a). The duration of vocalizations in the audible (A, C) and ultrasonic (B, D) ranges evoked by innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) stimulation of the knee was measured for a period of 1 min starting with the onset of the stimulus. Total duration (A, B) represents the arithmetic sum of individual vocalization events. (A) and (B) In normal animals intrathecal application of CGRP8-37 (1 μM; 15 min) had no effect on vocalizations evoked by innocuous or noxious stimuli. (C) and (D) To determine drug effects in the arthritis pain model, vocalizations were measured in another group of animals before (normal) and 5-6 h after arthritis induction (arthritis) and during intrathecal application of CGRP8-37 (1 μM; 15 min). Increased audible and ultrasonic vocalizations of arthritic animals were significantly inhibited by CGRP8-37 (n = 5; paired t-test). * P < 0.05 (paired t-test).
3.2.1.2 Effect of BIBN4096BS
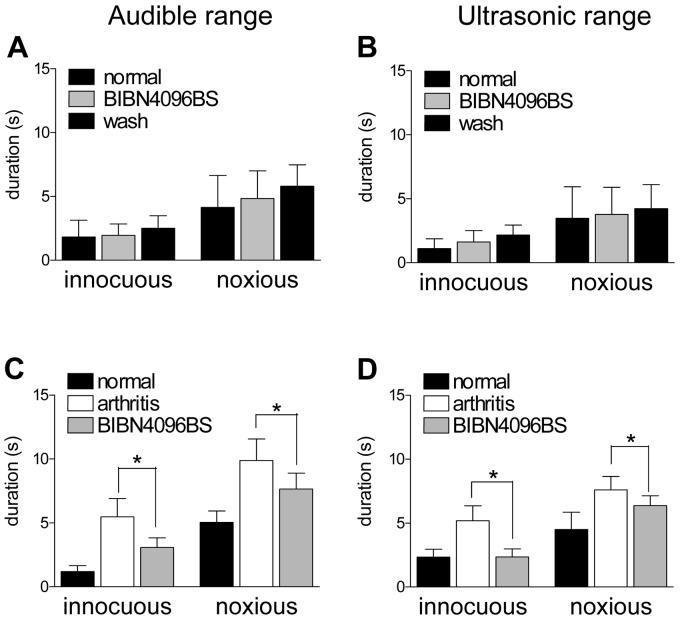
Intrathecal administration of the non-peptide CGRP1 receptor antagonist BIBN4096BS reduced the duration of vocalizations evoked by noxious and (normally) innocuous stimulation of the arthritic knee 5-6 h postinduction of arthritis (n = 8) but had no effect in normal animals (n = 3) (Fig. 7). The inhibitory effects of BIBN4096BS in arthritic animals were significant (P < 0.05, paired t-test).
Figure 7. Spinal administration of BIBN4096BS inhibits increased vocalizations of arthritic animals.
(A) and (B) In normal animals, BIBN4096BS (1 μM; 15 min) had no effect on the vocalizations in the audible (A, C) and ultrasonic (B, D) ranges evoked by innocuous (500 g/30 mm2) and noxious (2000 g/30 mm2) stimulation of the knee (n = 3). (C) and (D) BIBN4096BS (1 μM; 15 min) significantly (P < 0.05) decreased the vocalizations of arthritic animals (n = 8). In this group of animals, vocalizations were recorded before (normal) and 5-6 h after arthritis induction (arthritis) and during intrathecal application of BIBN4096BS (1 μM; 15 min). * P < 0.05 (paired t-test).
These data suggest that CGRP1 receptor-mediated spinal nociceptive transmission is important for evoking supraspinally generated vocalizations in the arthritis pain model but not under normal conditions.
3.2.2 Spinal reflexes
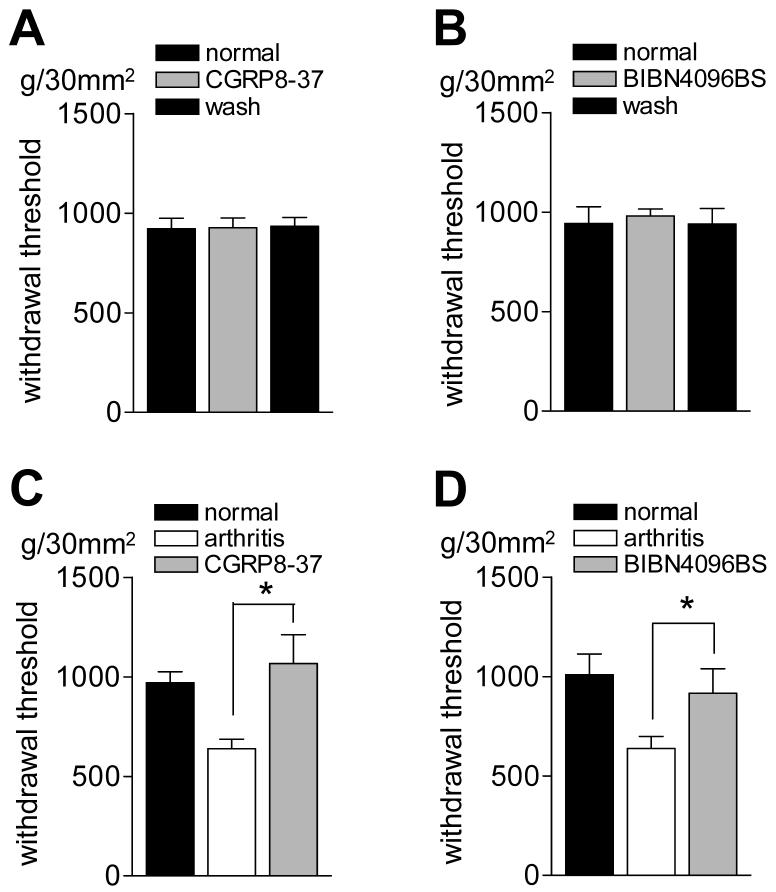
Thresholds for hindlimb withdrawal reflexes (Fig. 8) were determined by compressing the knee joint with gradually increasing stimulus intensities using a calibrated forceps whose output was displayed on an LCD screen (see 2.2.3). In normal animals, intrathecal application of CGRP8-37 (1 μM, 15 min; n = 4) or BIBN4096BS (1 mM, 15 min; n = 3) had no effect on the mechanical thresholds (Fig. 8 A and B). Arthritic animals (Fig. 8 C and D) showed decreased reflex thresholds, reflecting allodynic pain behavior. In arthritic animals, intrathecal application of CGRP8-37 (1 μM, 15 min; n = 4) or BIBN4096BS (1 mM, 15 min; n = 8) increased the withdrawal thresholds significantly (P < 0.05, paired t-test). These data suggest that spinal CGRP1 receptors are important for pain-related spinal reflex behavior in the arthritis model.
Figure 8. Spinal administration of CGRP1 antagonists reverses the decreased hindlimb withdrawal thresholds in arthritic animals.
(A) and (B) In normal animals, intrathecal application of CGRP8-37 (1 μM, 15 min; n = 4) or BIBN4096BS (1 mM, 15 min; n = 3) had no effect on the mechanical thresholds (g/30 mm2) for hindlimb withdrawal reflexes in response to stimulation of the knee. To determine the effects of CGRP1 receptor antagonists in the arthritis pain model, mechanical reflex thresholds were measured in another group of rats before (normal) and 5-6 h after arthritis induction (arthritis) and during intrathecal application of CGRP8-37 (C) or BIBN4096BS (D). Intrathecal application of CGRP8-37 (1 μM, 15 min; n = 4) or BIBN4096BS (1 mM, 15 min; n = 8) increased (reversed) the withdrawal thresholds in arthritic rats significantly (P < 0.05, paired t-test). Mechanical stimuli of increasing intensity were applied to the knee joint by means of a forceps pressure transducer (see Methods). * P < 0.05 (paired t-test).
4. Discussion
This study addressed the role of spinal CGRP1 receptors in the processing of nociceptive information in the amygdala and in spinal and supraspinal forms of pain behavior. We used selective CGRP1 receptor antagonists, the standard peptide fragment CGRP8-37 and the novel non-peptide antagonist BIBN4096BS (Doods et al., 2000; Poyner 1996; Van Rossum et al., 1997; Wu et al., 2002).
The major findings are as follows: (1) Spinal (intrathecal) application of CGRP1 receptor antagonists CGRP8-37 or BIBN4096BS inhibited the increased responsiveness of amygdala neurons in arthritis. This suggests that CGRP1 receptors in the spinal dorsal horn mediate ascending transmission of nociceptive information to the amygdala. (2) Intrathecally administered CGRP1 receptor antagonists inhibited the vocalizations of arthritic rats. Thus spinal CGRP1 receptors contribute to supraspinally organized pain behavior (vocalizations) that is known to involve the amygdala (Borszcz and Leaton 2003; Han et al., 2005b; Han and Neugebauer 2005; Nandigama and Borszcz 2003). (3) Intrathecally administered CGRP1 receptor antagonists inhibited spinal motor reflexes in arthritic rats, confirming that spinal CGRP1 receptors contribute to spinal mechanisms of nociceptive behavioral responses.
A large body of evidence shows that the amygdala is critical to negative emotions and affect such as fear and anxiety (Charney 2003; Davidson 2002; Maren and Quirk 2004; Phelps and Ledoux 2005), which are strongly associated with pain states (Gallagher and Verma 2004; McWilliams et al., 2004), particularly arthritis pain (McWilliams et al., 2003). The amygdala is also believed to play an important role in the emotional-affective component of pain (Bernard et al., 1996; Nandigama and Borszcz 2003; Neugebauer et al., 2004; Schneider et al., 2001). Chemical deactivation of the amygdala has been shown to reduce affect-related pain behavior such as ultrasonic vocalizations and vocalization afterdischarges (Borszcz and Leaton 2003; Han et al., 2005b; Han and Neugebauer 2005; Nandigama and Borszcz 2003).
Vocalizations were used in the present study as a measure of higher integrated behavioral response to pain. Vocalizations during stimulation are organized in the brainstem (medullary level) whereas vocalization afterdischarges that outlast the actual stimulus are organized in the forebrain, including the amygdala (Borszcz 1995; Borszcz and Leaton 2003; Han and Neugebauer 2005; Nandigama and Borszcz 2003). Ultrasonic vocalizations in the 22-25 kHz range are emitted specifically in response to aversive stimuli and reflect the negative affective state of the animal (Borta et al., 2006; Han et al., 2005a; Han and Neugebauer 2005; Knutson et al., 2002; Ko et al., 2005). Our study did not reveal any apparent differences in the effect of spinal CGRP1 receptor blockade on different components of vocalizations, which is consistent with the ascending nociceptive transmission from the spinal cord to brain areas involved in the generation and modulation of vocalizations.
A technical aspect of this study deserves careful consideration. We recorded vocalizations of frequencies in the audible and ultrasonic ranges as described in our previous studies (see Methods). They were recorded simultaneously but with different microphones (condenser microphone with an upper limit of 16 kHz and bat detector ignoring frequencies outside the 25 ± 4 kHz range) and processed through different channels of the amplifier. Vocalizations in the audible and ultrasonic ranges involve different neural (Yajima et al., 1981) and physical (Roberts 1975) mechanisms. They are distinct, but it is very common for one to break very sharply into the other (see Roberts 1975). A previous study (Jourdan et al., 1995) described a temporally segregated (by ms) pattern of audible and ultrasonic vocalizations using brief (2 ms) electrical stimulation to evoke these responses. The mechanical stimuli used in our study lasted 10 s. Not surprisingly, our data show a great overlap of vocalizations recorded in the audible and ultrasonic ranges. Without spectrographic analysis it is impossible to determine if high and low frequency energies are entirely independent sound emissions and if the high frequency sounds represent ultrasonic harmonics of audible sounds or pure tones with an initial frequency modulation.
The importance of spinal processing for vocalization responses has been shown previously in normal naïve animals using intrathecal administration of serotonin or norepinephrine (Borszcz et al., 1996). The present study significantly advances these findings by showing the contribution of spinal mechanisms to vocalizations in a pain model. Further, the present study determines a mechanism through which spinal nociceptive processing gains access to the modulation of vocalizations, i.e., an ascending peptidergic projection to the amygdala.
The amygdala is central to the generation and modulation of audible and ultrasonic vocalizations during and after noxious stimulation as shown in our previous studies that used chemical inactivation of the amygdala by antagonists for metabotropic glutamate receptors and CGRP1 receptors (Han et al., 2005b; Han and Neugebauer 2005). The amygdala is linked to the pain system through the spino-parabrachio-amygdaloid pain pathway that arises from lamina I neurons in the spinal cord and projects to the CeLC of the amygdala through the lateral parabrachial nucleus (Bernard et al., 1996; Gauriau and Bernard 2002; Jasmin et al., 1997). Importantly, these lamina I neurons express NK1 receptors (Todd et al., 2000) and CGRP receptors (Cottrell et al., 2005) and receive peptidergic input from nociceptive afferents (Braz et al., 2005). Further, our previous studies showed that the nociceptive transmission to the CeLC from the lateral parabrachial area also utilizes CGRP (Han et al., 2005b). The peptidergic spino-parabrachio-amygdaloid pathway is different from non-peptidergic ascending pathways, which originate from lamina V projection neurons that are targeted by interneurons in lamina II. The amygdala is also an important projection site of these non-peptidergic pathways (Braz et al., 2005) and spino-amygdaloid fibers project to the CeLC (Cliffer et al., 1991).
To our knowledge, the functional role of the spino-parabrachio-amygdaloid pathway in pain behavior has been hypothesized but not shown. The present study using CGRP1 receptor antagonists suggests that it is this peptidergic pain pathway that is involved in the modulation of vocalizations as well as spinal motor reflexes. Consistent with this, the intrathecal application technique can be expected to target receptors more effectively in the superficial rather than deep laminae of the dorsal horn, further differentiating between the superficial peptidergic and deep non-peptidergic ascending pathways (see Braz et al., 2005).
The present electrophysiological studies of amygdala neurons receiving input from the parabrachial area and from CGRP1 receptor containing elements in the spinal cord show a mechanism through which the ascending peptidergic pathway modulates higher integrated pain behavior. Persistent pain is accompanied by plastic changes in the amygdala (Neugebauer et al., 2004). The behavioral consequence of pain-related activation of the amygdala is the production or facilitation of supraspinal and spinal pain behavior (Han et al., 2005b; Han and Neugebauer 2005). This study shows for the first time the significance of spinal processing for amygdala sensitization in a pain model that is known to produce CGRP receptor-dependent central sensitization in the spinal cord (Neugebauer et al., 1996) and in the amygdala (Han et al., 2005b).
CGRP1 receptors are expressed at particularly high levels in the central nucleus of the amygdala (Oliver et al., 1998). CGRP immunoreactive terminals of fibers from the lateral parabrachial area essentially delineate the CeLC (de Lacalle and Saper 2000; Harrigan et al., 1994; Kruger et al., 1988; Schwaber et al., 1988). These terminals target CeLC neurons that project to brainstem areas such as the periaqueductal gray (Harrigan et al., 1994; Schwaber et al., 1988; Xu et al., 2003), which is important for expression of behavior and descending pain modulation. Pain-related sensitization of CeLC neurons, i.e., enhanced responsiveness to afferent inputs, depends on CGRP1 receptor activation in the amygdala (Han et al., 2005b).
CGRP1 receptors are also important for spinal nociceptive transmission. Intrathecal or iontophoretic administration of a CGRP1 antagonist induced antinociception in normal naïve rats and in rats with hindpaw inflammation (Sun et al., 2003; Yu et al., 1994; Yu et al., 1996; Yu et al., 1998). CGRP8-37 also inhibited the responses of spinal dorsal horn neurons to transdermic electrical stimulation of the hindpaw (Yu et al., 1999) and to noxious mechanical stimulation of the knee joint (Neugebauer et al., 1996). Central sensitization of dorsal horn neurons in the arthritis and capsaicin pain models (Neugebauer et al., 1996; Sun et al., 2004a) and CGRP-induced sensitization of dorsal horn neurons (Sun et al., 2004b; Yu et al., 1999; Yu et al., 2002) were also inhibited by blocking CGRP1 receptors with CGRP8-37. These results suggest an important contribution of CGRP receptors to central sensitization of neurons along the spino-parabrachio-amygdaloid pain pathway.
In summary, our study shows that endogenous activation of spinal CGRP1 receptors in the arthritis pain model contributes to the sensitization of amygdala neurons and to amygdala-modulated pain behaviors. These data suggest that CGRP is utilized along the ascending peptidergic pathway to the amygdala and plays an important role in spinal and supraspinal pain behaviors.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CeA
central nucleus of the amygdala
- CeLC
latero-capsular division of the CeA
- CGRP
calcitonin gene-related peptide
- PB
pontine parabrachial area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernard J-F, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog Brain Res. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- Borszcz GS. Increases in vocalization and motor reflex thresholds are influenced by the site of morphine microinjection: comparisons following administration into the periaqueductal gray, ventral medulla, and spinal subarachnoid space. Behav Neurosci. 1995;109:502–522. doi: 10.1037//0735-7044.109.3.502. [DOI] [PubMed] [Google Scholar]
- Borszcz GS, Johnson CP, Williams DH. Increases in vocalization and motor reflex thresholds generated by the intrathecal administration of serotonin or norepinephrine. Behav Neurosci. 1996;110:809–822. doi: 10.1037//0735-7044.110.4.809. [DOI] [PubMed] [Google Scholar]
- Borszcz GS, Leaton RN. The effect of amygdala lesions on conditional and unconditional vocalizations in rats. Neurobiol Learn and Mem. 2003;79:212–225. doi: 10.1016/s1074-7427(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Borta A, Wohr M, Schwarting RKW. Rat ultrasonic vocalization in aversively motivated situations and the role of individual differences in anxiety-related behavior. Behavioural Brain Research. 2006;166:271–280. doi: 10.1016/j.bbr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Carlton SM, McNeill DL, Chung K, Coggeshall RE. Organization of calcitonin gene-related peptide-immunoreactive terminals in the primate dorsal horn. J Comp Neurol. 1988;276:527–536. doi: 10.1002/cne.902760407. [DOI] [PubMed] [Google Scholar]
- Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003;38:50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res Brain Res Rev. 1997;24:28–66. doi: 10.1016/s0165-0173(97)00010-6. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnett NW, Grady EF. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol. 2005;490:239–255. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- Cridland RA, Henry JL. Effects of intrathecal administration of neuropeptides on a spinal nociceptive reflex in the rat: VIP, galanin, CGRP, TRH, somatostatin and angiotensin II. Neuropeptides. 1988;11:23–32. doi: 10.1016/0143-4179(88)90024-8. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davis M. Anatomic and physiologic substrates of emotion in an animal model. J Clin Neurophysiol. 1998;15:378–387. doi: 10.1097/00004691-199809000-00002. [DOI] [PubMed] [Google Scholar]
- de Lacalle S, Saper CB. Calcitonin gene-related peptide-like immunoreactivity marks putative visceral sensory pathways in human brain. Neuroscience. 2000;100:115–130. doi: 10.1016/s0306-4522(00)00245-1. [DOI] [PubMed] [Google Scholar]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharm. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeazza MT, Garry MG, Yost HJ, Strait KA, Hargreaves KM, Seybold VS. Plasticity in the synthesis and storage of substance P and calcitonin gene-related peptide in primary afferent neurons during peripheral inflammation. Neuroscience. 1995;66:443–458. doi: 10.1016/0306-4522(94)00545-g. [DOI] [PubMed] [Google Scholar]
- Gallagher RM, Verma S. Mood and anxiety disorders in chronic pain. Progress in Pain Res and Management. 2004;27:139–178. [Google Scholar]
- Gauriau C, Bernard J-F. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard J-F. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol. 2004;468:24–56. doi: 10.1002/cne.10873. [DOI] [PubMed] [Google Scholar]
- Han JS, Bird GC, Li W, Neugebauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standarized measure of pain-related behavior. Neurosci Meth. 2005a;141:261–269. doi: 10.1016/j.jneumeth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Han JS, Li W, Neugebauer V. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J Neurosci. 2005b;25:10717–10728. doi: 10.1523/JNEUROSCI.4112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain. 2005;113:211–222. doi: 10.1016/j.pain.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Harrigan EA, Magnuson DJ, Thunstedt GM, Gray TS. Corticotropin releasing factor neurons are innervated by calcitonin gene-related peptide terminals in the rat central amygdaloid nucleus. Brain Res Bull. 1994;33:529–534. doi: 10.1016/0361-9230(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Honor P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Card JP, Basbaum AI. Transneuronal labeling of a nociceptive pathway, the spino-(trigemino- )parabrachio-amygdaloid, in the rat. J Neurosci. 1997;17:3751–3765. doi: 10.1523/JNEUROSCI.17-10-03751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan D, Ardid D, Chapuy E, Eschalier A, Le BD. Audible and ultrasonic vocalization elicited by single electrical nociceptive stimuli to the tail in the rat. Pain. 1995;63:237–249. doi: 10.1016/0304-3959(95)00049-X. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Ko SW, Chatila T, Zhuo M. Contribution of CaMKIV to injury and fear- induced ultrasonic vocalizations in adult mice. Mol Pain. 2005;1:10. doi: 10.1186/1744-8069-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger L, Sternini C, Brecha NC, Mantyh PW. Distribution of calcitonin gene-related peptide immunoreactivity in relation to the rat central somatosensory projection. J Comp Neurol. 1988;273:149–162. doi: 10.1002/cne.902730203. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain. 2004a;110:112–122. doi: 10.1016/j.pain.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J Neurophysiol. 2004b;91:13–24. doi: 10.1152/jn.00485.2003. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic Mechanisms of Associative Memory in the Amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Nandigama P, Borszcz GS. Affective analgesia following the administration of morphine into the amygdala of rats. Brain Res. 2003;959:343–354. doi: 10.1016/s0006-8993(02)03884-2. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Chen PS, Willis WD. Role of metabotropic glutamate receptor subtype mGluR1 in brief nociception and central sensitization of primate STT cells. J Neurophysiol. 1999;82:272–282. doi: 10.1152/jn.1999.82.1.272. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol. 2002;87:103–112. doi: 10.1152/jn.00264.2001. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol. 2003;89:716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. The Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Rumenapp P, Schaible H-G. Calcitonin gene-related peptide is involved in the spinal processing of mechanosensory input from the rat's knee joint and in the generation and maintenance of hyperexcitability of dorsal horn neurons during development of acute inflammation. Neuroscience. 1996;71:1095–1109. doi: 10.1016/0306-4522(95)00473-4. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Wainwright A, Heavens RP, Hill RG, Sirinathsinghji DJS. Distribution of novel CGRP1 receptor and adrenomedullin receptor mRNAs in the rat central nervous system. Mol Brain Res. 1998;57:149–154. doi: 10.1016/s0169-328x(98)00052-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ledoux JE. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Poyner D. Pharmacology of receptors for calcitonin gene-related peptide and amylin. TIPS. 1996;16:424–429. doi: 10.1016/s0165-6147(00)89093-8. [DOI] [PubMed] [Google Scholar]
- Roberts LH. The rodent ultrasound production mechanism. Ultrasonics. 1975;13:83–88. doi: 10.1016/0041-624x(75)90052-9. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- Schaible H-G. On the role of tachykinins and calcitonin gene-related peptide in the spinal mechanisms of nociception and in the induction and maintenance of inflammation-evoked hyperexcitability in spinal cord neurons (with special reference to nociception in joints) Prog Brain Res. 1996;113:423–441. doi: 10.1016/s0079-6123(08)61102-4. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Holthusen H, Kessler C, Posse S, Muller-Gartner HW, Arndt JO. Subjective ratings of pain correlate with subcortical-limbic blood flow: an fMRI study. Neuropsychobiology. 2001;43:175–185. doi: 10.1159/000054887. [DOI] [PubMed] [Google Scholar]
- Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol. 1988;270:416–426. doi: 10.1002/cne.902700310. [DOI] [PubMed] [Google Scholar]
- Spike RC, Puskar Z, Andrew D, Todd AJ. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci. 2003;18:2433–2448. doi: 10.1046/j.1460-9568.2003.02981.x. [DOI] [PubMed] [Google Scholar]
- Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–172. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Lawand NB, Lin Q, Willis WD. Role of Calcitonin Gene-Related Peptide in the Sensitization of Dorsal Horn Neurons to Mechanical Stimulation After Intradermal Injection of Capsaicin. J Neurophysiol. 2004a;92:320–326. doi: 10.1152/jn.00086.2004. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/s0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin Gene-Related Peptide Receptor Activation Produces PKA- and PKC-Dependent Mechanical Hyperalgesia and Central Sensitization. J Neurophysiol. 2004b;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection Neurons in Lamina I of Rat Spinal Cord with the Neurokinin 1 Receptor Are Selectively Innervated by Substance P-Containing Afferents and Respond to Noxious Stimulation. J Neurosci. 2002;22:4103–4113. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rossum D, Hanish U-K, Quirion R. Neuroanatomical Localization, Pharmacological Characterization and Functions of CGRP, Related Peptides and Their Receptors. Neuroscience & Biobehavioral Reviews. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord. Plenum; New York: 2004. [Google Scholar]
- Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- Wu D, Doods H, Arndt K, Schindler M. Development and potential of non-peptide antagonists for calcitonin-gene-related peptide (CGRP) receptors: evidence for CGRP receptor heterogeneity. Biochem Soc Trans. 2002;30:468–473. doi: 10.1042/bst0300468. [DOI] [PubMed] [Google Scholar]
- Xu W, Lundeberg T, Wang YT, Li Y, Yu L-C. Antinociceptive effect of calcitonin gene-related peptide in the central nucleus of amygdala: activating opioid receptors through amygdala-periaqueductal gray pathway. Neuroscience. 2003;118:1015–1022. doi: 10.1016/s0306-4522(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Hayashi Y, Yoshii N. Identification of ultrasonic vocalization substrates determined by electrical stimulation applied to the medulla oblongata in the rat. Brain Research. 1981;229:353–362. doi: 10.1016/0006-8993(81)90999-9. [DOI] [PubMed] [Google Scholar]
- Yu LC, Hansson P, Lundeberg T. The calcitonin gene-related peptide antagonist CGRP8-37 increases the latency to withdrawal responses in rats. Brain Res. 1994;653:223–230. doi: 10.1016/0006-8993(94)90393-x. [DOI] [PubMed] [Google Scholar]
- Yu L-C, Hansson P, Brodda-Jansen G, Theodorsson E, Lundeberg T. Intrathecal CGRP8-37-induced bilateral increase in hindpaw withdrawal latency in rats with unilateral inflammation. Br J Pharmacol. 1996;117:43–50. doi: 10.1111/j.1476-5381.1996.tb15152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LC, Hansson P, Lundeberg S, Lundeberg T. Effects of calcitonin gene-related peptide-(8-37) on withdrawal responses in rats with inflammation. European Journal of Pharmacology. 1998;347:275–282. doi: 10.1016/s0014-2999(98)00102-2. [DOI] [PubMed] [Google Scholar]
- Yu LC, Zheng EM, Lundeberg T. Calcitonin gene-related peptide 8-37 inhibits the evoked discharge frequency of wide dynamic range neurons in dorsal horn of the spinal cord in rats. Regulatory Peptides. 1999;83:21–24. doi: 10.1016/s0167-0115(99)00046-4. [DOI] [PubMed] [Google Scholar]
- Yu Y, Lundeberg T, Yu LC. Role of calcitonin gene-related peptide and its antagonist on the evoked discharge frequency of wide dynamic range neurons in the dorsal horn of the spinal cord in rats. Regulatory Peptides. 2002;103:23–27. doi: 10.1016/s0167-0115(01)00326-3. [DOI] [PubMed] [Google Scholar]