SUMMARY
The Mre11-Rad50-Nbs1 (MRN) complex is a primary sensor of DNA double-strand breaks (DSBs). Upon recruitment to DSBs it plays a critical role in catalyzing 5′→3′ single-strand resection that is required for repair by homologous recombination (HR). Unknown mechanisms repress HR in G1 phase of the cell cycle during which non-homologous end joining (NHEJ) is the favored mode of DSB repair. Here we describe fission yeast Ctp1, so-named because it shares conserved domains with the mammalian tumor suppressor CtIP. Ctp1 is recruited to DSBs where it is essential for repair by HR. Ctp1 is required for efficient formation of RPA-coated single-strand DNA adjacent to DSBs, indicating that it functions with the MRN complex in 5′→3′ resection. Ctp1 is periodically expressed during the cell cycle, with the onset of its expression coinciding with the start of DNA replication. These data suggest that regulation of Ctp1 underlies cell cycle control of HR.
INTRODUCTION
Eukaryotes have two primary mechanisms of repairing DNA double-strand breaks (DSBs): non-homologous end joining (NHEJ) and homologous recombination (HR). The critical components of these pathways are evolutionary conserved from yeast to humans. Their controlling elements include the Ku70-Ku80 DNA end-binding complex required for NHEJ and the Mre11-Rad50-Nbs1 (MRN) complex required for HR. Defects in either pathway have profound effects on human health in ways that are directly linked to genome instability. Notably, hypomorphic mutations of Mre11 and Nbs1 cause Ataxia-Telangiectasia-Like Disorder (ATLD) and Nijmegen Breakage Syndrome (NBS), respectively (D’Amours and Jackson, 2002). These are clinically related diseases of which NBS is characterized by chromosomal instability, radiation sensitivity, microcephaly, growth retardation, immunodeficiency, and predisposition to cancer.
Recent studies of model organisms support the long-standing notion that the two modes of DSB repair are controlled during the cell cycle, with NHEJ favored in the pre-replicative phase and HR favored in post-replicative phases (Aylon et al., 2004; Ferreira and Cooper, 2004; Ira et al., 2004). There are logical reasons for this pattern of regulation. In haploid organisms the absence of a sister chromatid during G1 precludes HR repair in unique DNA sequences, leaving NHEJ as the only viable option. Replication fork breakage during S phase creates a single-ended break (SEB) that cannot be repaired by NHEJ, necessitating repair by a fork recapture mechanism dependent on HR (McGlynn and Lloyd, 2002). In principle either pathway can suffice in G2, although HR is favored in yeasts, presumably because it is error free. A bias for DSB repair pathways also exists in meiosis, in which HR repair of programmed DSBs is favored because it generates genetic diversity and forms chiasmata between homologous chromosomes that are essential for their proper segregation.
While the underlying reasons for the bias towards one DSB repair pathway over the other are clear, the exact mechanism through which specific repair modes are coordinated with cell-cycle stage has remained enigmatic. Recent studies have indicated that cyclin-dependent kinase (CDK) activity plays a pivotal role in that control (Aylon et al., 2004; Henderson et al., 2006; Ira et al., 2004). CDK activity first increases in late G1 to bring about S phase and it is strongly activated in late G2 to drive entry into mitosis. Therefore, one possible way to regulate HR during the cell cycle is to make it dependent on CDK activity. Studies of the budding yeast Saccharomyces cerevisiae support a model in which CDK activity promotes the 5′→3′ single-strand resection of a DSB that is required for HR repair (Aylon et al., 2004; Ira et al., 2004). This resection largely depends on the independent activities of Exo1 5′→3′ exonuclease and Mre11-Rad50-Xrs2 (MRX) complex (Ira et al., 2004; Llorente and Symington, 2004; Nakada et al., 2004). Paradoxically, Mre11 has endonuclease and 3′→5′ exonuclease activities in vitro (Assenmacher and Hopfner, 2004; D’Amours and Jackson, 2002), and the nuclease activity of Mre11 is apparently not required for MRN-dependent resection of DSBs (Llorente and Symington, 2004)
Another plausible mechanism for restricting HR repair to the post-replicative phase is by coupling it to sensors that are specifically laid on chromatin during replication. Factors involved in chromosome cohesion are good candidates for this type of sensor; indeed, cohesion factors have been implicated in HR repair in several species (Losada and Hirano, 2005).
A third form of cell-cycle dependent control of HR could involve reliance on cell cycle oscillation of gene expression and protein degradation of HR repair factor(s). This form of periodic expression is universally employed for the control of replication initiation and prevention of re-replication (Diffley, 2004; Jensen et al., 2006). The Schizosaccharomyces pombe gene encoding the replication initiation protein Cdc18 (Cdc6 in budding yeast and humans) is transcriptionally induced at G1/S by the MBF/DSC-1 (MCB-binding factor/DNA Synthesis Control-1) transcription factor. Cdc18 is later inactivated by CDK-mediated phosphorylation and degraded to prevent reinitiation of replication (Kelly and Brown, 2000). Since DNA replication produces the sister chromatids required for HR repair, it seems reasonable to propose that HR could be linked to DNA replication through the MBF-regulated expression of a gene required for HR. In support of this hypothesis we describe an MBF-regulated gene that encodes a previously uncharacterized factor of the MRN epistasis group in fission yeast.
RESULTS
ctp1+ is a Cell Cycle-Regulated Gene Required for DSB Repair
We hypothesized that transcriptional regulation of a gene required for HR might underlie cell cycle control of DSB repair in fission yeast. To explore this model we compiled a list of putative MBF-regulated genes that are periodically transcribed in S phase (Oliva et al., 2005; Peng et al., 2005; Rustici et al., 2004). This list of genes was narrowed to those that are also induced in early meiosis and in G2 cells exposed to ionizing radiation (IR) (Mata et al., 2002; Watson et al., 2004). The final list of 12 genes (Supplemental Table 1) included genes involved in DNA replication (e.g. cdc18+ and cdc22+), cell cycle control (cdt2+ and cig2+) and the replication checkpoint (mrc1+). Of the 4 uncharacterized genes, 2 encode predicted transcription factors (SPAP14E8.02 and SPBC21B10.13c), 1 encodes a predicted dUTPase (SPAC644.05c), and the remaining gene (SPCC338.08) is annotated as encoding a 285-aa sequence orphan. For reasons described below we named this gene ctp1+.
Tetrad dissection of a ctp1Δ/+ diploid revealed that ctp1Δ cells are viable. However, they grow slowly with a doubling time of ~200 minutes versus ~150 minutes in wild type. This phenotype is typical of mutants defective in HR repair. Consistent with this possibility, we found that ctp1Δ cells are profoundly sensitive to IR (Fig. 1A), the toxic effects of which are largely attributable to DSBs (Iliakis, 1991). Acute IR-sensitivity is characteristic of HR mutants or those defective in the DNA damage checkpoint. It is unlike that of NHEJ-defective mutants, which are insensitive to IR except when synchronized in G1 (Ferreira and Cooper, 2004). Microscopic examination revealed that ctp1Δ cells arrest division in response to IR (Fig. 1B), hence their DNA damage checkpoint is intact. This conclusion was confirmed by immunoblot analysis of the DNA damage checkpoint kinase Chk1 (Fig. 1C), which is hyper-phosphorylated when activated. From these results we concluded that Ctp1 is likely required for repair of DSBs by HR.
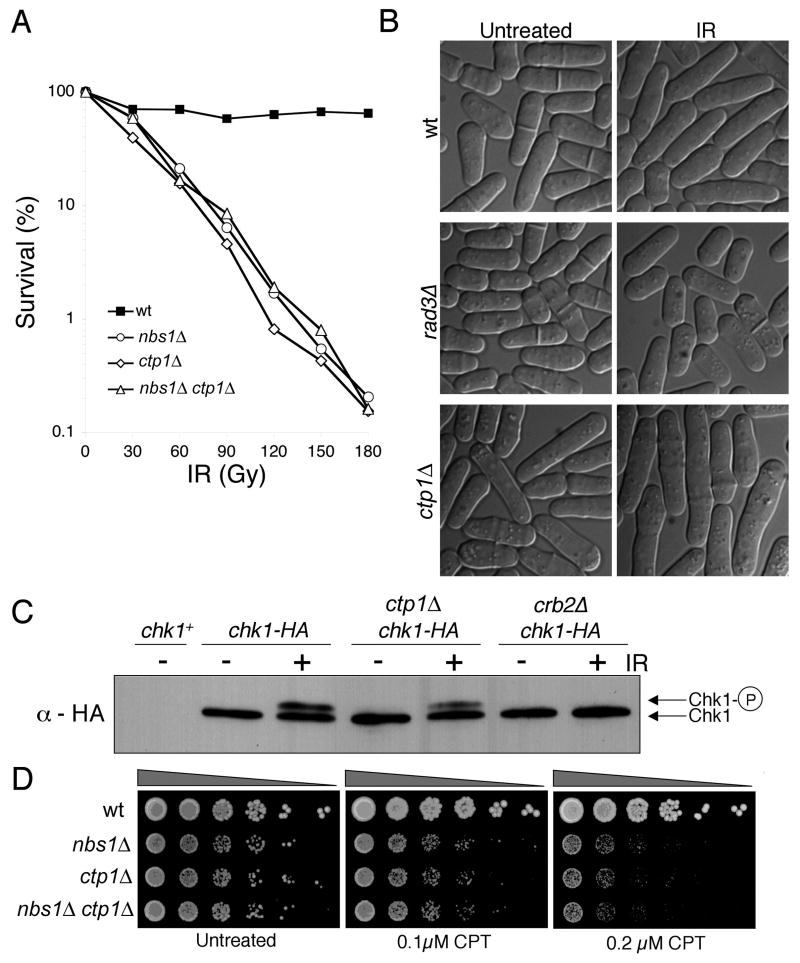
Figure 1. Ctp1 is Required for Survival of IR and CPT.
(A) ctp1Δ cells are sensitive to IR. Their sensitivity is equivalent to nbs1Δ cells and combining the mutations has no additive effect.
(B) ctp1ΔΔ cells arrest division in response to IR, with the septation index dropping to <2%, showing that the DNA damage checkpoint is intact. The rad3Δ strain is checkpoint defective.
(C) Chk1 undergoes activating phosphorylation in response to IR in ctp1ΔΔ cells as indicated by the appearance of a slow mobility species of Chk1 that is hyper-phosphorylated. The crb2Δ strain is checkpoint defective.
(D) ctp1Δ cells are sensitive to CPT. Their sensitivity is equivalent to nbs1Δ cells and combining the mutations has no additive effect.
Ctp1 and the MRN Complex Collaborate in DSB Repair
5′→3′ resection of DSBs channels their repair into the HR pathway. The resultant 3′ ssDNA tail loads recombination proteins that promote strand invasion into the sister chromatid. In S. cerevisiae the MRX complex is required for fully efficient 5′→3′ resection (Ira et al., 2004; Llorente and Symington, 2004; Nakada et al., 2004). If Ctp1 controls HR it might function with the MRN complex. Support for this model comes from the observations that ctp1Δ and nbs1Δ cells are equally sensitive to IR and the mutations have no additive effect (Fig. 1A). Identical findings are obtained when ctp1Δ is combined with mre11Δ (rad32Δ) or rad50Δ mutations (data not shown). From these results we conclude that Ctp1 and the MRN complex have mutually dependent functions in repair of IR-induced DSBs.
Replication fork collapse is thought to be the most common source of spontaneous DSBs (McGlynn and Lloyd, 2002). The resulting SEBs are repaired by a fork recapture mechanism requiring the MRN complex and other HR proteins. Camptothecin (CPT) breaks forks by trapping topoisomerase I as it unwinds DNA, creating a strand discontinuity that is converted into a SEB during S phase (Pommier, 2006). We observed that ctp1Δ cells are highly sensitive to CPT (Fig. 1D). As seen for IR survival, ctp1Δ and nbs1Δ cells are equally sensitive to CPT and the mutations are not additive in their effects (Fig. 1D), providing further evidence that Ctp1 functions in an MRN-dependent pathway of DSB repair.
Ctp1 Is Required For Meiosis
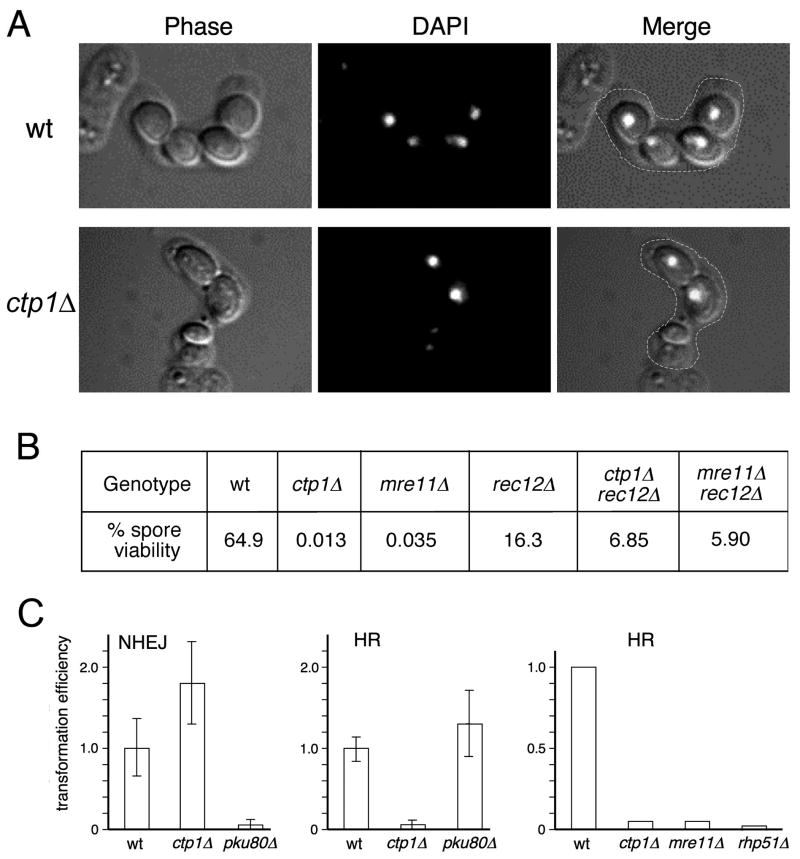
Formation of viable spores depends on HR repair of meiotic DSBs. To ascertain the role of Ctp1 in meiosis we mated ctp1Δ haploids. As seen for wild type, ctp1Δ cells readily conjugate and form asci containing spores. However, unlike the 4 spores of equal size seen in wild type, the asci from a ctp1Δ × ctp1Δ mating have an erratic number of spores of variable sizes. Nuclear DNA is improperly segregated with many of the smaller spores having little or no nuclear DNA (Fig. 2A). As anticipated from these phenotypes, ctp1Δ spore viability was very poor (0.013%) compared to wild type (64.9%) (Fig. 3B). A comparable defect was observed in an mre11Δ × mre11Δ mating (0.035%), as expected (Young et al., 2004).
Figure 2. Ctp1 is Essential for Meiosis and for HR Repair of DSBs in Mitotic Cells.
(A) Asci from a ctp1Δ × ctp1Δ mating are abnormal. DNA is stained with DAPI.
(B) Spore viability in a ctp1Δ × ctp1Δ mating is very low. This defect is partially suppressed in a rec12Δ background that is unable to form programmed meiotic DSBs.
(C) Ctp1 is required for HR but not NHEJ. HR was measured by integration of a transformed linearized plasmid into homologous sequences. NHEJ was measured by circularization of a transformed linearized plasmid. Values are calculated with respect to transformants obtained with an uncut plasmid and are normalized to wild type.
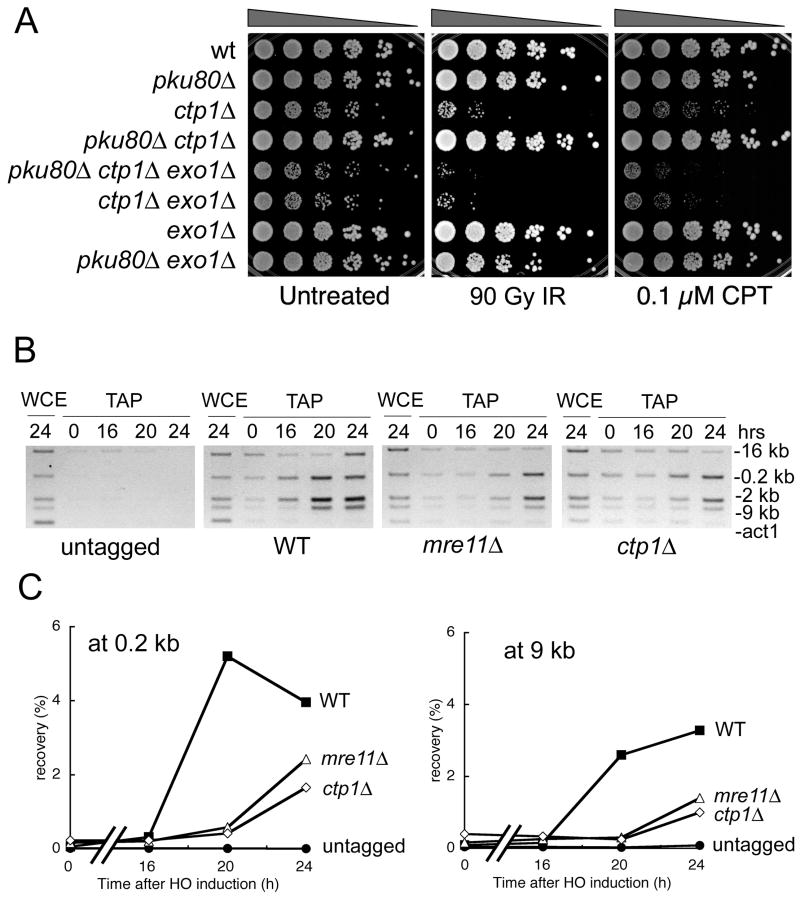
Figure 3. Exo1 Can Substitute for Ctp1 in Repair of DSBs and Recruitment of RPA to a DSB is Defective in ctp1Δ Cells.
(A) The IR and CPT survival defects of ctp1Δ cells are suppressed by eliminating Ku80. This rescue depends on Exo1.
(B) Recruitment of RPA to a DSB is reduced in ctp1Δ and mre11Δ strains relative to wild type. ChIP analysis of RPA (rad11-TAP) around an HO-induced DSB. Expression of HO endonuclease was controlled using the thiamine-repressible nmt1 promoter. Sites located 0.2, 2, 9 and 16 kb from the DSB were assayed for enrichment of RPA (see Figure 4 for map of probes). The act1 probe is included as a negative control. Microscopic analyses confirmed that >90% of the cells in all strains arrested division as a result of HO expression, confirming highly efficient cutting by HO endonuclease.
(C) Recruitment of RPA to a DSB is reduced in ctp1Δ and mre11Δ strains relative to wild type. Quantitative real-time PCR was used to measure enrichment of RPA at sites located 0.2 or 9 kb from the HO-induced DSB.
This experiment was repeated in a rec12Δ mutant that is unable to make meiotic DSBs (Young et al., 2004). The rec12Δ mutation strongly suppressed ctp1Δ spore inviability, with spore viability rising to 6.85% (Fig. 2B). We observed a similar rec12Δ rescue of mre11Δ (Fig. 2B). The absence of a fully epistatic relationship between rec12Δ and mrn or ctp1Δ mutations is likely attributable to spontaneous SEBs arising during pre-meiotic S phase, with their repair likely dependent on the MRN complex and Ctp1. From these results we conclude that Ctp1 is required for repair of programmed meiotic DSBs.
Ctp1 is Required for HR
We next performed plasmid-based yeast transformation assays to establish whether Ctp1 is required for NHEJ or HR (Ferreira and Cooper, 2004; Orr-Weaver et al., 1981). The NHEJ assay involves transformation of cells with a replication origin-containing plasmid that is linearized within sequences that lack homology to genomic sequences. Transformation efficiency depends on plasmid circularization proceeding through NHEJ. An uncut plasmid is transformed in parallel to normalize for transformation efficiencies. As expected, plasmid end-joining efficiency in ctp1Δ cells was comparable to wild type and unlike that of pku80Δ cells that are NHEJ-deficient (Fig. 2C). We conclude that Ctp1 is not required for NHEJ.
HR was measured by transforming leu1-32 cells with a plasmid lacking a replication origin and linearized within leu1+, in which formation of stable leucine prototrophs requires HR-dependent integration of the plasmid at the leu1-32 locus. This analysis revealed similar large reductions of HR in ctp1Δ and mre11Δ cells (Fig. 2C), as expected if the MRN complex and Ctp1 function together. In separate gene targeting experiments, which relies on homologous recombination with the ends of linear DNA fragments, we observed >10-fold decreases in the rate of gene targeting in ctp1Δ and mrn mutants relative to wild type (our unpublished data). These observations demonstrate that Ctp1 is required for HR repair of DSBs.
Ctp1 Function Can be Rescued by Exo1 5′→3′ Exonuclease
MRN-defective strains are extremely sensitive to IR because they cannot carry out DSB repair by HR. Elimination of NHEJ in mrn mutants might be expected to further impair IR survival, but in fact the opposite is true (Tomita et al., 2003). A rad50Δ pku70Δ strain is much more resistant to IR than a rad50Δ strain. Interestingly, this effect depends on the 5′→3′ exonuclease Exo1 (also known as ExoI) (Szankasi and Smith, 1995), which is not normally required for IR survival but is essential in a rad50Δ pku70Δ background (Tomita et al., 2003). The pku70Δ mutation does not suppress mutants defective in later steps of HR, which strongly suggests that inactivating Ku70-Ku80 complex allows Exo1 to carry out the 5′→3′ resection of DSBs that is normally performed by an MRN-dependent nuclease.
To determine the position of Ctp1 in this pathway, we created single, double and triple mutant strains involving ctp1Δ, pku80Δ and exo1Δ. While making these strains we observed that pku80Δ suppressed the slow growth phenotype of ctp1Δ (Fig. 3A). This genetic interaction supports our supposition that ctp1Δ cells grow slowly because of an HR repair defect. This suppression is not observed in an exo1Δ background, indicating that inactivating Ku70-Ku80 complex allows Exo1 to perform the resection activity that normally requires Ctp1 (Fig. 3A). These genetic relationships were replicated in IR and CPT survival assays (Fig. 3A). Therefore, in the absence of Ku70-Ku80 complex, Exo1 can substitute for Ctp1. This characteristic set of genetic interactions strengthens the evidence that the MRN complex and Ctp1 work together in DSB repair.
To further address whether Ctp1 is required for resection of DSBs, we used chromatin immunoprecipitation (ChIP) to determine whether a subunit of the RPA single-strand DNA binding complex associates with DNA near a site-specific DSB created by HO endonuclease (Du et al., 2003). This type of assay has been previously used to assay resection in budding yeast (Ira et al., 2004). We detected enrichment of RPA near the DSB in wild type cells (Fig. 3B). Some RPA enrichment was also detected in the ctp1Δ strain but it was diminished relative to wild type (Fig. 3B). An essentially identical defect was observed in mre11Δ cells (Fig. 3B). These data were confirmed by quantitative real-time PCR studies (Fig. 3C). Taken together with the suppression of ctp1Δ by Exo1, these data support the conclusion that Ctp1 and MRN are required for resection of DSBs.
Ctp1 Localizes at a DSB
To evaluate whether Ctp1 is directly involved in processing DSBs, we used ChIP to monitor Ctp1 localization near an HO DSB site. We engineered the ctp1+ genomic locus to encode Ctp1 with a C-terminal TAP-tag. However, we discovered that this strain is highly sensitive to genotoxins, being equivalent to a ctp1Δ strain (our unpublished data). This phenotype was explained when RACE-PCR analysis of SPCC338.08 transcripts revealed a previously unnoticed 3′ proximal intron. The correct Ctp1 open reading frame encodes a 294 amino acid protein with a different C-terminal 41-aa (Supplemental Fig. 1). Modifying the revised ctp1+ genomic locus to encode full-length Ctp1-TAP yielded a strain that is fully resistant to genotoxins (our unpublished data).
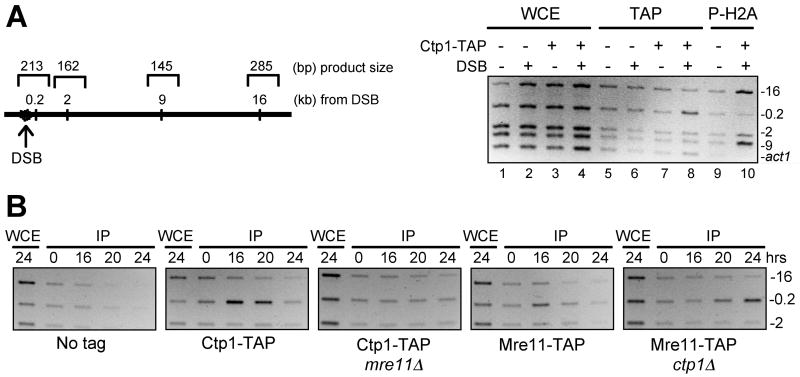
ChIP analysis revealed that Ctp1-TAP co-precipitates with DNA very near (0.2 kB) the HO break, but not at sites located 2, 9 or 16 kB further away (Fig. 4A). Phosphorylated histone H2A, which flags DNA damage when phosphorylated by the checkpoint kinases Rad3 and Tel1 (Nakamura et al., 2004), displays an inverse pattern relative to Ctp1, being found 2–16 kB but not 0.2 kB away from the break (Fig. 4A). The phospho-H2A pattern matches that seen in budding yeast (Shroff et al., 2004). These observations indicate that Ctp1 is directly involved in processing DSBs.
Figure 4. Ctp1 Localizes at a DSB by a Mre11-Dependent Mechanism.
(A) ChIP analysis of Ctp1 and phospho-H2A around an HO-induced DSB. Expression of HO endonuclease was controlled using the thiamine-repressible nmt1 promoter. Assays were performed in a ctp1+ background or a strain in which the endogenous copy of ctp1+ was modified to encode Ctp1-TAP. Ctp1-TAP was enriched 0.2 kb from the DSB (lane 8) whereas phospho-H2A was enriched 2, 9 and 16 kb from the DSB (lane 10). Specific enrichment of Ctp1-TAP at 0.2 kb from the DSB was confirmed with 4 independent strains. WCE, whole cell extract; IP, TAP, immunoprecipitated Ctp1-TAP; P-H2A, immunoprecipitated C-terminally phosphorylated histone H2A.
(B) ChIP analysis of Ctp1 and Mre11 around an HO-induced DSB. Ctp1-TAP is specifically detected 0.2 kb from the DSB in mre11+ but not mre11Δ cells. In contrast, Mre11-TAP is specifically detected 0.2 kb from the DSB in both ctp1+ and ctp1Δ cells. Genotoxin survival studies confirmed that Mre11-TAP is functional. The absence of Ctp1-TAP from the DSB in mre11Δ cells was confirmed with 4 independent strains. The 9 kb and act1 products are not shown. Microscopic analyses of the 24-hr samples confirmed that >90% of the cells in all strains arrested division as a result of HO expression, indicating highly efficient cutting by HO endonuclease. The delayed detection of Mre11-TAP at the HO break site in ctp1Δ cells may reflect delayed cutting at HO.
Localization of Ctp1 at a DSB Requires Mre11
To investigate whether recruitment of Ctp1 to a DSB requires the MRN complex, or vice versa, we carried out ChIP experiments with Ctp1-TAP and Mre11-TAP in wild type or mutant strains. These studies showed that recruitment of Ctp1 to the HO-DSB is abolished in mre11Δ cells (Fig. 4B). In contrast, Mre11 was detected at the HO-DSB in both ctp1+ and ctp1Δ cells (Fig. 4B). As seen for Ctp1, localization of Mre11 was restricted to the fragment 0.2 kb from the DSB and not at sites 2, 9, or 16 kb away from the DSB (Fig. 4B). These data indicate that the MRN complex can associate with a DSB independently of Ctp1, whereas the localization of Ctp1 at a DSB requires the MRN complex.
The Telomere Maintenance Function of the MRN Complex is Independent of Ctp1
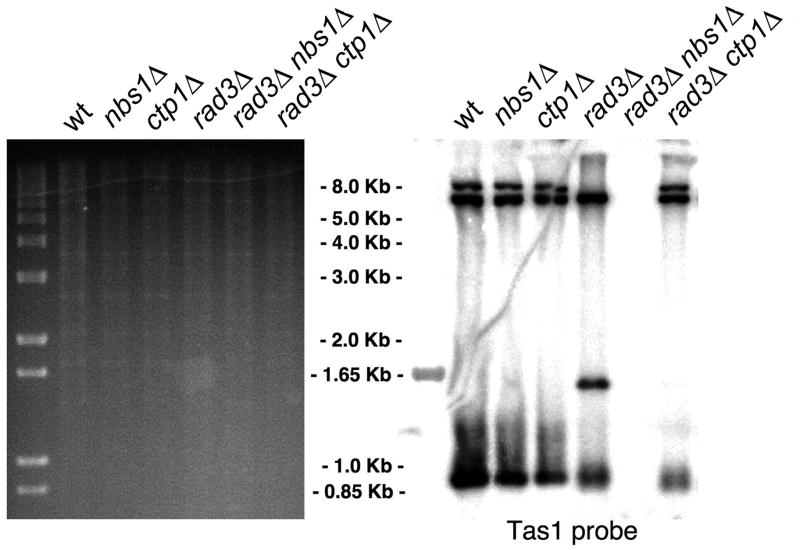
The checkpoint kinase Tel1, which is the fission yeast ortholog of human ATM, associates with DSBs and telomeres by binding to the C-terminus of Nbs1 (You et al., 2005). Since Ctp1 is required for the MRN-dependent repair of DSBs but it is not required for Mre11 binding to DSBs, we asked whether Ctp1 is required for the MRN-dependent function of Tel1 in telomere maintenance. Tel1 shares its telomere maintenance activity with the checkpoint kinase Rad3-Rad26 that is orthologous to human ATR-ATRIP. Consequently, Tel1 function is most easily assayed in a rad3Δ background in which Tel1 is essential to prevent telomere erosion and cellular senescence (Naito et al., 1998; Nakamura et al., 2002). This cellular senescence phenotype is not observed in a rad3Δ ctp1Δ strain. Southern analysis confirmed that the rad3Δ ctp1Δ strain maintains telomere-associated TAS1 sequences, unlike a rad3Δ nbs1Δ strain (Fig. 5). Since Tel1 function requires a functional MRN complex, these data indicate that MRN complexes assemble and recruit Tel1 to telomeres in the absence of Ctp1. Furthermore, Ctp1 is not required for the MRN-dependent signaling function of Tel1.
Figure 5. The Telomere Maintenance Function of MRN is Independent of Ctp1.
Southern blot analysis of EcoRI-digested genomic DNA from the indicated strains probed with the TAS1 (telomere associated-1) probe.
MBF Directly Regulates ctp1+ Expression
Having established that Ctp1 is required for HR repair of DSBs, and knowing that ctp1+ is periodically expressed during the cell cycle, we decided to explore whether MBF links HR repair with genome duplication by regulating ctp1+ expression. We monitored ctp1+ transcripts in wild type cells and those lacking Nrm1, which is a transcriptional co-repressor of MBF (de Bruin et al., 2006). Quantitative RT-PCR samples from wild type confirmed that ctp1+ is strongly transcriptionally induced in S phase (Fig. 6A). Transcription from ctp1+ is significantly elevated and only weakly periodic in nrm1Δ cells (Fig. 6A), showing that MBF regulates ctp1+ expression.
Figure 6. Cell Cycle Control of Ctp1 Abundance.
(A) Transcription of ctp1+ is regulated by MBF. Wild type and nrm1Δ strains were synchronized in G2 by centrifugal elutriation. Samples were taken as cells underwent mitosis and septation. The septation index approximately coincides with S phase. Transcript levels from ctp1+ and cdc22+ were determined by real-time PCR, normalized to act1+ transcript levels and shown as relative transcript levels (%) to maximum wild type levels during the cell cycle (wild type maximum is 100%).
(B) MBF localizes at the ctp1+ promoter region. Association of Cdc10-HA, Res2-HA and Nrm1-HA with the ctp1+, cdc22+ and act1+ promoters was determined by ChIP using log phase cultures. Tagged constructs were expressed from the endogenous loci. Whole cell extract (WCE) from the “no tag” strain was used as a control. Immunoblots confirmed that Nrm1 protein is expressed in res2Δ cells (data not shown).
(C) Immunoblot of Ctp1-TAP in asynchronous, irradiated and HU-treated cells. Immunoblot of Cdc2 with PSTAIR antisera is the loading control.
(D) Immunoblot of Ctp1-TAP in asynchronous, nitrogen-starved or carbon-starved cells. Immunoblot of tubulin is the loading control.
(E) Immunoblot of Ctp1-TAP in cells released from an HU arrest. Septation index indicates completion of the cell cycle.
The promoter region of ctp1+ contains an MCB (MluI cell cycle box) motif that is a potential binding site of MBF (Rustici et al., 2004). ChIP studies performed with three subunits of MBF (Cdc10, Res2 and Nrm1) show specific enrichment of MBF at the ctp1+ promoter region relative to an act1+ promoter negative control (Fig. 6B). The association of Nrm1 with the ctp1+ promoter is abolished in res2Δ cells (Fig. 6B), which agrees with evidence that Res2 is required for the association of Nrm1 with MBF (our unpublished data). From these results we conclude that MBF directly regulates ctp1+ expression.
Cell Cycle Regulation of Ctp1 Abundance
To be effective as a way of controlling protein abundance during the cell cycle, transcriptional control of a gene must be coupled to rapid turnover of its protein product. Accordingly, if Ctp1 acts as a controlling factor for DSB repair during the cell cycle, it should be present in S and G2 when HR is active and absent in G1 when HR is inactive. Ctp1-TAP is detected as single mobility protein species in cultures of asynchronous cells that are predominantly in G2 phase (Fig. 6C). Arrest in S phase by treatment with hydroxyurea (HU) leads to a substantial increase in Ctp1 abundance, a finding consistent with the transcriptional regulation of ctp1+ during the cell cycle and the increase of MBF-dependent transcripts in HU-arrested cells. Treatment with IR causes a more modest (~2-fold) increase in Ctp1 abundance, again consistent with the gene expression studies (Watson et al., 2004). In support of our model for the cell cycle control of HR repair, we found that Ctp1 is absent in cells arrested in G1 phase by nitrogen starvation (Fig. 6D). These findings are consistent with studies showing that cells arrested in G1 by nitrogen starvation favor NHEJ over HR (Ferreira and Cooper, 2004). Carbon starvation causes fission yeast cells to arrest in G2 phase (Costello et al., 1986). Importantly, Ctp1 remains abundant in carbon-starved cells (Fig. 6D), showing that the disappearance of Ctp1 in nitrogen-starved cells is not an indirect consequence of growth arrest. Since Ctp1 is an essential element of the MRN-dependent mechanism of repairing DSBs through HR, we conclude that the absence of Ctp1 in G1 phase could be sufficient to prevent HR repair of DSBs.
The absence of Ctp1 in G1-arrested cells suggests that Ctp1 protein is subject to rapid turnover. To explore this possibility further, cells were synchronized in early S phase by HU treatment and then released from the arrest by washing out the drug. Ctp1 remains abundant for the first ~60 minutes and then drops precipitously as cells complete the cell cycle (Fig. 6E).
Fission Yeast Ctp1 and Human CtIP are Conserved
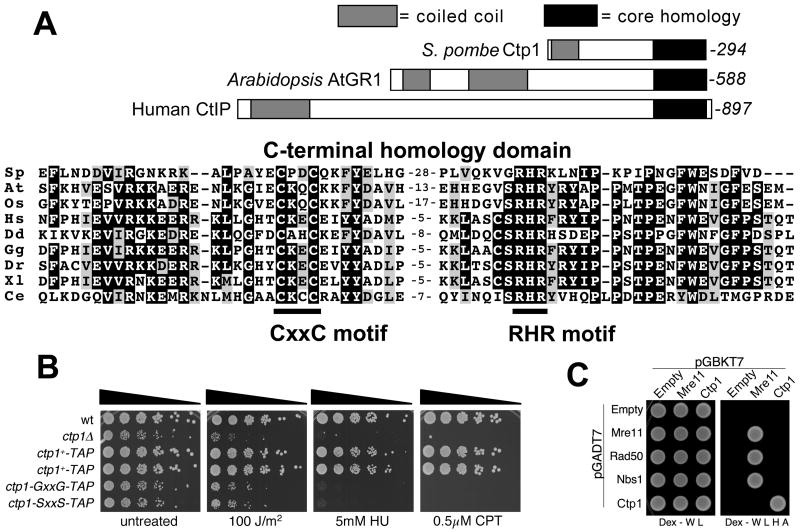
When we used the correct C-terminal sequence of Ctp1 to search for homologous proteins by Position-Specific Iterated BLAST (PSI-BLAST) (Schaffer et al., 2001) we found that the C-terminal region of Ctp1 has significant sequence similarity (Expect value = 4e-21) to the C-terminal region of Arabidopsis thaliana gamma response-1 (AtGR1) (Fig. 7A). AtGR1 is a gene of unknown function that is transcriptionally induced in response to ionizing radiation (Deveaux et al., 2000), sharing this property with ctp1+. Reiterative PSI-BLAST searches revealed that the C-terminal regions of Ctp1 and AtGR1 have significant sequence similarities to the C-terminal regions of proteins from diverse eukaryotic species (Fig. 7A). The similarities are centered on a CxxC motif that is potentially involved in zinc chelation (Fig. 7A). The human Ctp1-related protein, which is known as CtIP, is thought to be a multivalent adaptor that links pathways of transcriptional regulation, checkpoint control and tumor suppression (Wu and Lee, 2006). The relationship to human CtIP prompted us to choose the name Ctp1 for the CtIP-related protein in pombe.
Figure 7. Conserved C-terminal Domain of Ctp1.
(A) The domain structures of S. pombe Ctp1, A. thaliania AtGR1 and H. sapiens CTIP/RBBP8 are shown. These proteins share C-terminal core homology domains of ~70 amino acids that include the CxxC and RHR motifs. Alignments of the C-terminal core homology domains of S. pombe Ctp1 (Sp) and its homologs in A. thaliana, At (4e-21); Oryza sativa, Os (4e-19); H. sapiens, Hs (5e-17); Dictyostelium discoideum, Dd (9e-15); Gallus gallus, Gg (1e-17); Danio rerio, Dr (6e-16); Xenopus laevis, Xl (3e-16); and Caenorhabditis elegans, Ce (6e-10). The PSI-BLAST Expect values are shown in parentheses.
(B) The conserved CxxC motif is required for Ctp1 activity. The CxxC motif was mutated to GxxG or SxxS. These mutations and a C-terminal TAP tag were introduced into the genomic copy of ctp1+. Two wild type constructs with the C-terminal TAP tag serve as controls. Immunoblotting confirmed that the mutant proteins were expressed at levels at or above the wild type amount (unpublished data).
(C) Yeast two-hybrid assays show that Ctp1 self-associates but does not interact with Mre11, Rad50 or Nbs1. Mre11-Mre11, Mre11-Rad50 and Mre11-Nbs1 interactions are also detected. No interactions were detected involving Rad50-Rad50, Nbs1-Nbs1 or Rad50-Nbs1 (data not shown).
The functional relevance of the conserved CxxC motif in Ctp1 was investigated by replacing it with GxxG or SxxS. The mutant strains grew slowly and were hypersensitive to genotoxins, having phenotypes that are almost as severe as ctp1Δ cells (Fig. 7B). It is therefore likely that the conservation of C-terminal domains of Ctp1, AtGR1 and CtIP has functional significance.
It is noteworthy that Ctp1, AtGR1 and CtIP also have N-terminal regions that have predicted coiled-coil motifs. In CtIP these motifs mediate homodimerization (Dubin et al., 2004). To investigate whether Ctp1 shares this property, we performed yeast two-hybrid analysis with Ctp1 (Fig. 7C). This analysis detected a robust Ctp1-Ctp1 self-interaction, indicating that Ctp1 multimerizes in vivo.
Yeast two-hybrid analyses did not detect interactions between Ctp1 and MRN subunits (Fig. 7C), nor were interactions detected by co-immunoprecipitation or by mass spectrometry analysis of proteins that co-precipitate with Ctp1 (data not shown). From these results we conclude that Ctp1 is unlikely to have a stable association with soluble MRN complex in vivo, but these results does not exclude the possibility that they associate in the context of a DSB.
DISCUSSION
Resection of the 5′ ends of DNA at a DSB is the first step of HR repair. Therefore, DNA end resection represents the earliest step at which HR could be controlled. In S. pombe the generation of 3′ strand overhangs at DSBs is mediated by the action of two pathways: a major pathway that is MRN-dependent and a minor Exo1-dependent one. The lack of both activities phenocopies the DNA damage sensitivity of a strain devoid of Rhp51 recombinase activity (Tomita et al., 2003). In the hope of identifying factors involved in the cell cycle-mediated control of HR, we analyzed the published S. pombe gene expression data sets and identified Ctp1, a DNA repair factor involved in an early step of HR. Mutants lacking Ctp1 are hypersensitive to a variety of DNA damaging agents. The degrees of sensitivities match that of mrn mutants and they do not have additive effects when combined. There is an enhancement of IR and CPT sensitivity when ctp1Δ and exo1Δ mutations are combined, and the DNA damage sensitivity of ctp1Δ cells is suppressed by eliminating the Ku70/Ku80 complex, which allows Exo1 to substitute for Ctp1. This striking set of genetic interactions involving ctp1Δ precisely matches those of mrn mutations and is not seen for other mutations that impair later steps of HR-mediated DSB repair (Tomita et al., 2003). Moreover, as is the case for zygotes harboring homozygous mrn mutations, homozygous ctp1Δ zygotes have a severe spore viability defect that is partially rescued by rec12Δ. In addition, ctp1Δ cells are unable to perform HR-dependent plasmid integration and yet are fully competent at performing NHEJ-dependent plasmid re-circularization. These observations lead us to propose that Ctp1 is a fourth essential component of the MRN-dependent mechanism for HR repair of DSBs. Specifically, our data strongly support the suggestion that Ctp1 is an essential co-factor for the 5′-strand resection activity of the MRN complex.
MBF Coordinates HR with Genome Duplication
The control of Ctp1 abundance during the cell cycle is determined by MBF-regulated expression of ctp1+ peaking in S phase coupled to rapid turnover of Ctp1 protein. Our data suggest that Ctp1 degradation is accelerated late in the cell cycle, perhaps upon exit from M phase. In future experiments we plan to explore whether the APC/cyclosome regulates Ctp1 proteolysis. Degradation of Ctp1 upon exit from mitosis is logical because it marks the last point in the cell cycle at which sister chromatids are available for HR repair.
Most of the MBF-regulated genes in fission yeast have roles that are directly connected to DNA replication (Oliva et al., 2005; Rustici et al., 2004). Given the intimate connection between DNA replication and HR repair it seems logical that MBF controls both processes. By regulating genes required to initiate DNA replication and HR, we propose that MBF directly links the onset of S phase with the switch from NHEJ to HR as the favored mode of DSB repair. At the very least our data uncover a mechanism that can account for the decrease in HR in G1 phase (Ferreira and Cooper, 2004).
Our data do not exclude the possibility of additional mechanisms of controlling HR during the cell cycle. For example, Ctp1 or the MRN subunits might also be regulated in a positive manner by post-translational modification such as phosphorylation, possibly by checkpoint kinases. However, the growth rates of ctp1Δ and mrn mutants are slower than rad3Δ cells (our unpublished data), hence it appears that Ctp1 and the MRN complex can function in the absence of the Rad3-dependent DNA replication and damage checkpoints. This conclusion is consistent with the high spore viability of a rad3Δ/rad3Δ meiosis (Shimada et al., 2002) in contrast to a ctp1Δ/ctp1Δ meiosis (Fig. 2). Another possibility is that Ctp1 is regulated by CDK phosphorylation (Aylon et al., 2004; Ira et al., 2004), although Ctp1 lacks preferred CDK consensus phosphorylation sites.
If the absence of Ctp1 in G1 is the only condition that prevents HR, then constitutive expression of ctp1+ might be expected to promote HR in G1. In attempting to test this prediction we observed that constitutive expression of ctp1+ causes a poor growth phenotype that is accompanied by a cell cycle delay and increased formation of Rad22 foci (data not shown). Rad22 is a Rad52 ortholog that assembles onto single-strand DNA during HR. At face value this phenotype suggests that constitutive expression of ctp1+ increases HR, as predicted by the model. We must caution, however, that it is unknown whether this effect results from inappropriate expression of ctp1+ in G1 phase. It could be a consequence of over-expressing ctp1+ in S or G2. Unfortunately, this phenotype has made it impossible to obtain the nearly pure populations of G1 cells that are essential for assaying HR in G1 phase (Ferreira and Cooper, 2004).
Collaborative Roles of Ctp1 and the MRN Complex
Ctp1 has N-terminal coiled-coil motifs that likely mediate dimerization (Fig. 7). The coiled-coil domains are followed by amino acids rich in PEST sequences that may promote its rapid turnover (Rechsteiner and Rogers, 1996). No domain of known function could be found for the C-terminus of Ctp1 but this region is conserved amongst a broad range of eukaryotes. Given the striking phenotypic similarities between mrn and ctp1Δ mutants, particularly their rescue by Exo1 in a pku80Δ background, and our observation that Ctp1 localizes at the ends of a DSB, it is tempting to speculate that the C-terminal region of Ctp1 directly participates in DNA end resection. Ctp1 might modify the nuclease activity of the MRN complex or it might be a nuclease itself.
Although MRN and Ctp1 have mutually dependent functions, we have not detected physical interactions between MRN and Ctp1 using several different approaches. A transient interaction between Ctp1 and the MRN complex might have escaped detection or these proteins may only associate in the context of a DSB, therefore we plan to repeat the co-precipitation studies in IR-treated cells. Alternatively, Ctp1 and the MRN complex may collaborate to initiate HR repair of DSBs without having physical interactions. The MRX complex plays a pivotal role in the eviction of histones from regions flanking sites of DSBs (Tsukuda et al., 2005). Consistent with such a potential role in clearing ends for subsequent processing, human Mre11-Rad50 has been shown to slide along naked dsDNA in vitro (de Jager et al., 2001; Moreno-Herrero et al., 2005). Therefore, it is conceivable that the function of Ctp1 in HR repair might depend on the clearance of histones or other preparatory functions carried out by the MRN complex.
Is Ctp1 Related to Sae2?
SAE2/COM1 is a S. cerevisiae gene identified in screens for mutants defective in meiotic prophase I (McKee and Kleckner, 1997; Prinz et al., 1997). Mutant sae2Δ strains are also sensitive to a range of DNA damaging agents and are impaired in the 5′-resection of DSBs (Clerici et al., 2005; Keeney and Kleckner, 1995; Rattray et al., 2001). Sae2 is thought to function in collaboration with the MRX complex. These properties resemble those of Ctp1. Our phylogenetic clustering analysis indicates that, in addition to sharing a number of functional similarities, Ctp1 and Sae2 might share a common ancestry (Supplemental Fig. 2). The sequence similarities between Ctp1 and Sae2 are very weak but it may be significant that they are confined to the C-terminal region of the proteins. Sae2 was also found to self-interact in a large-scale two-hybrid screen (Uetz et al., 2000), suggesting that homo-dimerization might be another property that it shares with Ctp1.
On the other hand there appear to be a number of important differences between Ctp1 and Sae2. Unlike the situation in fission yeast in which ctp1Δ and mrn mutants are equally sensitive to DNA damaging agents, the genotoxin sensitivity of sae2Δ mutants is substantially less than that of mrx mutants (Rattray et al., 2001). The MRX complex apparently has Sae2-independent activities in DSB repair whereas the S. pombe MRN complex cannot repair DSBs without the assistance of Ctp1. Interestingly, the MRX/N complex is required for NHEJ in budding yeast but not in fission yeast (Hartsuiker et al., 2001; Moore and Haber, 1996). Another difference appears to involve the mechanisms of recruitment of Sae2 and Ctp1 to DSBs. Sae2-GFP forms IR-induced foci independently of Mre11 (Lisby et al., 2004), whereas localization of Ctp1 at an HO-induced DSB requires Mre11. Conceivably these differences could reflect the different assay systems. We have not been able to detect IR-induced Ctp1-GFP foci but this likely reflects the low amount of Ctp1 that is recruited to DSBs. Another difference between Sae2 and Ctp1 involves the CxxC motif that is conserved in Ctp1 and metazoan CtIP proteins but is absent in Sae2 (Supplemental Fig. 2). Mutation of this motif severely impairs the function of Ctp1 (Fig. 7B).
Given the functional similarities and the established role of Sae2 in DNA end processing, we currently favor the idea that Ctp1 and Sae2 are rapidly diverging orthologs whose functions are largely conserved. However, it should be noted that Sae2 abundance is not cell cycle regulated (Baroni et al., 2004), therefore the mechanism we have proposed for regulating DSB repair in fission yeast cannot operate in budding yeast.
Roles for AtGR1 and CtIP in DSB Repair?
We have noted that Ctp1 shares a conserved C-terminal domain with A. thaliana AtGR1, a gene of unknown function that is transcriptionally induced in response to ionizing radiation (Deveaux et al., 2000). Similarly, ctp1+ expression is induced in irradiated cells (Watson et al., 2004). It is tempting to propose that the shared properties of Ctp1 and AtGR1 are significant. We speculate that AtGR1 is a Ctp1 ortholog that participates in HR repair of DSBs.
Human CtIP is an 897 amino acid protein that was originally identified through its yeast two-hybrid interactions with the transcriptional repressor CtBP (C terminus-binding protein of adenovirus E1A), the retinoblastoma tumor suppressor protein RB, and the breast and ovarian cancer-specific tumor suppressor BRCA1 (Wu and Lee, 2006). Interestingly, CtIP−/− mice die much earlier in development than mice nullizygous for its known interacting partners (Chen et al., 2005). This fact may indicate that CtIP has an essential function that does not involve its known interacting partners. Interestingly, hemizygous CtIP+/− mice are viable but their life span is shortened by the development of multiple types of tumors, particularly large lymphomas (Chen et al., 2005). Perhaps these phenotypes arise because CtIP is required for HR repair of DSBs. This possibility is consistent with studies showing that homozygous disruption of MRN subunits in mice results in early embryonic lethality (D’Amours and Jackson, 2002). Furthermore, there is increased tumorigenesis in mice having a hypomorphic mutation of RAD50 or a hemizygous NBS1+/− genotype (Bender et al., 2002; Dumon-Jones et al., 2003). An additional connection between CtIP and the MRN complex is indicated by their co-precipitation with BRCA1/BARD1 complexes (Greenberg et al., 2006). CtIP undergoes BRCA1-dependent ubiquitination in response to DNA damage, it also co-localizes with BRCA1 in IR-induced foci, and it is required for the G2-M checkpoint and Chk1 phosphorylation in response to IR (Yu and Chen, 2004; Yu et al., 2006).
In the context of our studies it is fascinating to note that CtIP abundance is tightly regulated during the cell cycle. CtIP is absent or very low in G1 and its abundance increases dramatically upon entry into S (Liu and Lee, 2006; Yu and Baer, 2000; Yu and Chen, 2004). This pattern correlates with an increase in CtIP mRNA that occurs in late G1. Interestingly, the E2F/RB pathway regulates CtIP expression (Liu and Lee, 2006). This pathway is functionally analogous to the MBF pathway in yeasts (Cooper, 2006; de Bruin et al., 2006). Other targets of the E2F/RB pathway include the ribonucleotide reductase R2 gene and CDC6, which are the homologs of the MBF-regulated genes cdc22+ and cdc18+, respectively. These observations suggest that CtIP and Ctp1 are regulated in much the same way. If CtIP is required for HR repair of DSBs, these data suggest a mechanism for regulating DSB repair during the cell cycle in human cells.
EXPERIMENTAL PROCEDURES
General Methods
Strains used in these studies are listed in Supplemental Table 2. Survival assays were performed by counting colonies plated in triplicate after 4 days of growth post IR treatment at the indicated doses after normalizing to the untreated sample. Data shown are representative of two independent experiments. For checkpoint studies, log-phase cells were treated with 120Gy IR using a 137Cs source and allowed to recover for 60 min in YES media at 30°C. Spore viability assays were performed by mixing cells of opposite mating types incubating at 25°C for 3 days on SSA plates (Egel and Egel-Mitani, 1974). Asci were treated with 1% glusulase at 37°C for 1 hour followed by 30% ethanol for 30 min to obtain free spores. Spores were counted with a hemacytometer and plated on YES media (Boddy et al., 2001). Data shown are representative of two independent experiments. HR and NHEJ assays were performed as described (Ferreira and Cooper, 2004).
Real-Time PCR and RT-PCR
Experiments were performed as described (de Bruin et al., 2006). Total RNA was isolated using the Rneasy kit (Qiagen). Reactions were run on Chromo-4 qPCR I system (MJ Research) using Qiagen QuantiTech SYBR Green PCR kit or QuantiTech SYBR Green RT-PCR kit for ChIP and RT-PCR experiments respectively.
Microscopy
For meiosis experiment, cells of opposite mating types were mixed and incubated for 3 days on SSA plates. Zygotic asci were fixed in 3.7% formaldehyde and washed 3 times in PEM pH 6.9 (100mM PIPES, 1mM EGTA, 1mM MgSO4) and treated with Zymolyase 100T (0.1mg/ml in PEM+1M Sorbitol) for 10 min at 37°C. Asci were washed once in PEM+1M Sorbitol with 1% TritonX-100 and subsequently washed 3 times in PEM. Asci were resuspended in PBSA (phosphate buffered saline + 0.1% NaN3) with 0.5μg/ml DAPI (Boddy et al., 2001). Images were taken using a Nikon Eclipse E800 microscope, Photometrics Quantix CCD camera, and IPlab Spectrum software.
Immunoblotting
Whole-cell extracts were prepared from exponentially growing cells and disrupted in lysis buffer using a bead beater and resolved in 10% SDS-PAGE. Proteins were transferred to nitrocellulose membranes, blocked with 5% TBS with 0.5% Tween-20, and probed with PAP (Sigma), PSTAIR (Sigma), HA (Roche) and Tubulin (courtesy of Keith Gull) antibodies.
Telomere Analysis
Genomic DNA isolated from each strain was digested overnight with EcoRI and resolved in 2% TAE agarose gel. Ethidium bromide stained gel image was taken to show even loading. DNA was transferred via capillary method to a Hybond N membrane (GE Healthcare) and probed with 32P labeled TAS1 (Nakamura et al., 2002).
HO ChIP Assay
ChIP assay was performed as previously described (Du et al., 2006) with the following modifications: rabbit IgG conjugated to tosylactivated magnetic beads (Dynal/Invitrogen) and gamma-H2A antibody (courtesy of Christophe Redon) conjugated to anti-rabbit magnetic beads (Dynal/Invitrogen) were used to pull down Ctp1-TAP and gamma-H2A, respectively. Purelink PCR Purification Kit (Invitrogen) was used to recover DNA. In all assays >90% of the cells of all strains expressing HO displayed a checkpoint arrest indicating highly efficient cutting by HO. In a separate experiment that used Southern hybridization to measure cutting the intact fragment containing the HO site was diminished by >90% in all the strains examined (wt, ctp1Δ and mre11Δ).
Yeast 2-Hybrid
Indicated proteins were fused to Gal4 activating domain and DNA binding domain in pGADT7 and pGBKT7 respectively and expressed in S. cerevisiae reporter strain AH109 (Clonetech Matchmaker system). Strains positive for co-transformation with the indicated pair of plasmids were assayed by growth on Dex-WL plates (minimal glucose media lacking tryptophan and leucine). Positive interactions were assayed by growth on high stringency Dex-WLHA plates (minimal glucose media lacking tryptophan, leucine, histidine, and adenine).
Cell Cycle Arrest and Synchronization
Cells were arrested in G1 by growth in minimal media lacking a nitrogen source (NH4Cl). Cells were arrested in G2 by growth in low glucose (0.1%) minimal media. Samples were grown for 48 hours at 30°C. In a separate experiment cells were synchronized by 12mM HU treatment for 4 hours at 25°C. Cells were subsequently washed 3 times with water and resuspended in fresh YES with samples collected at the indicated time points.
Supplementary Material
Acknowledgments
We thank Nick Boddy, Santiago Cavero, Li-Lin Du, Tatyana Kalashnikova, Eunnie Kim, Michelle Krall, Victoria Martin, Christophe Redon, John Tainer, Scott Williams, James Wohlschlegel and John R. Yates 3rd for reagents, discussions, and technical support. Y.Y. is supported by a fellowship from The Uehara Memorial Foundation. This work was supported by NIH grants awarded to C.W. (GM059441) and P.R. (CA77325 and GM59447) and J.T./P.R. (CA117638).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assenmacher N, Hopfner KP. MRE11/RAD50/NBS1: complex activities. Chromosoma. 2004;113:157–166. doi: 10.1007/s00412-004-0306-4. [DOI] [PubMed] [Google Scholar]
- Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni E, Viscardi V, Cartagena-Lirola H, Lucchini G, Longhese MP. The functions of budding yeast Sae2 in the DNA damage response require Mec1- and Tel1-dependent phosphorylation. Mol Cell Biol. 2004;24:4151–4165. doi: 10.1128/MCB.24.10.4151-4165.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CF, Sikes ML, Sullivan R, Huye LE, Le Beau MM, Roth DB, Mirzoeva OK, Oltz EM, Petrini JH. Cancer predisposition and hematopoietic failure in Rad50(S/S) mice. Genes Dev. 2002;16:2237–2251. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy MN, Gaillard PH, McDonald WH, Shanahan P, Yates JR, 3rd, Russell P. Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell. 2001;107:537–548. doi: 10.1016/s0092-8674(01)00536-0. [DOI] [PubMed] [Google Scholar]
- Chen PL, Liu F, Cai S, Lin X, Li A, Chen Y, Gu B, Lee EY, Lee WH. Inactivation of CtIP leads to early embryonic lethality mediated by G1 restraint and to tumorigenesis by haploid insufficiency. Mol Cell Biol. 2005;25:3535–3542. doi: 10.1128/MCB.25.9.3535-3542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M, Mantiero D, Lucchini G, Longhese MP. The Saccharomyces cerevisiae Sae2 protein promotes resection and bridging of double strand break ends. J Biol Chem. 2005;280:38631–38638. doi: 10.1074/jbc.M508339200. [DOI] [PubMed] [Google Scholar]
- Cooper K. Rb, whi it’s not just for metazoans anymore. Oncogene. 2006;25:5228–5232. doi: 10.1038/sj.onc.1209630. [DOI] [PubMed] [Google Scholar]
- Costello G, Rodgers L, Beach D. Fission yeast enters stationary G0 state from either G1 or G2. Curr Genet. 1986;11:119–125. [Google Scholar]
- D’Amours D, Jackson SP. The Mre11 complex: at the crossroads of dna repair and checkpoint signalling. Nature Rev. 2002;3:317–327. doi: 10.1038/nrm805. [DOI] [PubMed] [Google Scholar]
- de Bruin RA, Kalashnikova TI, Chahwan C, McDonald WH, Wohlschlegel J, Yates J, 3rd, Russell P, Wittenberg C. Constraining G1-specific transcription to late G1 phase: the MBF-associated corepressor Nrm1 acts via negative feedback. Mol Cell. 2006;23:483–496. doi: 10.1016/j.molcel.2006.06.025. [DOI] [PubMed] [Google Scholar]
- de Jager M, van Noort J, van Gent DC, Dekker C, Kanaar R, Wyman C. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol Cell. 2001;8:1129–1135. doi: 10.1016/s1097-2765(01)00381-1. [DOI] [PubMed] [Google Scholar]
- Deveaux Y, Alonso B, Pierrugues O, Godon C, Kazmaier M. Molecular cloning and developmental expression of AtGR1, a new growth-related Arabidopsis gene strongly induced by ionizing radiation. Radiation Res. 2000;154:355–364. doi: 10.1667/0033-7587(2000)154[0355:mcadeo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Diffley JF. Regulation of early events in chromosome replication. Curr Biol. 2004;14:R778–786. doi: 10.1016/j.cub.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Du LL, Nakamura TM, Moser BA, Russell P. Retention but not recruitment of Crb2 at double-strand breaks requires Rad1 and Rad3 complexes. Mol Cell Biol. 2003;23:6150–6158. doi: 10.1128/MCB.23.17.6150-6158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du LL, Nakamura TM, Russell P. Histone modification-dependent and -independent pathways for recruitment of checkpoint protein Crb2 to double-strand breaks. Genes Dev. 2006;20:1583–1596. doi: 10.1101/gad.1422606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin MJ, Stokes PH, Sum EY, Williams RS, Valova VA, Robinson PJ, Lindeman GJ, Glover JN, Visvader JE, Matthews JM. Dimerization of CtIP, a BRCA1- and CtBP-interacting protein, is mediated by an N-terminal coiled-coil motif. J Biol Chem. 2004;279:26932–26938. doi: 10.1074/jbc.M313974200. [DOI] [PubMed] [Google Scholar]
- Dumon-Jones V, Frappart PO, Tong WM, Sajithlal G, Hulla W, Schmid G, Herceg Z, Digweed M, Wang ZQ. Nbn heterozygosity renders mice susceptible to tumor formation and ionizing radiation-induced tumorigenesis. Cancer Res. 2003;63:7263–7269. [PubMed] [Google Scholar]
- Egel R, Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp Cell Res. 1974;88:127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- Ferreira MG, Cooper JP. Two modes of DNA double-strand break repair are reciprocally regulated through the fission yeast cell cycle. Genes Dev. 2004;18:2249–2254. doi: 10.1101/gad.315804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RA, Sobhian B, Pathania S, Cantor SB, Nakatani Y, Livingston DM. Multifactorial contributions to an acute DNA damage response by BRCA1/BARD1-containing complexes. Genes Dev. 2006;20:34–46. doi: 10.1101/gad.1381306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsuiker E, Vaessen E, Carr AM, Kohli J. Fission yeast Rad50 stimulates sister chromatid recombination and links cohesion with repair. EMBO J. 2001;20:6660–6671. doi: 10.1093/emboj/20.23.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson KA, Kee K, Maleki S, Santini PA, Keeney S. Cyclin-dependent kinase directly regulates initiation of meiotic recombination. Cell. 2006;125:1321–1332. doi: 10.1016/j.cell.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliakis G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. Bioessays. 1991;13:641–648. doi: 10.1002/bies.950131204. [DOI] [PubMed] [Google Scholar]
- Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LJ, Jensen TS, de Lichtenberg U, Brunak S, Bork P. Co-evolution of transcriptional and post-translational cell-cycle regulation. Nature. 2006;443:594–597. doi: 10.1038/nature05186. [DOI] [PubMed] [Google Scholar]
- Keeney S, Kleckner N. Covalent protein-DNA complexes at the 5′ strand termini of meiosis-specific double-strand breaks in yeast. Proc Natl Acad Sci USA. 1995;92:11274–11278. doi: 10.1073/pnas.92.24.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TJ, Brown GW. Regulation of chromosome replication. Annu Rev Biochem. 2000;69:829–880. doi: 10.1146/annurev.biochem.69.1.829. [DOI] [PubMed] [Google Scholar]
- Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Liu F, Lee WH. CtIP activates its own and cyclin D1 promoters via the E2F/RB pathway during G1/S progression. Mol Cell Biol. 2006;26:3124–3134. doi: 10.1128/MCB.26.8.3124-3134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente B, Symington LS. The Mre11 nuclease is not required for 5′ to 3′ resection at multiple HO-induced double-strand breaks. Mol Cell Biol. 2004;24:9682–9694. doi: 10.1128/MCB.24.21.9682-9694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 2005;19:1269–1287. doi: 10.1101/gad.1320505. [DOI] [PubMed] [Google Scholar]
- Mata J, Lyne R, Burns G, Bahler J. The transcriptional program of meiosis and sporulation in fission yeast. Nat Genet. 2002;32:143–147. doi: 10.1038/ng951. [DOI] [PubMed] [Google Scholar]
- McGlynn P, Lloyd RG. Recombinational repair and restart of damaged replication forks. Nat Rev. 2002;3:859–870. doi: 10.1038/nrm951. [DOI] [PubMed] [Google Scholar]
- McKee AH, Kleckner N. A general method for identifying recessive diploid-specific mutations in Saccharomyces cerevisiae, its application to the isolation of mutants blocked at intermediate stages of meiotic prophase and characterization of a new gene SAE2. Genetics. 1997;146:797–816. doi: 10.1093/genetics/146.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JK, Haber JE. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Herrero F, de Jager M, Dekker NH, Kanaar R, Wyman C, Dekker C. Mesoscale conformational changes in the DNA-repair complex Rad50/Mre11/Nbs1 upon binding DNA. Nature. 2005;437:440–443. doi: 10.1038/nature03927. [DOI] [PubMed] [Google Scholar]
- Naito T, Matsuura A, Ishikawa F. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat Genet. 1998;20:203–206. doi: 10.1038/2517. [DOI] [PubMed] [Google Scholar]
- Nakada D, Hirano Y, Sugimoto K. Requirement of the Mre11 complex and exonuclease 1 for activation of the Mec1 signaling pathway. Mol Cell Biol. 2004;24:10016–10025. doi: 10.1128/MCB.24.22.10016-10025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TM, Du LL, Redon C, Russell P. Histone H2A phosphorylation controls Crb2 recruitment at DNA breaks, maintains checkpoint arrest, and influences DNA repair in fission yeast. Mol Cell Biol. 2004;24:6215–6230. doi: 10.1128/MCB.24.14.6215-6230.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TM, Moser BA, Russell P. Telomere binding of checkpoint sensor and DNA repair proteins contributes to maintenance of functional fission yeast telomeres. Genetics. 2002;161:1437–1452. doi: 10.1093/genetics/161.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Rosebrock A, Ferrezuelo F, Pyne S, Chen H, Skiena S, Futcher B, Leatherwood J. The cell cycle-regulated genes of Schizosaccharomyces pombe. PLoS Biol. 2005;3:e225. doi: 10.1371/journal.pbio.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver TL, Szostak JW, Rothstein RJ. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci USA. 1981;78:6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Karuturi RK, Miller LD, Lin K, Jia Y, Kondu P, Wang L, Wong LS, Liu ET, Balasubramanian MK, Liu J. Identification of cell cycle-regulated genes in fission yeast. Mol Biol Cell. 2005;16:1026–1042. doi: 10.1091/mbc.E04-04-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- Prinz S, Amon A, Klein F. Isolation of COM1, a new gene required to complete meiotic double-strand break-induced recombination in Saccharomyces cerevisiae. Genetics. 1997;146:781–795. doi: 10.1093/genetics/146.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray AJ, McGill CB, Shafer BK, Strathern JN. Fidelity of mitotic double-strand-break repair in Saccharomyces cerevisiae: a role for SAE2/COM1. Genetics. 2001;158:109–122. doi: 10.1093/genetics/158.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- Rustici G, Mata J, Kivinen K, Lio P, Penkett CJ, Burns G, Hayles J, Brazma A, Nurse P, Bahler J. Periodic gene expression program of the fission yeast cell cycle. Nat Genet. 2004;36:809–817. doi: 10.1038/ng1377. [DOI] [PubMed] [Google Scholar]
- Schaffer AA, Aravind L, Madden TL, Shavirin S, Spouge JL, Wolf YI, Koonin EV, Altschul SF. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nuc Acids Res. 2001;29:2994–3005. doi: 10.1093/nar/29.14.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Nabeshima K, Tougan T, Nojima H. The meiotic recombination checkpoint is regulated by checkpoint rad+ genes in fission yeast. EMBO J. 2002;21:2807–2818. doi: 10.1093/emboj/21.11.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff R, Arbel-Eden A, Pilch D, Ira G, Bonner WM, Petrini JH, Haber JE, Lichten M. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr Biol. 2004;14:1703–1711. doi: 10.1016/j.cub.2004.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szankasi P, Smith GR. A role for exonuclease I from S. pombe in mutation avoidance and mismatch correction. Science. 1995;267:1166–1169. doi: 10.1126/science.7855597. [DOI] [PubMed] [Google Scholar]
- Tomita K, Matsuura A, Caspari T, Carr AM, Akamatsu Y, Iwasaki H, Mizuno K, Ohta K, Uritani M, Ushimaru T, et al. Competition between the Rad50 complex and the Ku heterodimer reveals a role for Exo1 in processing double-strand breaks but not telomeres. Mol Cell Biol. 2003;23:5186–5197. doi: 10.1128/MCB.23.15.5186-5197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukuda T, Fleming AB, Nickoloff JA, Osley MA. Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature. 2005;438:379–383. doi: 10.1038/nature04148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- Watson A, Mata J, Bahler J, Carr A, Humphrey T. Global gene expression responses of fission yeast to ionizing radiation. Mol Biol Cell. 2004;15:851–860. doi: 10.1091/mbc.E03-08-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Lee WH. CtIP, a multivalent adaptor connecting transcriptional regulation, checkpoint control and tumor suppression. Cell Cycle. 2006;5:1592–1596. doi: 10.4161/cc.5.15.3127. [DOI] [PubMed] [Google Scholar]
- You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol. 2005;25:5363–5379. doi: 10.1128/MCB.25.13.5363-5379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JA, Hyppa RW, Smith GR. Conserved and nonconserved proteins for meiotic DNA breakage and repair in yeasts. Genetics. 2004;167:593–605. doi: 10.1534/genetics.103.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Baer R. Nuclear localization and cell cycle-specific expression of CtIP, a protein that associates with the BRCA1 tumor suppressor. J Biol Chem. 2000;275:18541–18549. doi: 10.1074/jbc.M909494199. [DOI] [PubMed] [Google Scholar]
- Yu X, Chen J. DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains. Mol Cell Biol. 2004;24:9478–9486. doi: 10.1128/MCB.24.21.9478-9486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Fu S, Lai M, Baer R, Chen J. BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev. 2006;20:1721–1726. doi: 10.1101/gad.1431006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.