Abstract
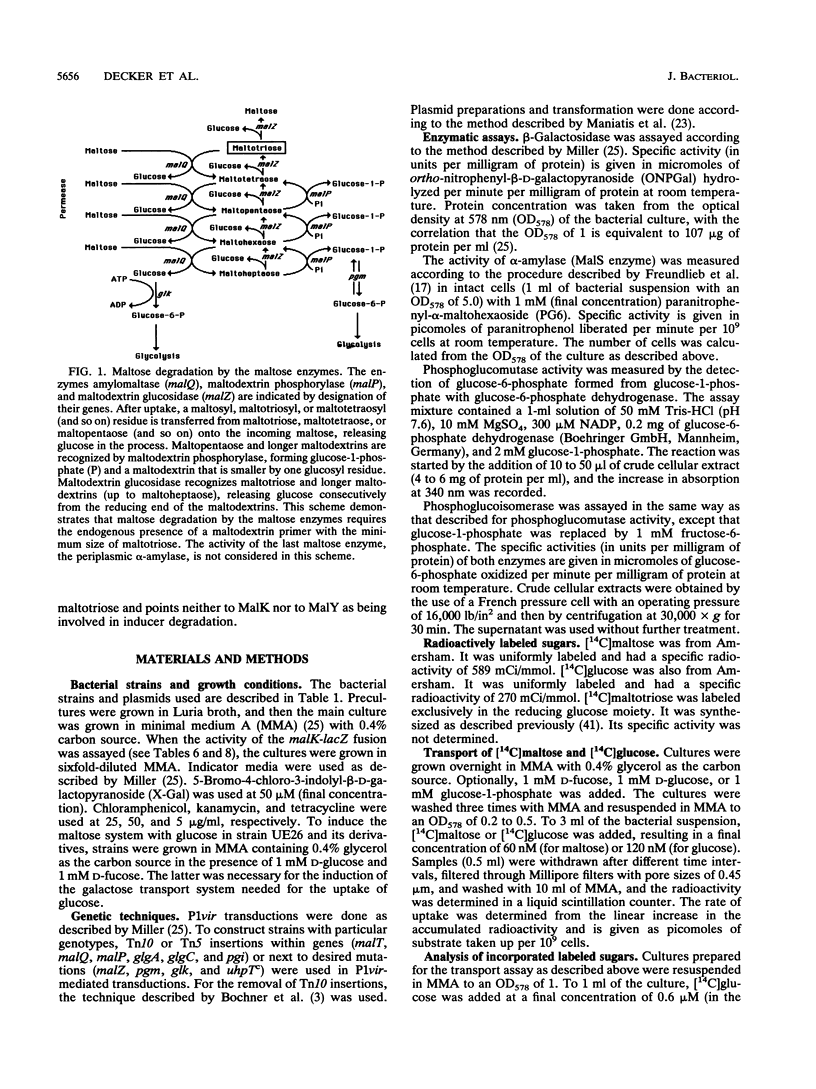
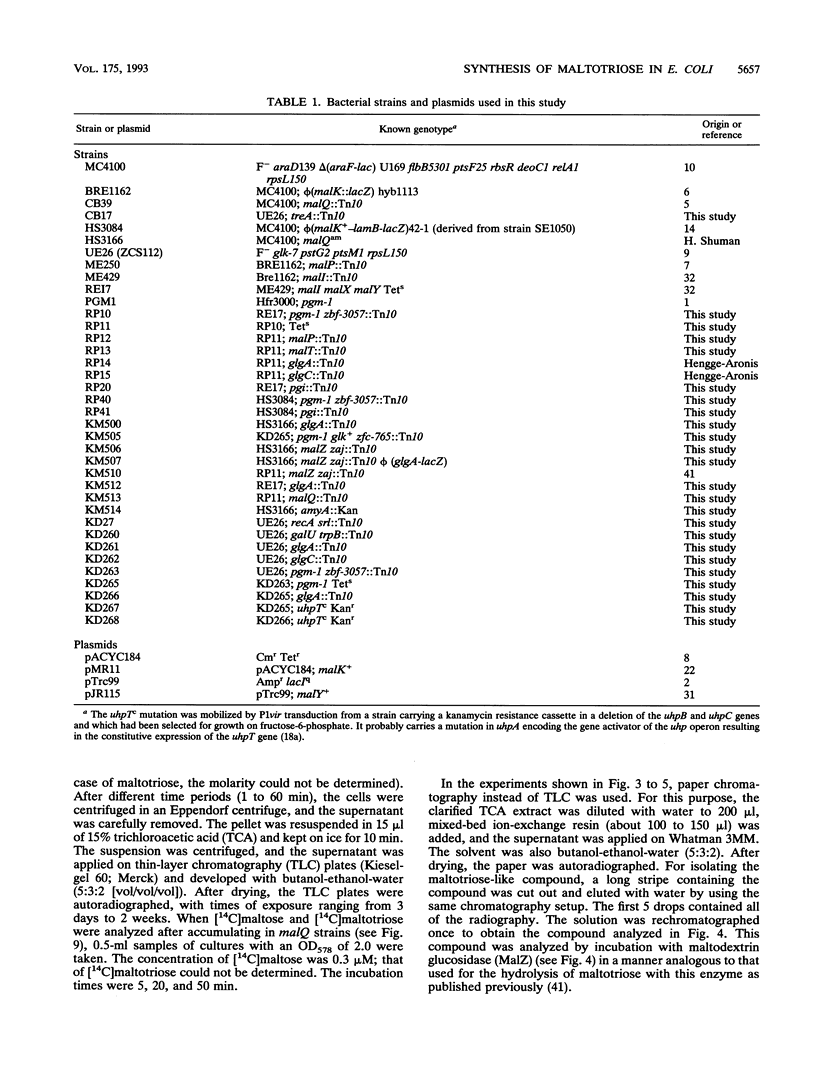
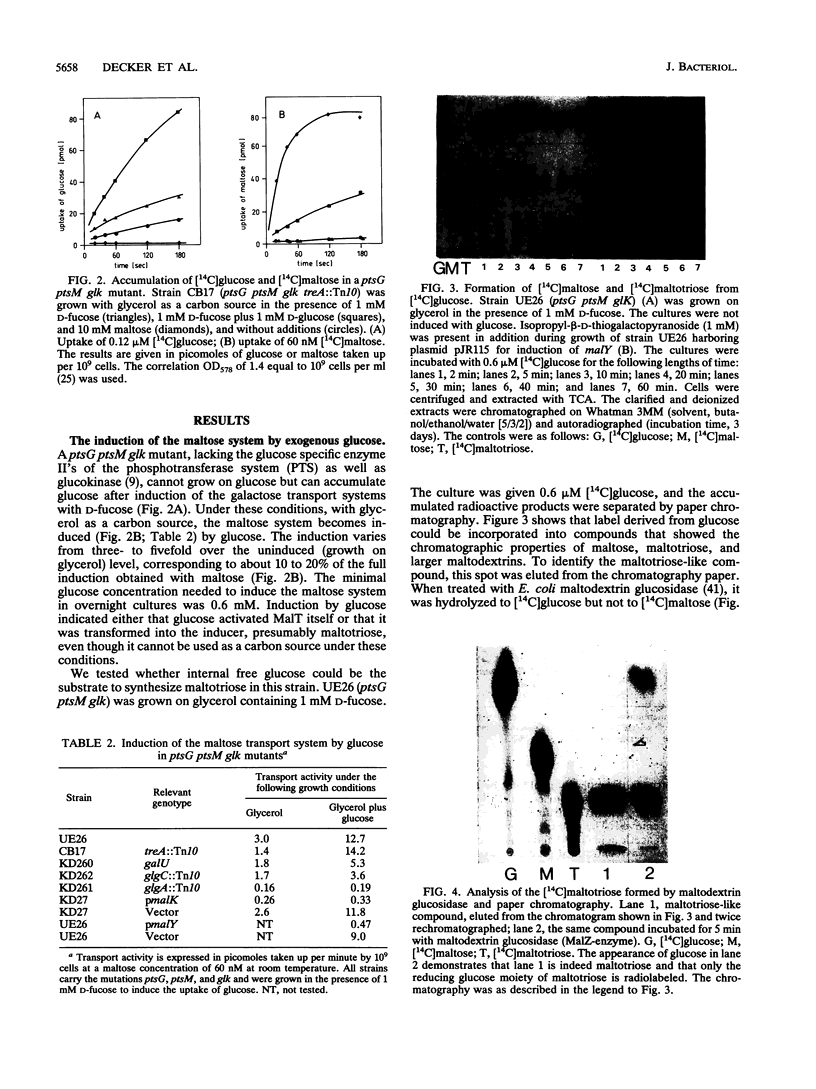
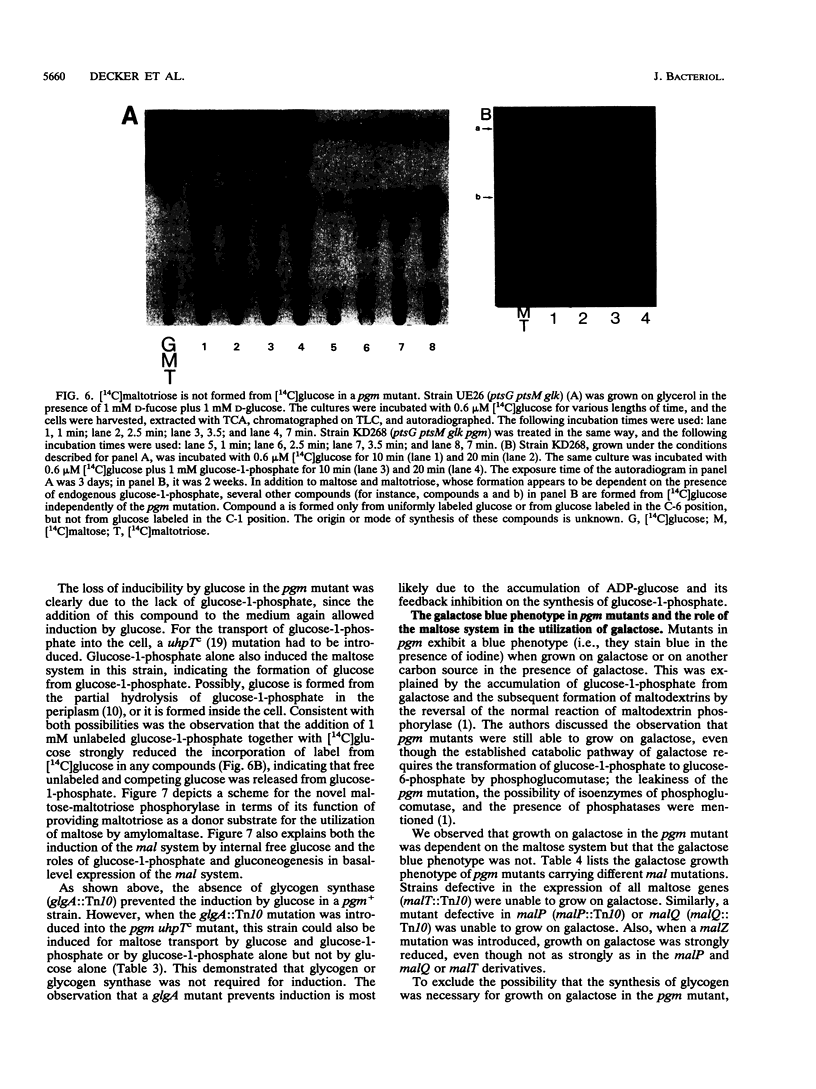
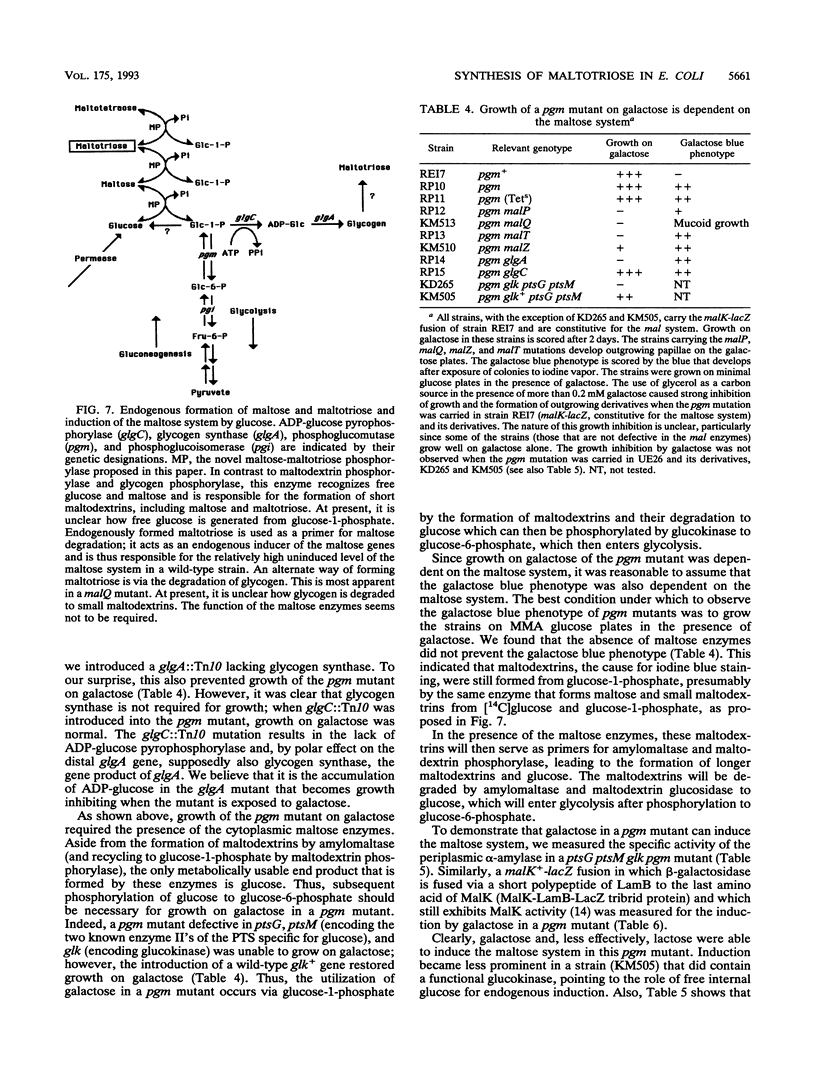
The maltose system in Escherichia coli consists of cell envelope-associated proteins and enzymes that catalyze the uptake and utilization of maltose and alpha,1-4-linked maltodextrins. The presence of these sugars in the growth medium induces the maltose system (exogenous induction), even though only maltotriose has been identified in vitro as an inducer (O. Raibaud and E. Richet, J. Bacteriol., 169:3059-3061, 1987). Induction is dependent on MalT, the positive regulator protein of the system. In the presence of exogenous glucose, the maltose system is normally repressed because of catabolite repression and inducer exclusion brought about by the phosphotransferase-mediated vectorial phosphorylation of glucose. In contrast, the increase of free, unphosphorylated glucose in the cell induces the maltose system. A ptsG ptsM glk mutant which cannot grow on glucose can accumulate [14C]glucose via galactose permeases. In this strain, internal glucose is polymerized to maltose, maltotriose, and maltodextrins in which only the reducing glucose residue is labeled. This polymerization does not require maltose enzymes, since it still occurs in malT mutants. Formation of maltodextrins from external glucose as well as induction of the maltose system is absent in a mutant lacking phosphoglucomutase, and induction by external glucose could be regained by the addition of glucose-1-phosphate entering the cells via a constitutive glucose phosphate transport system. malQ mutants, which lack amylomaltase, are constitutive for the expression of the maltose genes. This constitutive nature is due to the formation of maltose and maltodextrins from the degradation of glycogen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Schwartz M. Phosphoglucomutase mutants of Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):621–626. doi: 10.1128/jb.108.2.621-626.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann E., Ochs B., Abel K. J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988 Sep 30;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Ehmann U., Forkl H., Klein W., Rimmele M., Postma P. Trehalose transport and metabolism in Escherichia coli. J Bacteriol. 1990 Jun;172(6):3450–3461. doi: 10.1128/jb.172.6.3450-3461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand B., Boos W. Maltose transacetylase of Escherichia coli. Mapping and cloning of its structural, gene, mac, and characterization of the enzyme as a dimer of identical polypeptides with a molecular weight of 20,000. J Biol Chem. 1991 Jul 25;266(21):14113–14118. [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weinstock G. M. Transposable lambda placMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1092–1099. doi: 10.1128/jb.162.3.1092-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B., Ehrmann M., Boos W. Osmoregulation of the maltose regulon in Escherichia coli. J Bacteriol. 1986 Jun;166(3):884–891. doi: 10.1128/jb.166.3.884-891.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa J., Marck C., Boquet P. L. The complete nucleotide sequence of the Escherichia coli gene appA reveals significant homology between pH 2.5 acid phosphatase and glucose-1-phosphatase. J Bacteriol. 1990 Sep;172(9):5497–5500. doi: 10.1128/jb.172.9.5497-5500.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. L., Nikaido H. Purification and characterization of the membrane-associated components of the maltose transport system from Escherichia coli. J Biol Chem. 1991 May 15;266(14):8946–8951. [PubMed] [Google Scholar]
- Davidson A. L., Shuman H. A., Nikaido H. Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2360–2364. doi: 10.1073/pnas.89.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann M., Boos W. Identification of endogenous inducers of the mal regulon in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3539–3545. doi: 10.1128/jb.169.8.3539-3545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Mutations affecting localization of an Escherichia coli outer membrane protein, the bacteriophage lambda receptor. J Mol Biol. 1980 Jul 25;141(1):63–90. doi: 10.1016/s0022-2836(80)80029-5. [DOI] [PubMed] [Google Scholar]
- Ferenci T. The recognition of maltodextrins by Escherichia coli. Eur J Biochem. 1980 Jul;108(2):631–636. doi: 10.1111/j.1432-1033.1980.tb04758.x. [DOI] [PubMed] [Google Scholar]
- Freundlieb S., Boos W. Alpha-amylase of Escherichia coli, mapping and cloning of the structural gene, malS, and identification of its product as a periplasmic protein. J Biol Chem. 1986 Feb 25;261(6):2946–2953. [PubMed] [Google Scholar]
- Freundlieb S., Ehmann U., Boos W. Facilitated diffusion of p-nitrophenyl-alpha-D-maltohexaoside through the outer membrane of Escherichia coli. Characterization of LamB as a specific and saturable channel for maltooligosaccharides. J Biol Chem. 1988 Jan 5;263(1):314–320. [PubMed] [Google Scholar]
- Hofnung M., Schwartz M., Hatfield D. Complementation studies in the maltose-A region of the Escherichia coli K12 genetic map. J Mol Biol. 1971 Nov 14;61(3):681–694. doi: 10.1016/0022-2836(71)90072-6. [DOI] [PubMed] [Google Scholar]
- Island M. D., Wei B. Y., Kadner R. J. Structure and function of the uhp genes for the sugar phosphate transport system in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1992 May;174(9):2754–2762. doi: 10.1128/jb.174.9.2754-2762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann O., Szmelcman S. Active transport of maltose in Escherichia coli K12. Involvement of a "periplasmic" maltose binding protein. Eur J Biochem. 1974 Aug 15;47(1):139–149. doi: 10.1111/j.1432-1033.1974.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Klein W., Boos W. Induction of the lambda receptor is essential for effective uptake of trehalose in Escherichia coli. J Bacteriol. 1993 Mar;175(6):1682–1686. doi: 10.1128/jb.175.6.1682-1686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnau S., Reyes M., Sievertsen A., Shuman H. A., Boos W. The activities of the Escherichia coli MalK protein in maltose transport, regulation, and inducer exclusion can be separated by mutations. J Bacteriol. 1991 Apr;173(7):2180–2186. doi: 10.1128/jb.173.7.2180-2186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D., Boos W., Bassford P. J., Jr, Rasmussen B. A. Dependence of maltose transport and chemotaxis on the amount of maltose-binding protein. J Biol Chem. 1985 Aug 15;260(17):9727–9733. [PubMed] [Google Scholar]
- Palmer T. N., Ryman B. E., Whelan W. J. The action pattern of amylomaltase from Escherichia coli. Eur J Biochem. 1976 Oct 1;69(1):105–115. doi: 10.1111/j.1432-1033.1976.tb10863.x. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Dubreuil C. Molecular characterization of malQ, the structural gene for the Escherichia coli enzyme amylomaltase. Mol Microbiol. 1988 Jul;2(4):473–479. doi: 10.1111/j.1365-2958.1988.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Raha M., Kawagishi I., Müller V., Kihara M., Macnab R. M. Escherichia coli produces a cytoplasmic alpha-amylase, AmyA. J Bacteriol. 1992 Oct;174(20):6644–6652. doi: 10.1128/jb.174.20.6644-6652.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Richet E. Maltotriose is the inducer of the maltose regulon of Escherichia coli. J Bacteriol. 1987 Jul;169(7):3059–3061. doi: 10.1128/jb.169.7.3059-3061.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidl J., Boos W. The malX malY operon of Escherichia coli encodes a novel enzyme II of the phosphotransferase system recognizing glucose and maltose and an enzyme abolishing the endogenous induction of the maltose system. J Bacteriol. 1991 Aug;173(15):4862–4876. doi: 10.1128/jb.173.15.4862-4876.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidl J., Römisch K., Ehrmann M., Boos W. MalI, a novel protein involved in regulation of the maltose system of Escherichia coli, is highly homologous to the repressor proteins GalR, CytR, and LacI. J Bacteriol. 1989 Sep;171(9):4888–4899. doi: 10.1128/jb.171.9.4888-4899.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richet E., Raibaud O. MalT, the regulatory protein of the Escherichia coli maltose system, is an ATP-dependent transcriptional activator. EMBO J. 1989 Mar;8(3):981–987. doi: 10.1002/j.1460-2075.1989.tb03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel R., Palm D. Escherichia coli maltodextrin phosphorylase: contribution of active site residues glutamate-637 and tyrosine-538 to the phosphorolytic cleavage of alpha-glucans. Biochemistry. 1990 Oct 23;29(42):9956–9962. doi: 10.1021/bi00494a028. [DOI] [PubMed] [Google Scholar]
- Schneider E., Freundlieb S., Tapio S., Boos W. Molecular characterization of the MalT-dependent periplasmic alpha-amylase of Escherichia coli encoded by malS. J Biol Chem. 1992 Mar 15;267(8):5148–5154. [PubMed] [Google Scholar]
- Schwartz M., Hofnung M. La maltodextrine phosphorylase d'Escherichia coli. Eur J Biochem. 1967 Sep;2(2):132–145. doi: 10.1111/j.1432-1033.1967.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Sur l'existence chez Escherichia coli K 12 d'une régulation commune à la biosynthèse des récepteurs du bactériophage et au métabolisme du maltose. Ann Inst Pasteur (Paris) 1967 Nov;113(5):685–704. [PubMed] [Google Scholar]
- Shuman H. A. Active transport of maltose in Escherichia coli K12. Role of the periplasmic maltose-binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. J Biol Chem. 1982 May 25;257(10):5455–5461. [PubMed] [Google Scholar]
- Silhavy T. J., Hartig-Beecken I., Boos W. Periplasmic protein related to the sn-glycerol-3-phosphate transport system of Escherichia coli. J Bacteriol. 1976 May;126(2):951–958. doi: 10.1128/jb.126.2.951-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Tapio S., Yeh F., Shuman H. A., Boos W. The malZ gene of Escherichia coli, a member of the maltose regulon, encodes a maltodextrin glucosidase. J Biol Chem. 1991 Oct 15;266(29):19450–19458. [PubMed] [Google Scholar]
- WIESMEYER H., COHN M. The characterization of the pathway of maltose utilization by Escherichia coli. II. General properties and mechanism of action of amylomaltase. Biochim Biophys Acta. 1960 Apr 22;39:427–439. doi: 10.1016/0006-3002(60)90195-5. [DOI] [PubMed] [Google Scholar]
- Wu H. C. Role of the galactose transport system in the establishment of endogenous induction of the galactose operon in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):213–223. doi: 10.1016/0022-2836(67)90327-0. [DOI] [PubMed] [Google Scholar]
- Yu F., Jen Y., Takeuchi E., Inouye M., Nakayama H., Tagaya M., Fukui T. Alpha-glucan phosphorylase from Escherichia coli. Cloning of the gene, and purification and characterization of the protein. J Biol Chem. 1988 Sep 25;263(27):13706–13711. [PubMed] [Google Scholar]