Abstract
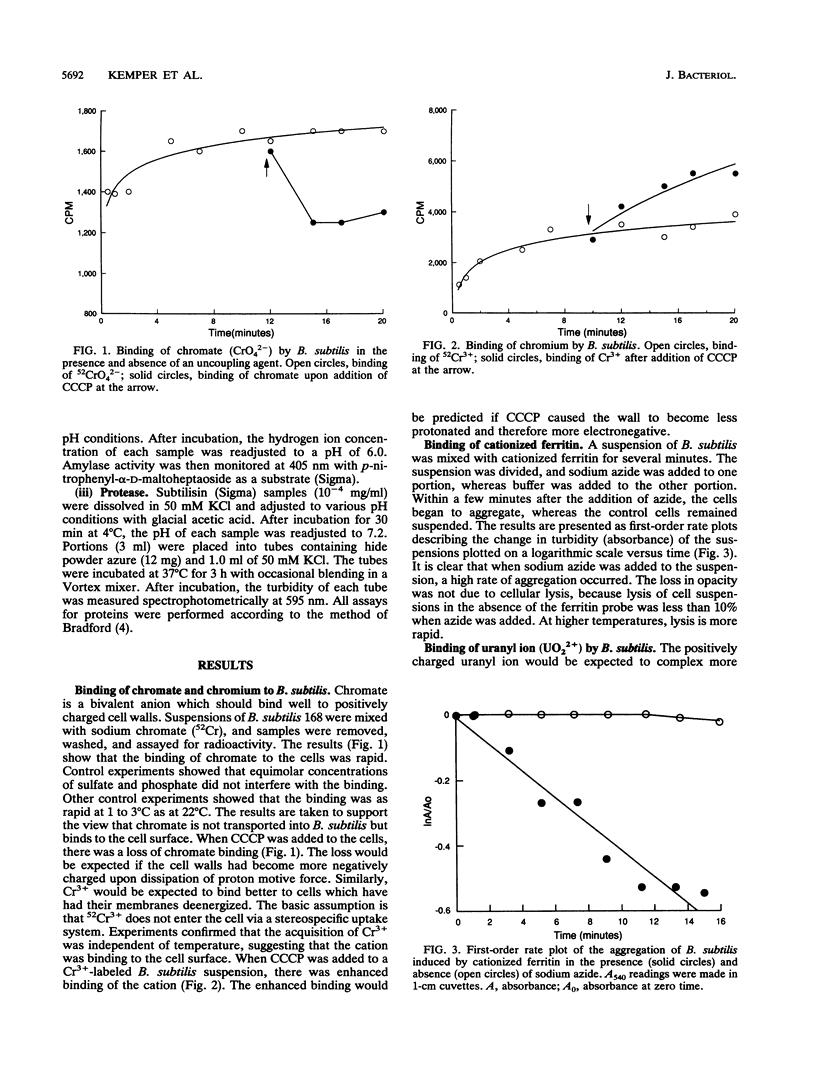
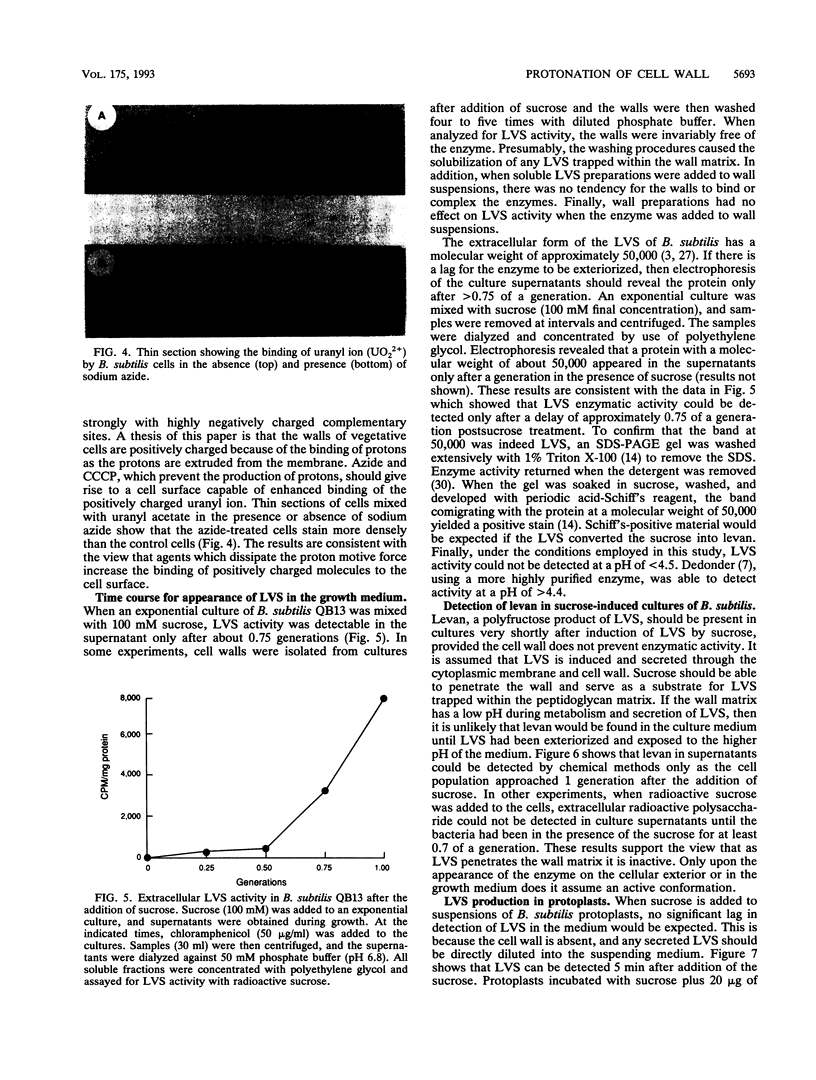
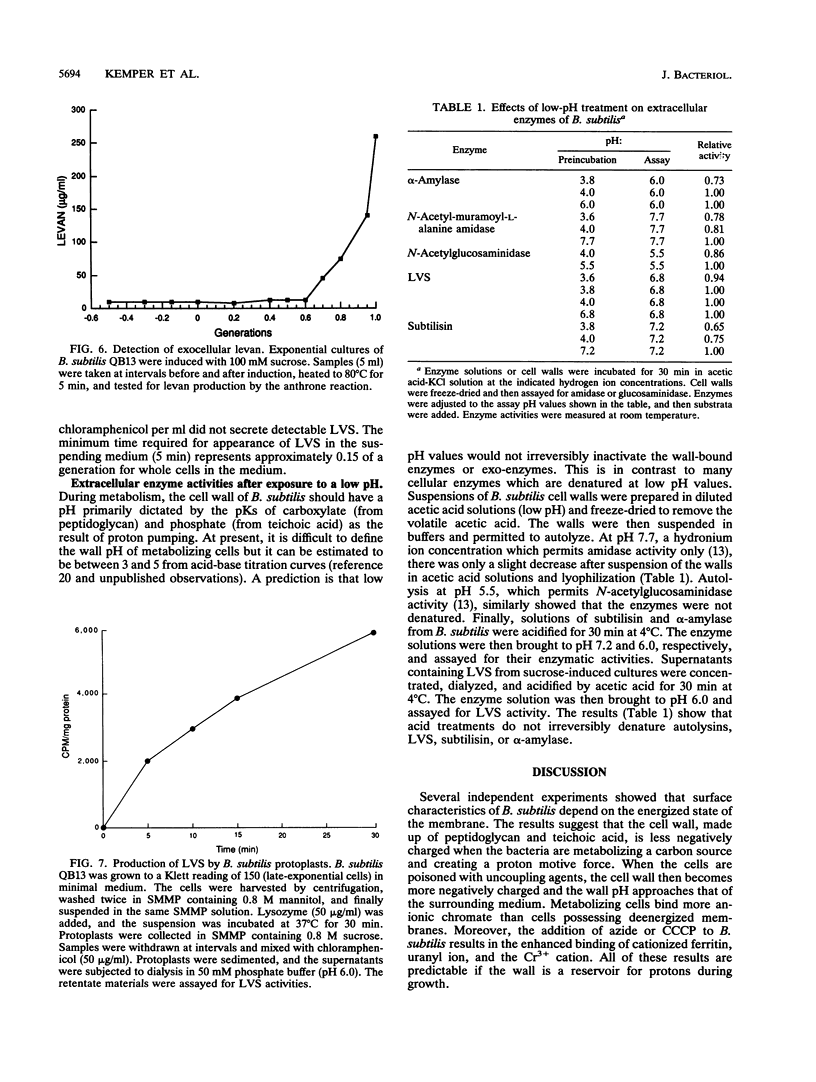
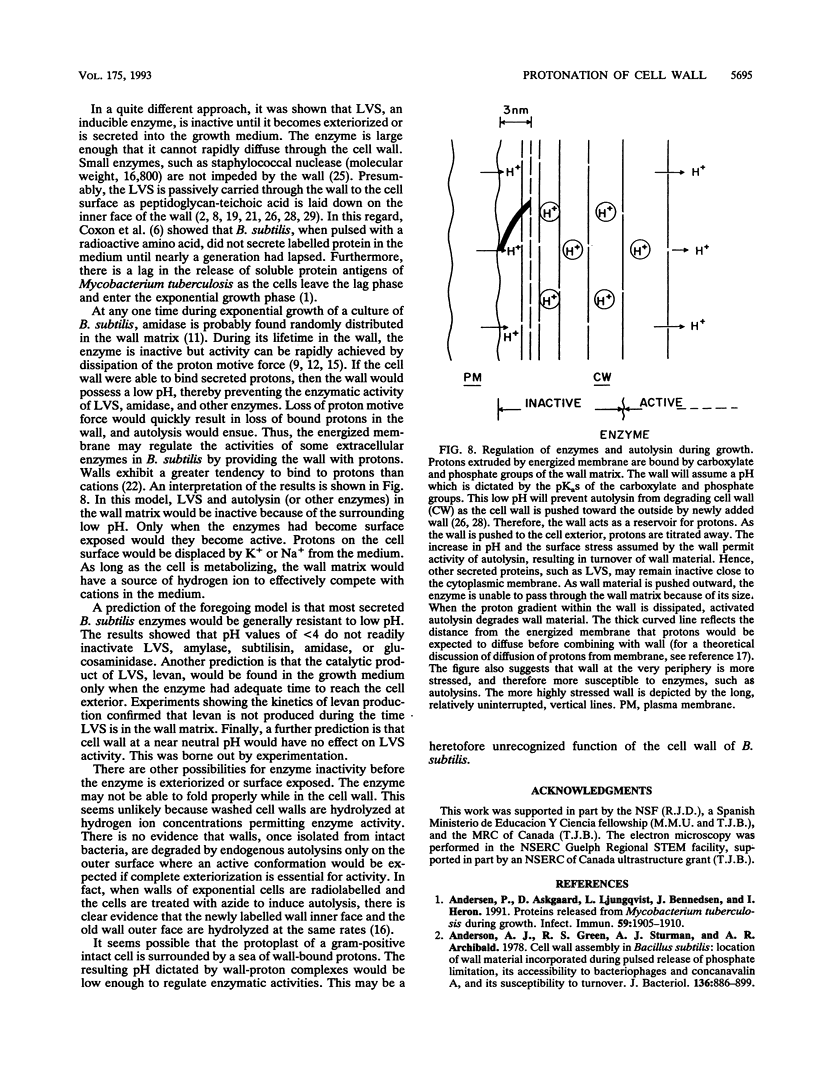
Bacterial metabolism excretes protons during normal metabolic processes. The protons may be recycled by chemiosmosis, diffuse through the wall into the medium, or bind to cell surface constituents. Calculations by Koch (J. Theor. Biol. 120:73-84, 1986) have suggested that the cell wall of gram-positive bacteria may serve as a reservoir of protons during growth and metabolism, causing the wall to have a relatively low pH. That the cell wall may possess a pH lower than the surrounding medium has now been tested in Bacillus subtilis by several independent experiments. When cultures of B. subtilis were treated with the proton conductors azide and carbonylcyanide m-chlorophenylhydrazone, the cells bound larger amounts of positively charged probes, including the chromium (Cr3+) and uranyl (UO2(2+) ions and were readily agglutinated by cationized ferritin. In contrast, the same proton conductors caused a decrease in the binding of the negatively charged probe chromate (CrO4(2-)). Finally, when levansucrase was induced in cultures by the addition of sucrose, the enzyme was inactive as it traversed the wall during the first 0.7 to 1.0 generation of growth. The composite interpretation of the foregoing observations suggests that the wall is positively charged during metabolism, thereby decreasing its ability to complex with cations while increasing its ability to bind with anions. This may be one reason why some enzymes, such as autolysins, are unable to hydrolyze their substrata until they reach the wall periphery or are in the medium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Askgaard D., Ljungqvist L., Bennedsen J., Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991 Jun;59(6):1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A. J., Green R. S., Sturman A. J., Archibald A. R. Cell wall assembly in Bacillus subtilis: location of wall material incorporated during pulsed release of phosphate limitation, its accessibility to bacteriophages and concanavalin A, and its susceptibility to turnover. J Bacteriol. 1978 Dec;136(3):886–899. doi: 10.1128/jb.136.3.886-899.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert T. V., Nagarajan V. Effect of signal sequence alterations on export of levansucrase in Bacillus subtilis. J Bacteriol. 1991 Jan;173(1):276–282. doi: 10.1128/jb.173.1.276-282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Chaloupka J., Vinter V. Turnover of cell walls in microorganisms. Microbiol Rev. 1988 Dec;52(4):554–567. doi: 10.1128/mr.52.4.554-567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Koch A. L. The functions of autolysins in the growth and division of Bacillus subtilis. Crit Rev Microbiol. 1987;15(2):169–222. doi: 10.3109/10408418709104457. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Hobot J. A., Rogers H. J. Intracellular location of the autolytic N-acetylmuramyl-L-alanine amidase in Bacillus subtilis 168 and in an autolysis-deficient mutant by immunoelectron microscopy. J Bacteriol. 1991 Feb;173(3):961–967. doi: 10.1128/jb.173.3.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Jolliffe L. K., Langemeier S. O., Doyle R. J. Hydrogen ion control of autolysin-dependent functions in Bacillus subtilis. Microbios. 1983;38(153-154):187–194. [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Doyle R. J. Inside-to-outside growth and turnover of the wall of gram-positive rods. J Theor Biol. 1985 Nov 7;117(1):137–157. doi: 10.1016/s0022-5193(85)80169-7. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The pH in the neighborhood of membranes generating a protonmotive force. J Theor Biol. 1986 May 7;120(1):73–84. doi: 10.1016/s0022-5193(86)80018-2. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Woeste S. Elasticity of the sacculus of Escherichia coli. J Bacteriol. 1992 Jul;174(14):4811–4819. doi: 10.1128/jb.174.14.4811-4819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., Mayzel K., Carstensen E. L. Cation exchange in cell walls of gram-positive bacteria. Can J Microbiol. 1976 Jul;22(7):975–982. doi: 10.1139/m76-142. [DOI] [PubMed] [Google Scholar]
- Merad T., Archibald A. R., Hancock I. C., Harwood C. R., Hobot J. A. Cell wall assembly in Bacillus subtilis: visualization of old and new wall material by electron microscopic examination of samples stained selectively for teichoic acid and teichuronic acid. J Gen Microbiol. 1989 Mar;135(3):645–655. doi: 10.1099/00221287-135-3-645. [DOI] [PubMed] [Google Scholar]
- Miller J. R., Kovacevic S., Veal L. E. Secretion and processing of staphylococcal nuclease by Bacillus subtilis. J Bacteriol. 1987 Aug;169(8):3508–3514. doi: 10.1128/jb.169.8.3508-3514.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Koch A. L., Doyle R. J., Streips U. N. Insertion and fate of the cell wall in Bacillus subtilis. J Bacteriol. 1984 Apr;158(1):169–179. doi: 10.1128/jb.158.1.169-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Glatron M. F., Chambert R., Steinmetz M. Levansucrase of Bacillus subtilis. Characterization of a form isolated from phenol-treated cells and activated by Triton X-100. Eur J Biochem. 1980 Jan;103(1):189–195. doi: 10.1111/j.1432-1033.1980.tb04303.x. [DOI] [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Use of triton X-100 to overcome the inhibition of fructosyltransferase by SDS. Anal Biochem. 1979 Aug;97(1):173–175. doi: 10.1016/0003-2697(79)90342-7. [DOI] [PubMed] [Google Scholar]
- Saunders C. W., Schmidt B. J., Mallonee R. L., Guyer M. S. Secretion of human serum albumin from Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):2917–2925. doi: 10.1128/jb.169.7.2917-2925.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenfeld E. M., Beveridge T. J., Koch A. L., Doyle R. J. Asymmetric distribution of charge on the cell wall of Bacillus subtilis. J Bacteriol. 1985 Sep;163(3):1167–1171. doi: 10.1128/jb.163.3.1167-1171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia Mera M., Kemper M., Doyle R., Beveridge T. J. The membrane-induced proton motive force influences the metal binding ability of Bacillus subtilis cell walls. Appl Environ Microbiol. 1992 Dec;58(12):3837–3844. doi: 10.1128/aem.58.12.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]