Abstract
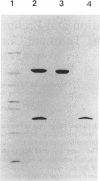
The terminal oxygenase component (ISPNAP) of naphthalene dioxygenase from Pseudomonas putida NCIB 9816-4 was purified to homogeneity. The protein contained approximately 4 g-atoms each of iron and acid-labile sulfide per mol of ISPNAP, and enzyme activity was stimulated significantly by addition of exogenous iron. The large (alpha) and small (beta) subunits of ISPNAP were isolated by two different procedures. The NH2-terminal amino acid sequences of the alpha and beta subunits were identical to the deduced amino acid sequences reported for the ndoB and ndoC genes from P. putida NCIB 9816 and almost identical to the NH2-terminal amino acid sequences determined for the large and small subunits of ISPNAP from P. putida G7. Gel filtration in the presence of 6 M urea gave an alpha subunit with an absorption maximum at 325 nm and broad absorption between 420 and 450 nm. The alpha subunit contained approximately 2 g-atoms each of iron and acid-labile sulfide per mol of the subunit. The beta subunit did not contain iron or acid-labile sulfide. These results, taken in conjunction with the deduced amino acid sequences of the large subunits from several iron-sulfur oxygenases, indicate that each alpha subunit of ISPNAP contains a Rieske [2Fe-2S] center.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batie C. J., LaHaie E., Ballou D. P. Purification and characterization of phthalate oxygenase and phthalate oxygenase reductase from Pseudomonas cepacia. J Biol Chem. 1987 Feb 5;262(4):1510–1518. [PubMed] [Google Scholar]
- Bernhardt F. H., Heymann E., Traylor P. S. Chemical and spectral properties of putidamonooxin, the iron-containing and acid-labile-sulfur-containing monooxygenase of a 4-methoxybenzoate O-demethylase from Pseudomonas putida. Eur J Biochem. 1978 Dec 1;92(1):209–223. doi: 10.1111/j.1432-1033.1978.tb12739.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cline J. F., Hoffman B. M., Mims W. B., LaHaie E., Ballou D. P., Fee J. A. Evidence for N coordination to Fe in the [2Fe-2S] clusters of Thermus Rieske protein and phthalate dioxygenase from Pseudomonas. J Biol Chem. 1985 Mar 25;260(6):3251–3254. [PubMed] [Google Scholar]
- Crutcher S. E., Geary P. J. Properties of the iron--sulphur proteins of the benzene dioxygenase system from Pseudomonas putida. Biochem J. 1979 Feb 1;177(2):393–400. doi: 10.1042/bj1770393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E., Ohnishi T., Atta-Asafo-Adjei E., Daldal F. Potential ligands to the [2Fe-2S] Rieske cluster of the cytochrome bc1 complex of Rhodobacter capsulatus probed by site-directed mutagenesis. Biochemistry. 1992 Apr 7;31(13):3342–3351. doi: 10.1021/bi00128a006. [DOI] [PubMed] [Google Scholar]
- Davies J. I., Evans W. C. Oxidative metabolism of naphthalene by soil pseudomonads. The ring-fission mechanism. Biochem J. 1964 May;91(2):251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T., Laborde A. L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982 Mar;149(3):948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T. Naphthalene dioxygenase: purification and properties of a terminal oxygenase component. J Bacteriol. 1983 Aug;155(2):505–511. doi: 10.1128/jb.155.2.505-511.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson B. D., Mondello F. J. Nucleotide sequencing and transcriptional mapping of the genes encoding biphenyl dioxygenase, a multicomponent polychlorinated-biphenyl-degrading enzyme in Pseudomonas strain LB400. J Bacteriol. 1992 May;174(9):2903–2912. doi: 10.1128/jb.174.9.2903-2912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee J. A., Findling K. L., Yoshida T., Hille R., Tarr G. E., Hearshen D. O., Dunham W. R., Day E. P., Kent T. A., Münck E. Purification and characterization of the Rieske iron-sulfur protein from Thermus thermophilus. Evidence for a [2Fe-2S] cluster having non-cysteine ligands. J Biol Chem. 1984 Jan 10;259(1):124–133. [PubMed] [Google Scholar]
- Geary P. J., Saboowalla F., Patil D., Cammack R. An investigation of the iron-sulphur proteins of benzene dioxygenase from Pseudomonas putida by electron-spin-resonance spectroscopy. Biochem J. 1984 Feb 1;217(3):667–673. doi: 10.1042/bj2170667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T., Cruden D. L., Haddock J. D., Zylstra G. J., Brand J. M. Oxidation of polychlorinated biphenyls by Pseudomonas sp. strain LB400 and Pseudomonas pseudoalcaligenes KF707. J Bacteriol. 1993 Jul;175(14):4561–4564. doi: 10.1128/jb.175.14.4561-4564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbiel R. J., Batie C. J., Sivaraja M., True A. E., Fee J. A., Hoffman B. M., Ballou D. P. Electron-nuclear double resonance spectroscopy of 15N-enriched phthalate dioxygenase from Pseudomonas cepacia proves that two histidines are coordinated to the [2Fe-2S] Rieske-type clusters. Biochemistry. 1989 May 30;28(11):4861–4871. doi: 10.1021/bi00437a051. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of ferredoxinNAP, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):465–468. doi: 10.1128/jb.172.1.465-468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Kok M., Neidle E. L. Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol. 1992;46:565–601. doi: 10.1146/annurev.mi.46.100192.003025. [DOI] [PubMed] [Google Scholar]
- Harayama S., Rekik M., Timmis K. N. Genetic analysis of a relaxed substrate specificity aromatic ring dioxygenase, toluate 1,2-dioxygenase, encoded by TOL plasmid pWW0 of Pseudomonas putida. Mol Gen Genet. 1986 Feb;202(2):226–234. doi: 10.1007/BF00331641. [DOI] [PubMed] [Google Scholar]
- Irie S., Doi S., Yorifuji T., Takagi M., Yano K. Nucleotide sequencing and characterization of the genes encoding benzene oxidation enzymes of Pseudomonas putida. J Bacteriol. 1987 Nov;169(11):5174–5179. doi: 10.1128/jb.169.11.5174-5179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey A. M., Yeh H. J., Jerina D. M., Patel T. R., Davey J. F., Gibson D. T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975 Feb 11;14(3):575–584. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Jeffrey A. M., Gibson D. T. Cis-1,2-dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971 Jan;142(1):394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- Kurkela S., Lehväslaiho H., Palva E. T., Teeri T. H. Cloning, nucleotide sequence and characterization of genes encoding naphthalene dioxygenase of Pseudomonas putida strain NCIB9816. Gene. 1988 Dec 20;73(2):355–362. doi: 10.1016/0378-1119(88)90500-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Locher H. H., Leisinger T., Cook A. M. 4-Sulphobenzoate 3,4-dioxygenase. Purification and properties of a desulphonative two-component enzyme system from Comamonas testosteroni T-2. Biochem J. 1991 Mar 15;274(Pt 3):833–842. doi: 10.1042/bj2740833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A., Krekel D., Lingens F. Purification and some properties of component A of the 4-chlorophenylacetate 3,4-dioxygenase from Pseudomonas species strain CBS. J Biol Chem. 1986 Sep 25;261(27):12883–12888. [PubMed] [Google Scholar]
- Neidle E. L., Hartnett C., Ornston L. N., Bairoch A., Rekik M., Harayama S. Nucleotide sequences of the Acinetobacter calcoaceticus benABC genes for benzoate 1,2-dioxygenase reveal evolutionary relationships among multicomponent oxygenases. J Bacteriol. 1991 Sep;173(17):5385–5395. doi: 10.1128/jb.173.17.5385-5395.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauber K., Fröhner C., Rosenberg G., Eberspächer J., Lingens F. Purification and properties of pyrazon dioxygenase from pyrazon-degrading bacteria. Eur J Biochem. 1977 Mar 15;74(1):89–97. doi: 10.1111/j.1432-1033.1977.tb11370.x. [DOI] [PubMed] [Google Scholar]
- Simon M. J., Osslund T. D., Saunders R., Ensley B. D., Suggs S., Harcourt A., Suen W. C., Cruden D. L., Gibson D. T., Zylstra G. J. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816-4. Gene. 1993 May 15;127(1):31–37. doi: 10.1016/0378-1119(93)90613-8. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Subramanian V., Liu T. N., Yeh W. K., Gibson D. T. Toluene dioxygenase: purification of an iron-sulfur protein by affinity chromatography. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1131–1139. doi: 10.1016/0006-291x(79)91998-3. [DOI] [PubMed] [Google Scholar]
- Taira K., Hirose J., Hayashida S., Furukawa K. Analysis of bph operon from the polychlorinated biphenyl-degrading strain of Pseudomonas pseudoalcaligenes KF707. J Biol Chem. 1992 Mar 5;267(7):4844–4853. [PubMed] [Google Scholar]
- Tsang H. T., Batie C. J., Ballou D. P., Penner-Hahn J. E. X-ray absorption spectroscopy of the [2Fe-2S] Rieske cluster in Pseudomonas cepacia phthalate dioxygenase. Determination of core dimensions and iron ligation. Biochemistry. 1989 Sep 5;28(18):7233–7240. doi: 10.1021/bi00444a015. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Fujisawa H. Subunit structure of oxygenase component in benzoate-1,2-dioxygenase system from Pseudomonas arvilla C-1. J Biol Chem. 1982 Nov 10;257(21):12497–12502. [PubMed] [Google Scholar]
- Yen K. M., Serdar C. M. Genetics of naphthalene catabolism in pseudomonads. Crit Rev Microbiol. 1988;15(3):247–268. doi: 10.3109/10408418809104459. [DOI] [PubMed] [Google Scholar]
- Zylstra G. J., Gibson D. T. Toluene degradation by Pseudomonas putida F1. Nucleotide sequence of the todC1C2BADE genes and their expression in Escherichia coli. J Biol Chem. 1989 Sep 5;264(25):14940–14946. [PubMed] [Google Scholar]