Abstract
The avirulence gene D (avrD) from Pseudomonas syringae pv. tomato comprises the first open reading frame (ORF) of a putative operon consisting of at least five tandem ORFs. The promoter of the avrD operon was localized to a 150-bp DNA fragment occurring 5' to the avrD gene by using the Tn7-lux and gus reporter systems. The avrD promoter in P. syringae pv. tomato and P. syringae pv. glycinea was poorly expressed when bacteria were grown in complex culture media but was activated during bacterial growth in plants. The timing and level of induction were similar in compatible and incompatible plant-pathogen interactions. When bacteria were grown in minimal culture medium, promoter activity was repressed by certain carbon sources, high concentrations of nitrogen compounds, and pH values above 6.5. Primer extension experiments on RNA from bacteria grown in minimal medium identified two transcription initiation sites 87 and 41 nucleotides upstream from the translational start site. Only the -41 transcriptional start site was identified in bacteria grown in soybean leaves. A sigma 54 promoter consensus sequence (GG-10 bp-GC) occurred 14 bp upstream of the -41 transcriptional start, and 3' deletions into this region completely abolished promoter activity. Little expression was observed when a gus fusion with the avrD promoter was introduced into an ntrA mutant strain of P. syringae pv. phaseolicola deficient in the sigma 54 cofactor. Expression from the avrD promoter also required the hrp regulatory genes, hrpS and hrpL. Deletions from the 5' end of the promoter region and base substitution analyses also identified two upstream elements important for expression. Sequence comparison of these elements with other cloned avirulence genes revealed the presence of a conserved consensus sequence elements with other cloned avirulence genes revealed the presence of a conserved consensus sequence (GGAACC-N15/16-CCAC) in the promoters of nine different avirulence genes from P. syringae pathovers.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlat M., Gough C. L., Zischek C., Barberis P. A., Trigalet A., Boucher C. A. Transcriptional organization and expression of the large hrp gene cluster of Pseudomonas solanacearum. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):187–193. doi: 10.1094/mpmi-5-187. [DOI] [PubMed] [Google Scholar]
- Barry G. F. A broad-host-range shuttle system for gene insertion into the chromosomes of gram-negative bacteria. Gene. 1988 Nov 15;71(1):75–84. doi: 10.1016/0378-1119(88)90079-0. [DOI] [PubMed] [Google Scholar]
- Boucher C. A., Van Gijsegem F., Barberis P. A., Arlat M., Zischek C. Pseudomonas solanacearum genes controlling both pathogenicity on tomato and hypersensitivity on tobacco are clustered. J Bacteriol. 1987 Dec;169(12):5626–5632. doi: 10.1128/jb.169.12.5626-5632.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney B. F., Denny T. P. A cloned avirulence gene from Pseudomonas solanacearum determines incompatibility on Nicotiana tabacum at the host species level. J Bacteriol. 1990 Sep;172(9):4836–4843. doi: 10.1128/jb.172.9.4836-4843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J. L., Ritter C., Gibbon M. J., Mur L. A., Wood J. R., Goss S., Mansfield J., Taylor J. D., Vivian A. Functional homologs of the Arabidopsis RPM1 disease resistance gene in bean and pea. Plant Cell. 1992 Nov;4(11):1359–1369. doi: 10.1105/tpc.4.11.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Simon M., Silverman M. Measuring gene expression with light. Science. 1985 Mar 15;227(4692):1345–1347. doi: 10.1126/science.2983423. [DOI] [PubMed] [Google Scholar]
- Grimm C., Panopoulos N. J. The predicted protein product of a pathogenicity locus from Pseudomonas syringae pv. phaseolicola is homologous to a highly conserved domain of several procaryotic regulatory proteins. J Bacteriol. 1989 Sep;171(9):5031–5038. doi: 10.1128/jb.171.9.5031-5038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E., Ausubel F. M. The central domain of Rhizobium meliloti NifA is sufficient to activate transcription from the R. meliloti nifH promoter. J Bacteriol. 1989 Jun;171(6):3354–3365. doi: 10.1128/jb.171.6.3354-3365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. C., Schuurink R., Denny T. P., Atkinson M. M., Baker C. J., Yucel I., Hutcheson S. W., Collmer A. Molecular cloning of a Pseudomonas syringae pv. syringae gene cluster that enables Pseudomonas fluorescens to elicit the hypersensitive response in tobacco plants. J Bacteriol. 1988 Oct;170(10):4748–4756. doi: 10.1128/jb.170.10.4748-4756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T. V., Dahlbeck D., Staskawicz B. J. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science. 1989 Sep 22;245(4924):1374–1377. doi: 10.1126/science.2781284. [DOI] [PubMed] [Google Scholar]
- Innes R. W., Bent A. F., Kunkel B. N., Bisgrove S. R., Staskawicz B. J. Molecular analysis of avirulence gene avrRpt2 and identification of a putative regulatory sequence common to all known Pseudomonas syringae avirulence genes. J Bacteriol. 1993 Aug;175(15):4859–4869. doi: 10.1128/jb.175.15.4859-4869.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Ebina Y., Nakazawa A., Nakazawa T. Nucleotide sequence surrounding transcription initiation site of xylABC operon on TOL plasmid of Pseudomonas putida. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1688–1691. doi: 10.1073/pnas.81.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Nucleotide sequence of the regulatory gene xylS on the Pseudomonas putida TOL plasmid and identification of the protein product. Gene. 1986;44(2-3):235–242. doi: 10.1016/0378-1119(86)90187-3. [DOI] [PubMed] [Google Scholar]
- Jenner C., Hitchin E., Mansfield J., Walters K., Betteridge P., Teverson D., Taylor J. Gene-for-gene interactions between Pseudomonas syringae pv. phaseolicola and Phaseolus. Mol Plant Microbe Interact. 1991 Nov-Dec;4(6):553–562. [PubMed] [Google Scholar]
- Johnson K., Parker M. L., Lory S. Nucleotide sequence and transcriptional initiation site of two Pseudomonas aeruginosa pilin genes. J Biol Chem. 1986 Nov 25;261(33):15703–15708. [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi D. Y., Tamaki S. J., Keen N. T. Cloned avirulence genes from the tomato pathogen Pseudomonas syringae pv. tomato confer cultivar specificity on soybean. Proc Natl Acad Sci U S A. 1989 Jan;86(1):157–161. doi: 10.1073/pnas.86.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
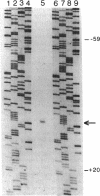
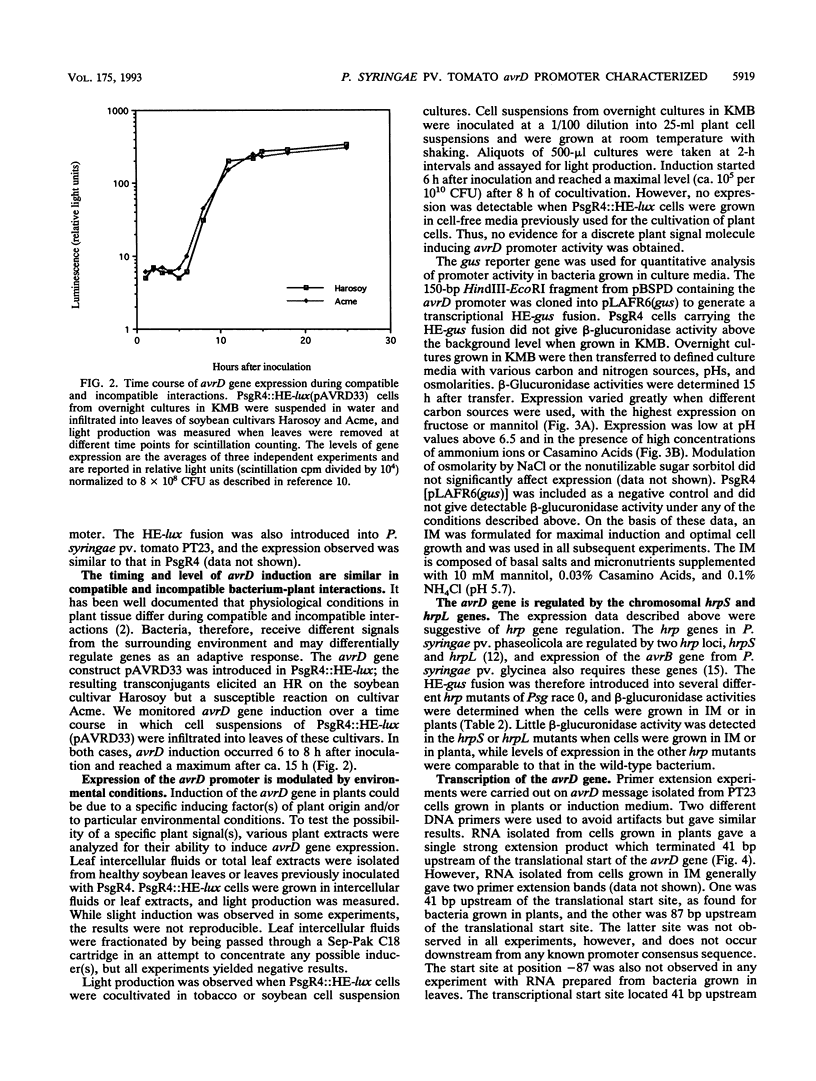
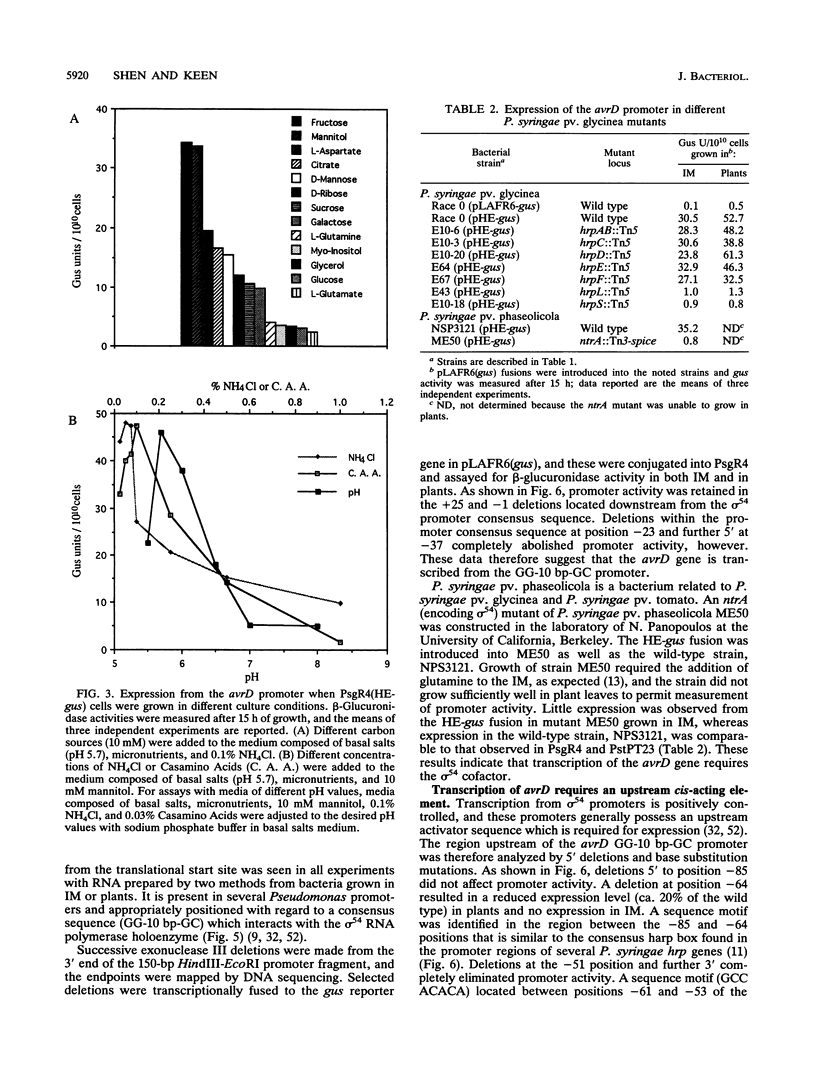
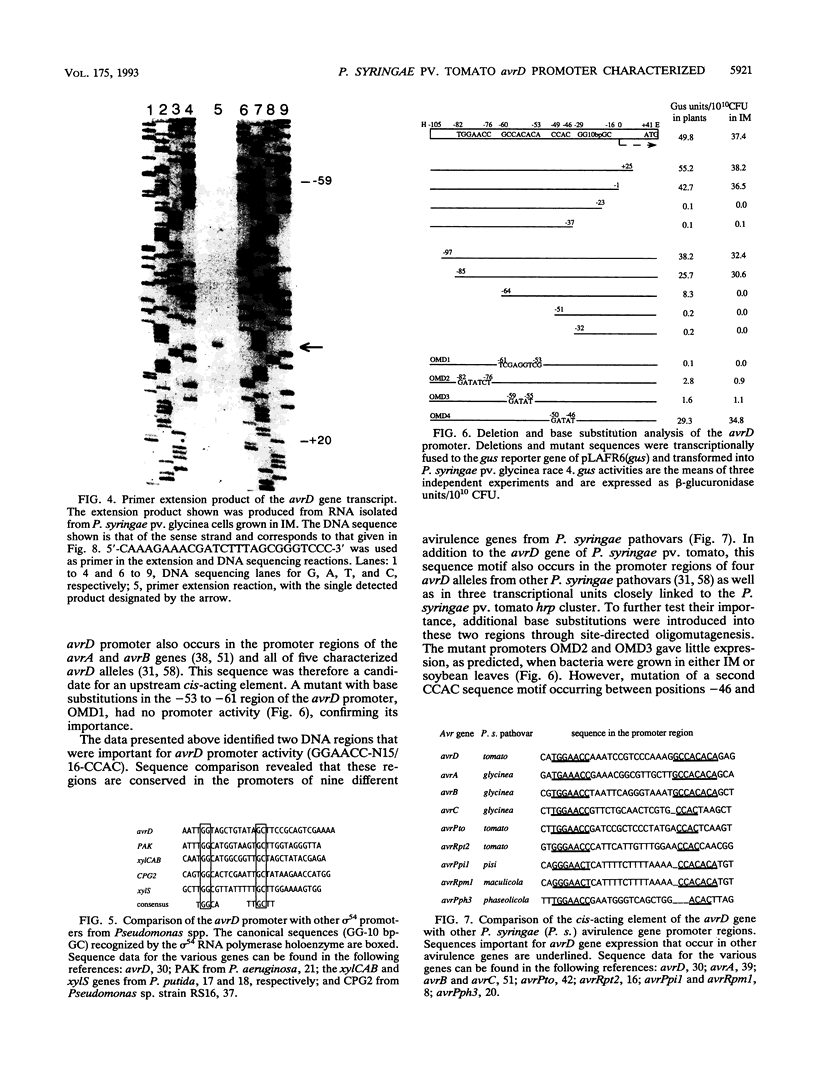
- Kobayashi D. Y., Tamaki S. J., Keen N. T. Molecular characterization of avirulence gene D from Pseudomonas syringae pv. tomato. Mol Plant Microbe Interact. 1990 Mar-Apr;3(2):94–102. doi: 10.1094/mpmi-3-094. [DOI] [PubMed] [Google Scholar]
- Kobayashi D. Y., Tamaki S. J., Trollinger D. J., Gold S., Keen N. T. A gene from Pseudomonas syringae pv. glycinea with homology to avirulence gene D from P. s. pv. tomato but devoid of the avirulence phenotype. Mol Plant Microbe Interact. 1990 Mar-Apr;3(2):103–111. doi: 10.1094/mpmi-3-103. [DOI] [PubMed] [Google Scholar]
- Kustu S., Santero E., Keener J., Popham D., Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989 Sep;53(3):367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren P. B., Peet R. C., Panopoulos N. J. Gene cluster of Pseudomonas syringae pv. "phaseolicola" controls pathogenicity of bean plants and hypersensitivity of nonhost plants. J Bacteriol. 1986 Nov;168(2):512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaelian I., Sergeant A. A general and fast method to generate multiple site directed mutations. Nucleic Acids Res. 1992 Jan 25;20(2):376–376. doi: 10.1093/nar/20.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton N. P., Clarke L. E. Identification of the promoter of the Pseudomonas gene coding for carboxypeptidase G2. J Mol Appl Genet. 1985;3(1):26–35. [PubMed] [Google Scholar]
- Napoli C., Staskawicz B. Molecular characterization and nucleic acid sequence of an avirulence gene from race 6 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Feb;169(2):572–578. doi: 10.1128/jb.169.2.572-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepold F., Anderson D., Mills D. Cloning determinants of pathogenesis from Pseudomonas syringae pathovar syringae. Proc Natl Acad Sci U S A. 1985 Jan;82(2):406–410. doi: 10.1073/pnas.82.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme L. G., Mindrinos M. N., Panopoulos N. J. Plant and environmental sensory signals control the expression of hrp genes in Pseudomonas syringae pv. phaseolicola. J Bacteriol. 1992 Jun;174(11):3499–3507. doi: 10.1128/jb.174.11.3499-3507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron J. M., Staskawicz B. J. Molecular characterization and hrp dependence of the avirulence gene avrPto from Pseudomonas syringae pv. tomato [corrected]. Mol Gen Genet. 1993 May;239(1-2):6–16. doi: 10.1007/BF00281595. [DOI] [PubMed] [Google Scholar]
- Schulte R., Bonas U. A Xanthomonas Pathogenicity Locus Is Induced by Sucrose and Sulfur-Containing Amino Acids. Plant Cell. 1992 Jan;4(1):79–86. doi: 10.1105/tpc.4.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte R., Bonas U. Expression of the Xanthomonas campestris pv. vesicatoria hrp gene cluster, which determines pathogenicity and hypersensitivity on pepper and tomato, is plant inducible. J Bacteriol. 1992 Feb;174(3):815–823. doi: 10.1128/jb.174.3.815-823.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Gold S. E., Tamaki S. J., Keen N. T. Construction of a Tn7-lux system for gene expression studies in gram-negative bacteria. Gene. 1992 Dec 1;122(1):27–34. doi: 10.1016/0378-1119(92)90028-n. [DOI] [PubMed] [Google Scholar]
- Staskawicz B. J., Dahlbeck D., Keen N. T. Cloned avirulence gene of Pseudomonas syringae pv. glycinea determines race-specific incompatibility on Glycine max (L.) Merr. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6024–6028. doi: 10.1073/pnas.81.19.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Dahlbeck D., Staskawicz B., Keen N. T. Characterization and expression of two avirulence genes cloned from Pseudomonas syringae pv. glycinea. J Bacteriol. 1988 Oct;170(10):4846–4854. doi: 10.1128/jb.170.10.4846-4854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöny B., Hennecke H. The -24/-12 promoter comes of age. FEMS Microbiol Rev. 1989 Dec;5(4):341–357. doi: 10.1016/0168-6445(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Wei Z. M., Sneath B. J., Beer S. V. Expression of Erwinia amylovora hrp genes in response to environmental stimuli. J Bacteriol. 1992 Mar;174(6):1875–1882. doi: 10.1128/jb.174.6.1875-1882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen M. C., Stall R. E., Staskawicz B. J. Characterization of a gene from a tomato pathogen determining hypersensitive resistance in non-host species and genetic analysis of this resistance in bean. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6743–6747. doi: 10.1073/pnas.85.18.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C. An Agrobacterium two-component regulatory system for the detection of chemicals released from plant wounds. Mol Microbiol. 1991 Oct;5(10):2345–2350. doi: 10.1111/j.1365-2958.1991.tb02080.x. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Lu Y., Heu S., Hutcheson S. W. Organization and environmental regulation of the Pseudomonas syringae pv. syringae 61 hrp cluster. J Bacteriol. 1992 Mar;174(6):1734–1741. doi: 10.1128/jb.174.6.1734-1741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]