Abstract
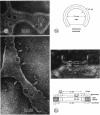
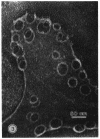
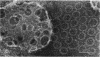
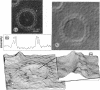
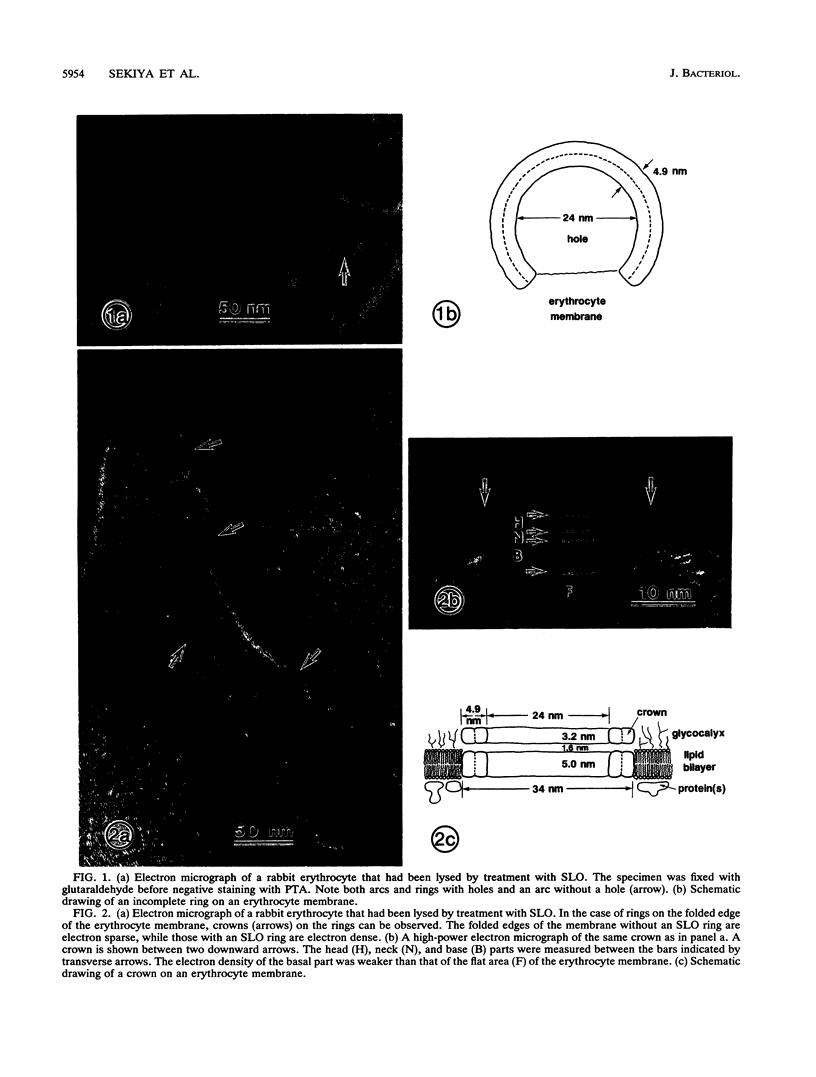
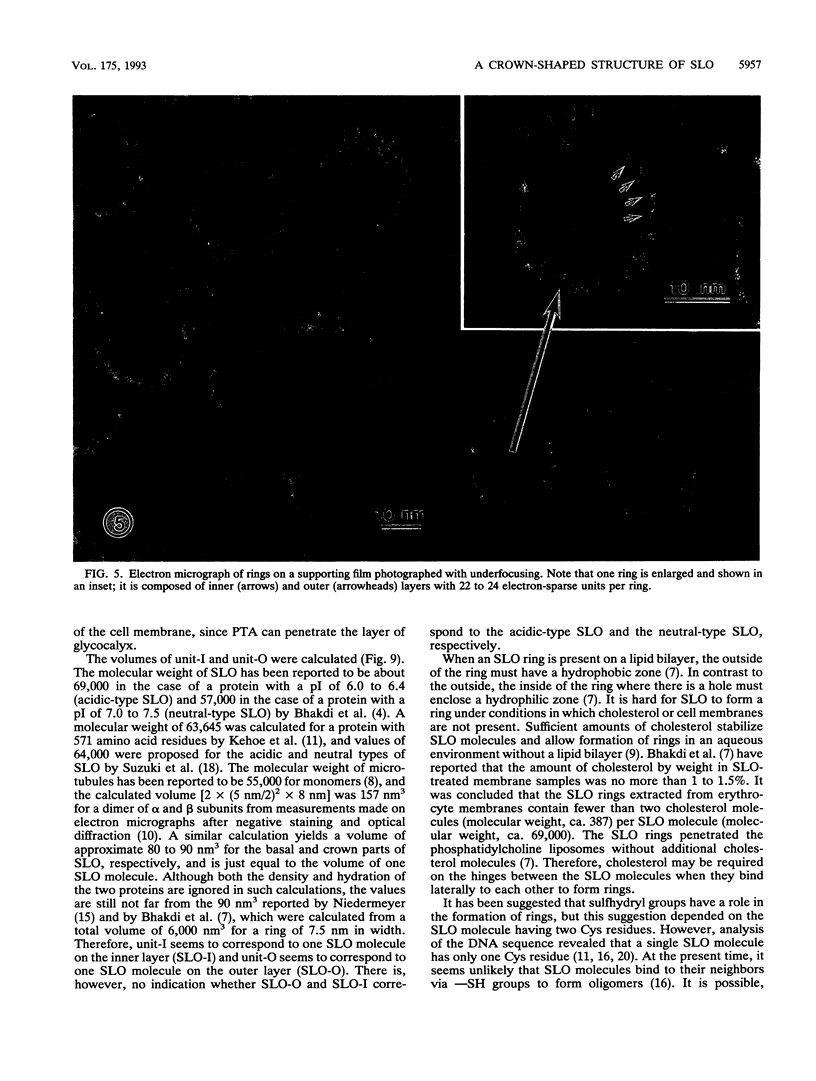
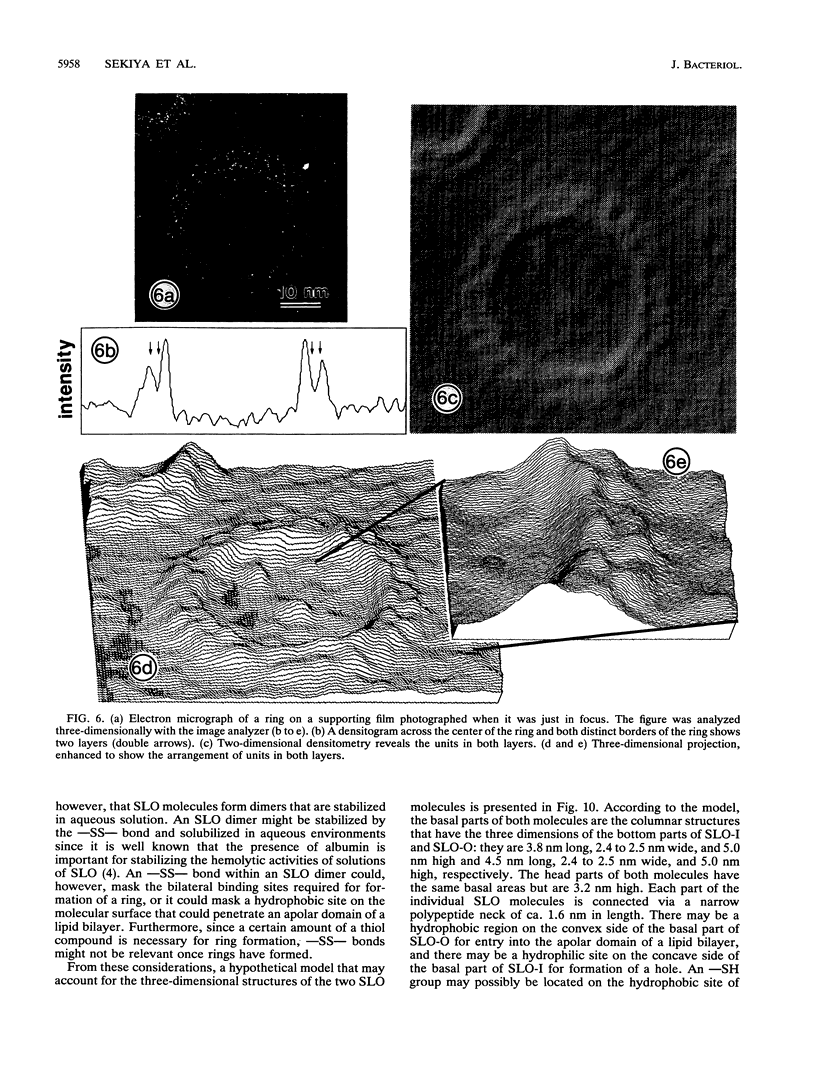
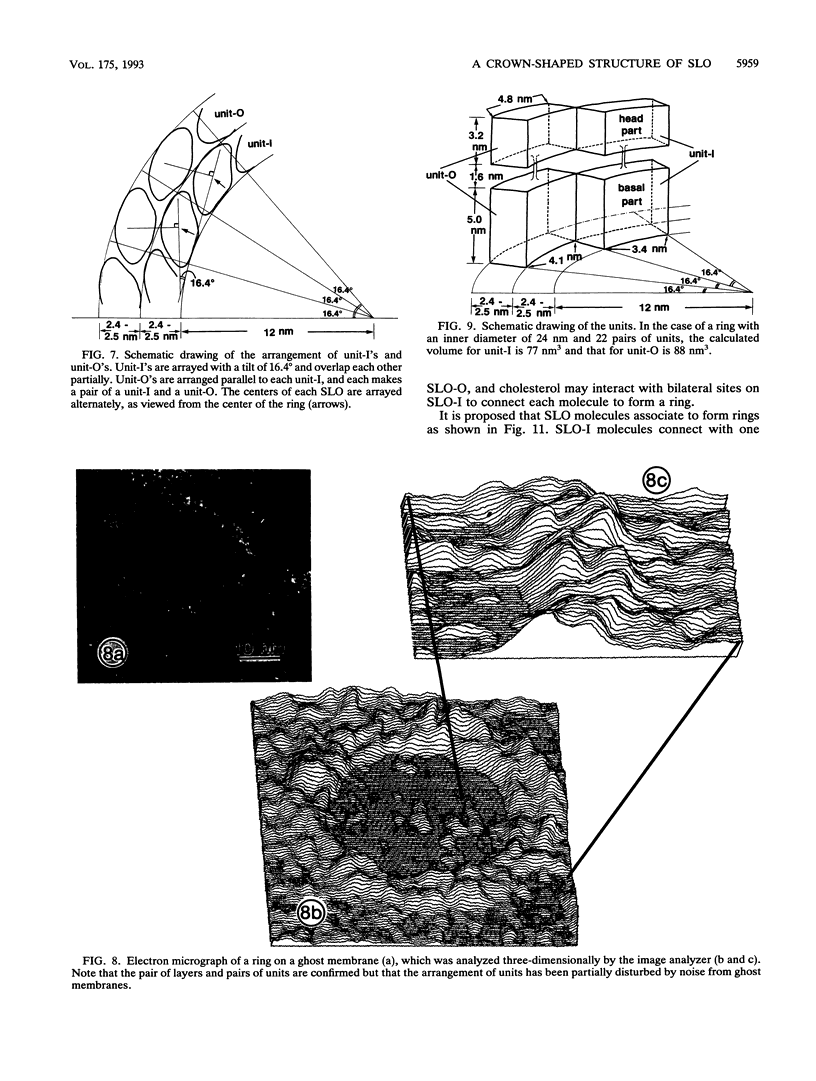
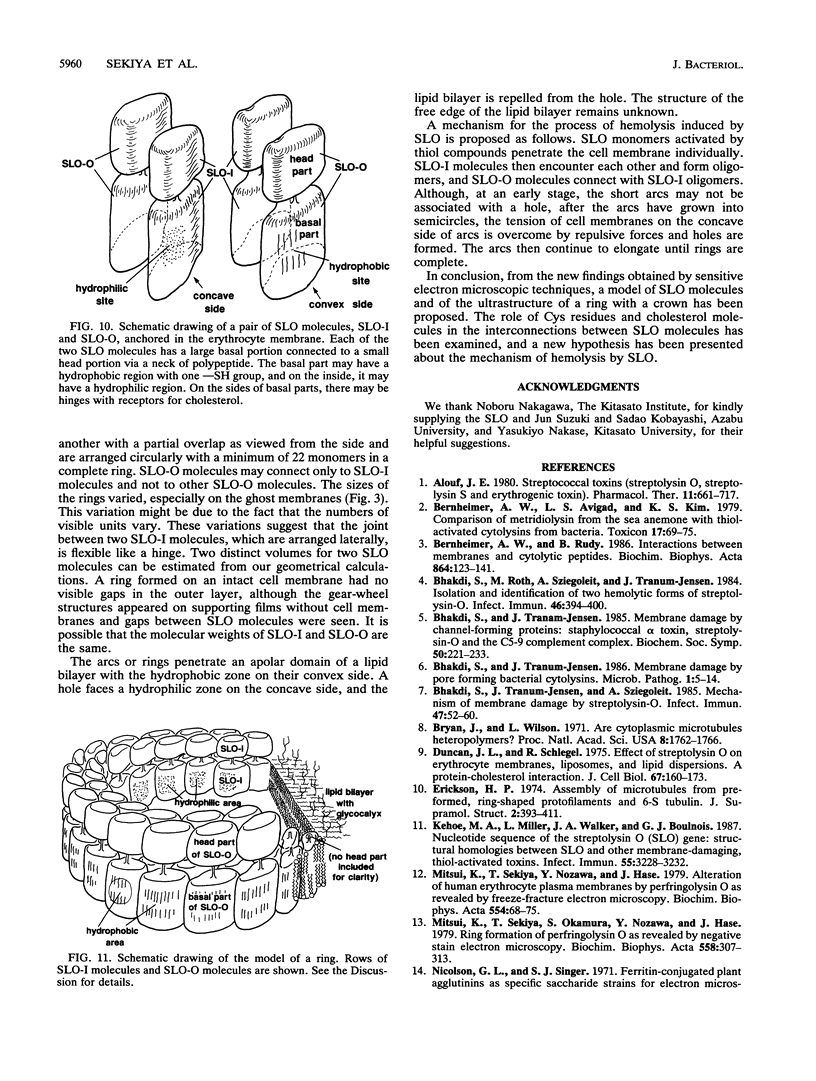
Streptolysin O (SLO) is a membrane-damaging toxin produced by most strains of group A beta-hemolytic streptococci. We performed ultrastructural analysis of SLO-derived lesions on erythrocyte membranes by examining electron micrographs of negatively stained preparations. SLO formed numerous arc- and ring-shaped structures with or without holes on membranes. Rings formed on intact cell membranes had an inner diameter of ca. 24 nm and had distinct borders of ca. 4.9 nm in width, but the diameter of rings varied from 24 to 30 nm on membranes of erythrocyte ghosts. Image analysis of electron micrographs demonstrated that each ring was composed of an inner and an outer layer. Each layer contained an array of 22 to 24 SLO molecules. On the top of the ring, we found a characteristic crown that projected from the cell membrane. The crown was separated by an electron-dense layer from the basal part of the ring that was embedded in the lipid bilayer of the erythrocyte membrane. Heights of the three parts, namely, the crown (head), the space (neck), and the basal portion (base), were ca. 3.2, 1.6, and 5.0 nm, respectively, and we postulated that these parts are the constituents of a single SLO molecule. The volumes of SLO molecules in the inner and outer layers were calculated to be 77 and 88 nm3. On the basis of a model of the structure of SLO, we propose some new details of the mechanisms of hemolysis by SLO toxin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S., Kim K. Comparison fo metridiolysin from the sea anemone with thiol-activated cytolysins from bacteria. Toxicon. 1979;17(1):69–75. doi: 10.1016/0041-0101(79)90257-5. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by channel-forming proteins: staphylococcal alpha-toxin, streptolysin-O and the C5b-9 complement complex. Biochem Soc Symp. 1985;50:221–233. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by pore-forming bacterial cytolysins. Microb Pathog. 1986 Feb;1(1):5–14. doi: 10.1016/0882-4010(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Wilson L. Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci U S A. 1971 Aug;68(8):1762–1766. doi: 10.1073/pnas.68.8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P. Assembly of microtubules from preformed, ring-shaped protofilaments and 6-S tubulin. J Supramol Struct. 1974;2(2-4):393–411. doi: 10.1002/jss.400020228. [DOI] [PubMed] [Google Scholar]
- Kehoe M. A., Miller L., Walker J. A., Boulnois G. J. Nucleotide sequence of the streptolysin O (SLO) gene: structural homologies between SLO and other membrane-damaging, thiol-activated toxins. Infect Immun. 1987 Dec;55(12):3228–3232. doi: 10.1128/iai.55.12.3228-3232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K., Sekiya T., Nozawa Y., Hase J. Alteration of human erythrocyte plasma membranes by perfringolysin O as revealed by freeze-fracture electron microscopy. Studies on Clostridium perfringens exotoxins V. Biochim Biophys Acta. 1979 Jun 13;554(1):68–75. doi: 10.1016/0005-2736(79)90007-5. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Sekiya T., Okamura S., Nozawa Y., Hase J. Ring formation of perfringolysin O as revealed by negative stain electron microscopy. Biochim Biophys Acta. 1979 Dec 12;558(3):307–313. doi: 10.1016/0005-2736(79)90265-7. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci U S A. 1971 May;68(5):942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer W. Interaction of streptolysin-O with biomembranes: kinetic and morphological studies on erythrocyte membranes. Toxicon. 1985;23(3):425–439. doi: 10.1016/0041-0101(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Pinkney M., Beachey E., Kehoe M. The thiol-activated toxin streptolysin O does not require a thiol group for cytolytic activity. Infect Immun. 1989 Aug;57(8):2553–2558. doi: 10.1128/iai.57.8.2553-2558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J., Kobayashi S., Kagaya K., Fukazawa Y. Heterogeneity of hemolytic efficiency and isoelectric point of streptolysin O. Infect Immun. 1988 Sep;56(9):2474–2478. doi: 10.1128/iai.56.9.2474-2478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J., Speth V. Complement lysis: the ultrastructure and orientation of the C5b-9 complex on target sheep erythrocyte membranes. Scand J Immunol. 1978;7(1):45–46. doi: 10.1111/j.1365-3083.1978.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Tweten R. K. Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from Clostridium perfringens: significant homology with the genes for streptolysin O and pneumolysin. Infect Immun. 1988 Dec;56(12):3235–3240. doi: 10.1128/iai.56.12.3235-3240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamaker L. W. Streptococcal toxins. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S723–S732. doi: 10.1093/clinids/5.supplement_4.s723. [DOI] [PubMed] [Google Scholar]