Abstract
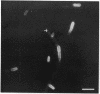
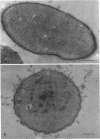
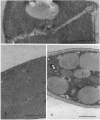
In vivo cells (hyphal bodies) of the hyphomycetous insect pathogen Beauveria bassiana collected from host Spodoptera exigua larval hemolymph were osmotically sensitive and lacked a well-defined cell wall. In light and electron microscope studies, a galactose-specific lectin purified from S. exigua hemolymph, concanavalin A (specific for alpha-mannose), and a polyclonal antibody to B. bassiana cell walls all bound to surfaces of in vitro-produced B. bassiana blastospores; however, none of these probes labelled the thin layer of extracellular material covering the plasma membranes of hyphal bodies. These cells were observed freely circulating in S. exigua hemolymph at 36 h postinfection, although immunocompetent hemocytes were known to be present. Additionally, association of hyphal bodies with hemocytes in monolayers was significantly less than for opsonized in vitro blastospores or submerged conidia. The absence of antigenically important galactomannan components on in vivo cells may therefore allow these cells to escape recognition and phagocytosis. Lack of structural components (e.g., chitin, as evidenced by the absence of binding of wheat germ agglutinin) may also be important with respect to evasion of host cellular defense mechanisms. Production of wall material resumed 48 to 60 h postinfection and therefore may coincide with loss of phagocytic capabilities of the hemocytes due to immunosuppressive effects of fungal metabolites. The protoplast-like cells may be formed by the action of hydrolytic enzymes in the hemocytes or by inhibition of fungal cell wall synthetases.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaxter M. L., Page A. P., Rudin W., Maizels R. M. Nematode surface coats: actively evading immunity. Parasitol Today. 1992 Jul;8(7):243–247. doi: 10.1016/0169-4758(92)90126-m. [DOI] [PubMed] [Google Scholar]
- Boucias D. G., Pendland J. C. The galactose binding lectin from the beet armyworm, Spodoptera exigua: distribution and site of synthesis. Insect Biochem Mol Biol. 1993 Mar;23(2):233–242. doi: 10.1016/0965-1748(93)90004-c. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Ultrastructural and biochemical studies of two dynamically expressed cell surface determinants on Candida albicans. Infect Immun. 1986 Jan;51(1):327–336. doi: 10.1128/iai.51.1.327-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Hanson L. H., Restrepo A., Stevens D. A. Intracellular multiplication of Paracoccidioides brasiliensis in macrophages: killing and restriction of multiplication by activated macrophages. Infect Immun. 1989 Aug;57(8):2289–2294. doi: 10.1128/iai.57.8.2289-2294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Bowers B., Sburlati A., Silverman S. J. Fungal cell wall synthesis: the construction of a biological structure. Microbiol Sci. 1988 Dec;5(12):370–375. [PubMed] [Google Scholar]
- Calderone R. A., Braun P. C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991 Mar;55(1):1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung S. Y., Boucias D. G., Vey A. J. Effect of Beauveria bassiana and Candida albicans on the cellular defense response of Spodoptera exigua. J Invertebr Pathol. 1993 Mar;61(2):179–187. doi: 10.1006/jipa.1993.1032. [DOI] [PubMed] [Google Scholar]
- Pendland J. C., Boucias D. G. Characteristics of a galactose-binding hemagglutinin (lectin) from hemolymph of Spodoptera exigua larvae. Dev Comp Immunol. 1986 Fall;10(4):477–487. doi: 10.1016/0145-305x(86)90169-2. [DOI] [PubMed] [Google Scholar]
- Pendland J. C., Boucias D. G. Variations in the ability of galactose and mannose-specific lectins to bind to cell wall surfaces during growth of the insect pathogenic fungus Paecilomyces farinosus. Eur J Cell Biol. 1993 Apr;60(2):322–330. [PubMed] [Google Scholar]
- Porchet-Henneré E., Dugimont T. Adaptability of the coccidian Coelotropha to parasitism. Dev Comp Immunol. 1992 Jul-Aug;16(4):263–274. doi: 10.1016/0145-305x(92)90001-s. [DOI] [PubMed] [Google Scholar]
- San-Blas G. The cell wall of fungal human pathogens: its possible role in host-parasite relationships. Mycopathologia. 1982 Sep 17;79(3):159–184. doi: 10.1007/BF01837196. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R. Dynamic changes of the cell wall surface of Candida albicans associated with germination and adherence. Eur J Cell Biol. 1989 Dec;50(2):285–290. [PubMed] [Google Scholar]