Abstract
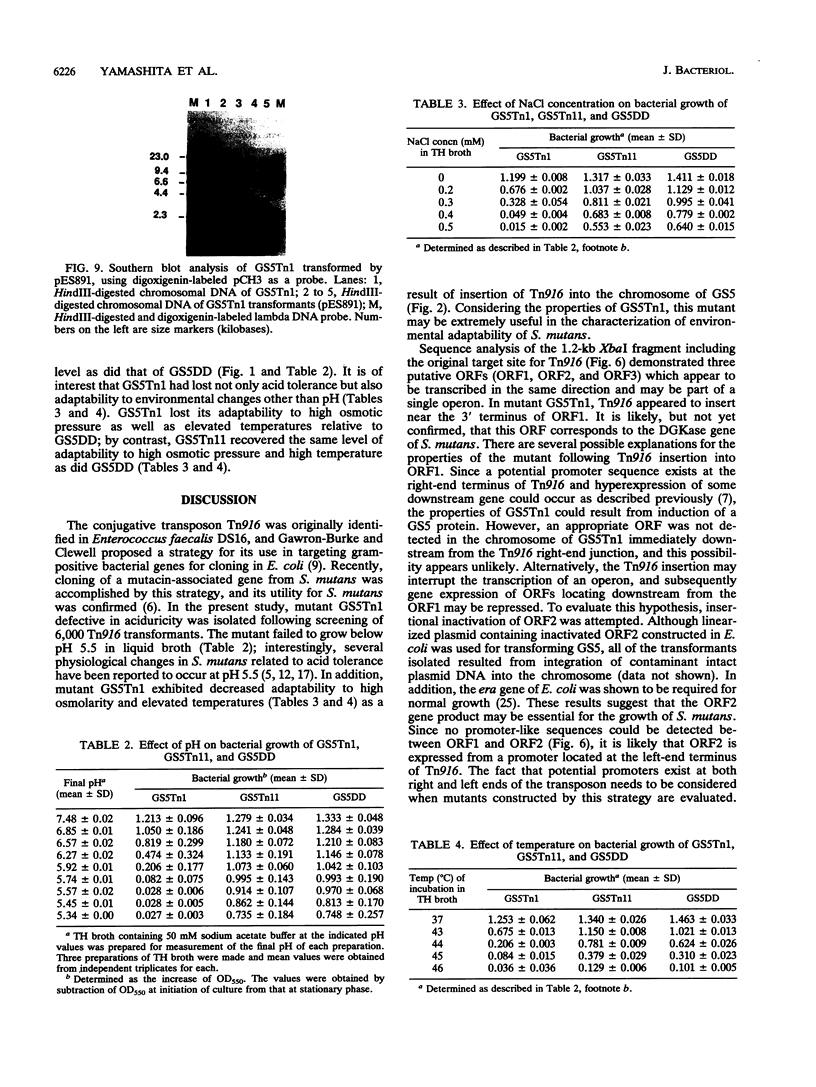
A mutant defective in aciduricity, GS5Tn1, was constructed following mutagenesis of Streptococcus mutans GS5 with the conjugative transposon Tn916. The mutant grew poorly at acidic pH levels and was sensitive to high osmolarity and elevated temperatures. These properties resulted from a single insertion of Tn916 into the GS5 chromosome, and the DNA fragment harboring the transposon was isolated into the cosmid vector, charomid 9-20. Spontaneous excision of Tn916 from the cosmid revealed that Tn916 inserted into a 8.6-kb EcoRI fragment. On the basis of the restriction analyses of insert fragments, it was found that Tn916 inserted into a 0.9-kb EcoRI-XbaI fragment. Nucleotide sequence analysis of this fragment indicated the presence of two open reading frames, ORF1 and ORF2. By using a marker rescue strategy, a 6.0-kb HindIII fragment including the target site for Tn916 insertion and the 5' end of ORF1 was isolated and sequenced. The deduced amino acid sequences of ORF1 and ORF2 showed significant homology with the diacylglycerol kinase and Era proteins, respectively, from Escherichia coli. Nucleotide sequence analysis of the Tn916 insertion junction region in the GS5Tn1 chromosome revealed that the transposon inserted near the 3' terminus of ORF1. Restoration of ORF1 to its original sequence in mutant GS5Tn1 was carried out following transformation with integration vector pVA891 containing an intact ORF1. The resultant transformant showed wild-type levels of aciduricity as well as resistance to elevated temperatures and high osmolarity. These results suggest that the S. mutans homolog of diacylglycerol kinase is important for adaptation of the organism to several environmental stress signals.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnn J., March P. E., Takiff H. E., Inouye M. A GTP-binding protein of Escherichia coli has homology to yeast RAS proteins. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8849–8853. doi: 10.1073/pnas.83.23.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender G. R., Sutton S. V., Marquis R. E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986 Aug;53(2):331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden G. H., Hamilton I. R. Competition between Streptococcus mutans and Lactobacillus casei in mixed continuous culture. Oral Microbiol Immunol. 1989 Jun;4(2):57–64. doi: 10.1111/j.1399-302x.1989.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Bowden G. H., Hamilton I. R. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and "S. mitior" growing in continuous culture. Can J Microbiol. 1987 Sep;33(9):824–827. doi: 10.1139/m87-143. [DOI] [PubMed] [Google Scholar]
- Caufield P. W., Shah G. R., Hollingshead S. K. Use of transposon Tn916 to inactivate and isolate a mutacin-associated gene from Streptococcus mutans. Infect Immun. 1990 Dec;58(12):4126–4135. doi: 10.1128/iai.58.12.4126-4135.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Flannagan S. E., Ike Y., Jones J. M., Gawron-Burke C. Sequence analysis of termini of conjugative transposon Tn916. J Bacteriol. 1988 Jul;170(7):3046–3052. doi: 10.1128/jb.170.7.3046-3052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Buckley N. D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol Immunol. 1991 Apr;6(2):65–71. doi: 10.1111/j.1399-302x.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Hanada N., Kuramitsu H. K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989 Jul;57(7):2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harper D. S., Loesche W. J. Growth and acid tolerance of human dental plaque bacteria. Arch Oral Biol. 1984;29(10):843–848. doi: 10.1016/0003-9969(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Iwami Y., Abbe K., Takahashi-Abbe S., Yamada T. Acid production by streptococci growing at low pH in a chemostat under anaerobic conditions. Oral Microbiol Immunol. 1992 Oct;7(5):304–308. doi: 10.1111/j.1399-302x.1992.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner V. A., Bell R. M., Modrich P. The DNA sequences encoding plsB and dgk loci of Escherichia coli. J Biol Chem. 1983 Sep 25;258(18):10856–10861. [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis C. R., Walsh J. P., Bell R. M. sn-1,2-Diacylglycerol kinase of Escherichia coli. Purification, reconstitution, and partial amino- and carboxyl-terminal analysis. J Biol Chem. 1985 Apr 10;260(7):4091–4097. [PubMed] [Google Scholar]
- Macrina F. L., Dertzbaugh M. T., Halula M. C., Krah E. R., 3rd, Jones K. R. Genetic approaches to the study of oral microflora: a review. Crit Rev Oral Biol Med. 1990;1(3):207–227. doi: 10.1177/10454411900010030401. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., Jones K. R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983 Nov;25(1):145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- March P. E., Lerner C. G., Ahnn J., Cui X., Inouye M. The Escherichia coli Ras-like protein (Era) has GTPase activity and is essential for cell growth. Oncogene. 1988 Jun;2(6):539–544. [PubMed] [Google Scholar]
- Perry D., Wondrack L. M., Kuramitsu H. K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983 Aug;41(2):722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotering H., Raetz C. R. Appearance of monoglyceride and triglyceride in the cell envelope of Escherichia coli mutants defective in diglyceride kinase. J Biol Chem. 1983 Jul 10;258(13):8068–8073. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroza T., Kuramitsu H. K. Construction of a model secretion system for oral streptococci. Infect Immun. 1993 Sep;61(9):3745–3755. doi: 10.1128/iai.61.9.3745-3755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. P., Fahrner L., Bell R. M. sn-1,2-diacylglycerol kinase of Escherichia coli. Diacylglycerol analogues define specificity and mechanism. J Biol Chem. 1990 Mar 15;265(8):4374–4381. [PubMed] [Google Scholar]
- Walsh J. P., Loomis C. R., Bell R. M. Regulation of diacylglycerol kinase biosynthesis in Escherichia coli. A trans-acting dgkR mutation increases transcription of the structural gene. J Biol Chem. 1986 Aug 25;261(24):11021–11027. [PubMed] [Google Scholar]
- Yamamoto M., Jones J. M., Senghas E., Gawron-Burke C., Clewell D. B. Generation of Tn5 insertions in streptococcal conjugative transposon Tn916. Appl Environ Microbiol. 1987 May;53(5):1069–1072. doi: 10.1128/aem.53.5.1069-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]