Abstract
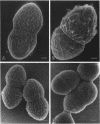
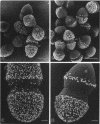
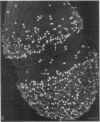
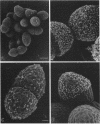
Enterococcus faecalis can acquire antibiotic resistance and virulence genes by transfer of pheromone-inducible conjugative plasmids such as pCF10, which encodes tetracycline resistance. Two pCF10-encoded cell surface proteins, Sec10 and Asc10, have been previously shown to play an important role in the transfer of this plasmid. We used high-resolution, field emission scanning electron microscopy to visualize these proteins on the surfaces of a series of isogenic strains of E. faecalis. Immunogold labeling, using both 6- and 12-nm colloidal gold, unambiguously demonstrated the expression and distribution of Sec10 and Asc10 on the surface of the E. faecalis cells. On unlabeled E. faecalis cells which expressed either Sec10 or Asc10, the former appeared to be more readily detected. Immunogold labeling of E. faecalis cells expressing both Asc10 and Sec10 clearly demonstrated the abundance and intermixing of both proteins on the cell surface except at septal regions. Sec10 was observed to be distributed over the cell surface. At regions of cell-cell contact, fine strands representing Asc10 were observed directly attaching adjacent cells to one another.
Full text
PDF



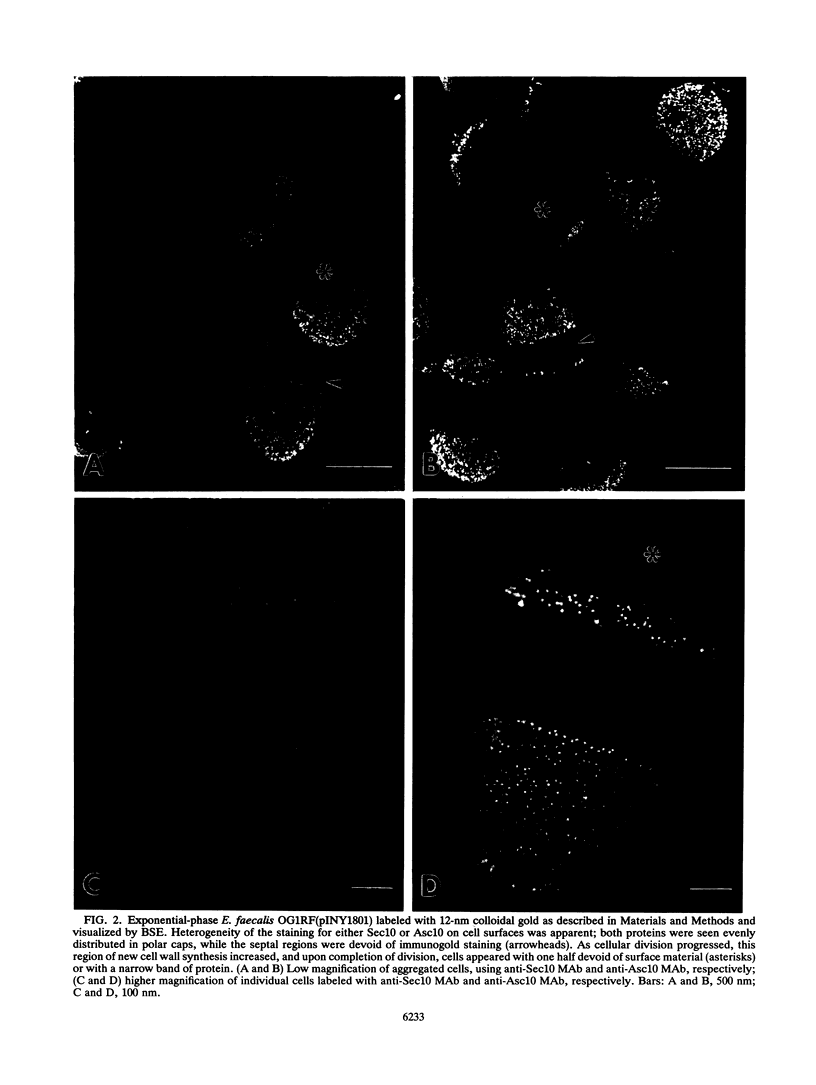

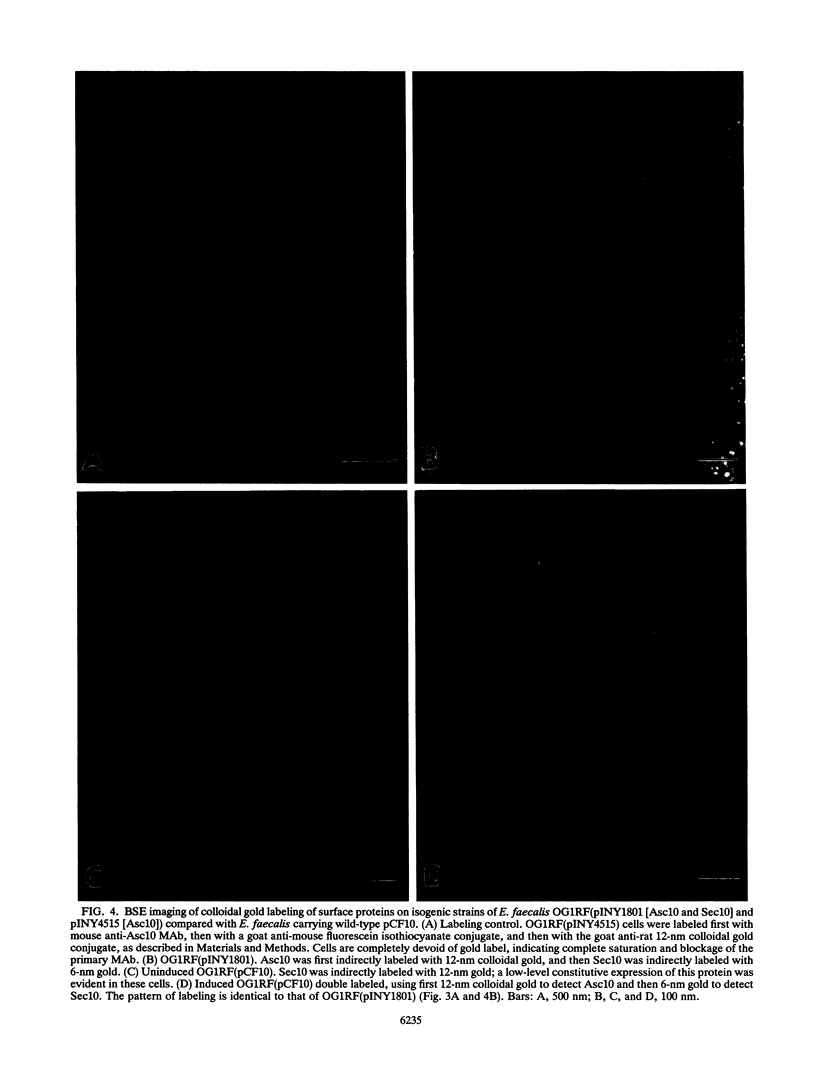


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitchison E. J., Lambert P. A., Farrell I. D. Antigenic composition of an endocarditis-associated isolate of Streptococcus faecalis and identification of its glycoprotein antigens by ligand blotting with lectins. J Med Microbiol. 1986 Mar;21(2):161–167. doi: 10.1099/00222615-21-2-161. [DOI] [PubMed] [Google Scholar]
- Aitchison E. J., Lambert P. A., Smith E. G., Farrell I. D. Serodiagnosis of Streptococcus faecalis endocarditis by immunoblotting of surface protein antigens. J Clin Microbiol. 1987 Feb;25(2):211–215. doi: 10.1128/jcm.25.2.211-215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyde A., Maconnachie E. Morphological correlations with dimensional change during SEM specimen preparation. Scan Electron Microsc. 1981;4:27–34. doi: 10.1002/sca.4950040104. [DOI] [PubMed] [Google Scholar]
- COLE R. M., HAHN J. J. Cell wall replication in Streptococcus pyogenes. Science. 1962 Mar 2;135(3505):722–724. doi: 10.1126/science.135.3505.722. [DOI] [PubMed] [Google Scholar]
- Christie P. J., Kao S. M., Adsit J. C., Dunny G. M. Cloning and expression of genes encoding pheromone-inducible antigens of Enterococcus (Streptococcus) faecalis. J Bacteriol. 1988 Nov;170(11):5161–5168. doi: 10.1128/jb.170.11.5161-5168.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Weaver K. E. Sex pheromones and plasmid transfer in Enterococcus faecalis. Plasmid. 1989 May;21(3):175–184. doi: 10.1016/0147-619x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979 Jul;2(3):454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Dunny G. M. Genetic functions and cell-cell interactions in the pheromone-inducible plasmid transfer system of Enterococcus faecalis. Mol Microbiol. 1990 May;4(5):689–696. doi: 10.1111/j.1365-2958.1990.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Lee L. N., LeBlanc D. J. Improved electroporation and cloning vector system for gram-positive bacteria. Appl Environ Microbiol. 1991 Apr;57(4):1194–1201. doi: 10.1128/aem.57.4.1194-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Zimmerman D. L., Tortorello M. L. Induction of surface exclusion (entry exclusion) by Streptococcus faecalis sex pheromones: use of monoclonal antibodies to identify an inducible surface antigen involved in the exclusion process. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8582–8586. doi: 10.1073/pnas.82.24.8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G., Funk C., Adsit J. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid. 1981 Nov;6(3):270–278. doi: 10.1016/0147-619x(81)90035-4. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E. E., Clewell D. B. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J Bacteriol. 1987 Aug;169(8):3473–3481. doi: 10.1128/jb.169.8.3473-3481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsen S. L., Frethem C., Autrata R. Workshop on high-resolution immunocytochemistry of cell surfaces using field emission SEM. J Histochem Cytochem. 1990 Dec;38(12):1779–1780. doi: 10.1177/38.12.2254643. [DOI] [PubMed] [Google Scholar]
- Erlandsen S. L., Hasslen S. R., Nelson R. D. Detection and spatial distribution of the beta 2 integrin (Mac-1) and L-selectin (LECAM-1) adherence receptors on human neutrophils by high-resolution field emission SEM. J Histochem Cytochem. 1993 Mar;41(3):327–333. doi: 10.1177/41.3.7679125. [DOI] [PubMed] [Google Scholar]
- Guzmàn C. A., Pruzzo C., LiPira G., Calegari L. Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infection and endocarditis. Infect Immun. 1989 Jun;57(6):1834–1838. doi: 10.1128/iai.57.6.1834-1838.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainfeld J. F. A small gold-conjugated antibody label: improved resolution for electron microscopy. Science. 1987 Apr 24;236(4800):450–453. doi: 10.1126/science.3563522. [DOI] [PubMed] [Google Scholar]
- Handley P. S., Jacob A. E. Some structural and physiological properties of fimbriae of Streptococcus faecalis. J Gen Microbiol. 1981 Dec;127(2):289–293. doi: 10.1099/00221287-127-2-289. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Craig R. A., White B. A., Yagi Y., Clewell D. B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. M., Olmsted S. B., Viksnins A. S., Gallo J. C., Dunny G. M. Molecular and genetic analysis of a region of plasmid pCF10 containing positive control genes and structural genes encoding surface proteins involved in pheromone-inducible conjugation in Enterococcus faecalis. J Bacteriol. 1991 Dec;173(23):7650–7664. doi: 10.1128/jb.173.23.7650-7664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Sakagami Y., Ishii Y., Isogai A., Kitada C., Fujino M., Adsit J. C., Dunny G. M., Suzuki A. Structure of cCF10, a peptide sex pheromone which induces conjugative transfer of the Streptococcus faecalis tetracycline resistance plasmid, pCF10. J Biol Chem. 1988 Oct 5;263(28):14574–14578. [PubMed] [Google Scholar]
- Olmsted S. B., Kao S. M., van Putte L. J., Gallo J. C., Dunny G. M. Role of the pheromone-inducible surface protein Asc10 in mating aggregate formation and conjugal transfer of the Enterococcus faecalis plasmid pCF10. J Bacteriol. 1991 Dec;173(23):7665–7672. doi: 10.1128/jb.173.23.7665-7672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorello M. L., Dunny G. M. Identification of multiple cell surface antigens associated with the sex pheromone response of Streptococcus faecalis. J Bacteriol. 1985 Apr;162(1):131–137. doi: 10.1128/jb.162.1.131-137.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorello M., Adsit J., Krug D., Antczak D., Dunny G. Monoclonal antibodies to cell surface antigens involved in sex pheromone induced mating in Streptococcus faecalis. J Gen Microbiol. 1986 Apr;132(4):857–864. doi: 10.1099/00221287-132-4-857. [DOI] [PubMed] [Google Scholar]
- Walther P., Autrata R., Chen Y., Pawley J. B. Backscattered electron imaging for high resolution surface scanning electron microscopy with a new type YAG-detector. Scanning Microsc. 1991 Jun;5(2):301–310. [PubMed] [Google Scholar]
- Wanner G., Formanek H., Galli D., Wirth R. Localization of aggregation substances of Enterococcus faecalis after induction by sex pheromones. An ultrastructural comparison using immuno labelling, transmission and high resolution scanning electron microscopic techniques. Arch Microbiol. 1989;151(6):491–497. doi: 10.1007/BF00454864. [DOI] [PubMed] [Google Scholar]
- Weidlich G., Wirth R., Galli D. Sex pheromone plasmid pAD1-encoded surface exclusion protein of Enterococcus faecalis. Mol Gen Genet. 1992 May;233(1-2):161–168. doi: 10.1007/BF00587575. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Erlandsen S. L. Localization of translocating Escherichia coli, Proteus mirabilis, and Enterococcus faecalis within cecal and colonic tissues of monoassociated mice. Infect Immun. 1991 Dec;59(12):4693–4697. doi: 10.1128/iai.59.12.4693-4697.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y., Kessler R. E., Shaw J. H., Lopatin D. E., An F., Clewell D. B. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J Gen Microbiol. 1983 Apr;129(4):1207–1215. doi: 10.1099/00221287-129-4-1207. [DOI] [PubMed] [Google Scholar]