Abstract
We evaluated the safety and immunogenicity of a chimeric alphavirus vaccine candidate in mice with selective immunodeficiencies. This vaccine candidate was highly attenuated in mice with deficiencies in the B and T cell compartments, as well as in mice with deficient gamma-interferon responsiveness. However, the level of protection varied among the strains tested. Wild type mice were protected against lethal VEEV challenge. In contrast, alpha/beta (αβ) TCR-deficient mice developed lethal encephalitis following VEEV challenge, while mice deficient in gamma/delta (γδ) T cells were protected. Surprisingly, the vaccine potency was diminished by 50% in animals lacking interferon-gamma receptor alpha chain (R1)-chain and a minority of vaccinated immunoglobulin heavy chain-deficient (μMT) mice survived challenge, which suggests that neutralizing antibody may not be absolutely required for protection. Prolonged replication of encephalitic VEEV in the brain of pre-immunized mice is not lethal and adoptive transfer experiments indicate that CD3+ T cells are required for protection.
Keywords: Alphavirus immunity, Recombinant vaccines, Venezuelan equine encephalitis virus, Pathogenesis
Introduction
To develop safe, attenuated vaccines and drug therapies against central nervous system (CNS)-invading viruses, it is critical to elucidate the mechanisms of pathogen clearance, host survival and pathogenesis resulting from viral encephalitis mediated by emerging and biothreat zoonotic pathogens. Several alphaviruses, namely eastern, western and Venezuelan equine encephalitis viruses, are etiological agents of encephalitis and are highly lethal in humans and experimental animal models (Weaver, 2005). Encephalitic alphaviruses are known to replicate to high titers in mice, and invasion of the CNS occurs rapidly in animals infected via the intranasal route leading to acute, lethal encephalitis (Paessler et al., 2003, Paessler et al., 2006a).
The alphavirus Venezuelan equine encephalitis virus (VEEV), a zoonotic pathogen with multiple subtypes, has been responsible for significant morbidity and mortality during epizootic outbreaks in equines and humans (Weaver et al., 1996). During the most recent outbreak in South America in 1983, approximately 100,000 human cases of infection were reported with over 300 clinical cases diagnosed (Weaver et al., 2004). The case-fatality rate in equine species is relatively high and can reach levels exceeding 80%, primarily due to the spread of infection to the CNS, which results in encephalitis. In humans, mortality rates are relatively low (< 1%), but clinical signs of neurological disease are prevalent, especially in children (de la Monte et al., 1985, Weaver et al., 2004).
Various animal models have been used to examine the distinct phases of infection, including the development of VEEV-induced encephalitis. Both hamster and mouse models have been used to understand the pathogenesis of the virus and the host immune response as well as to evaluate vaccines (Charles et al., 1995, Ryzhikov et al., 1991, Ryzhikov et al., 1995). Infection in the mouse by a peripheral route of challenge exposure (subcutaneous) results in a biphasic disease pattern, in which the virus initially replicates in lymphoid tissue and ultimately progresses into the CNS (Anishchenko et al., 2006, Paessler et al., 2003, Paessler et al., 2006b), while intranasal and intracranial challenge results in earlier CNS penetration. Once CNS infection is established in naïve animals, acute meningoencephalitis with neuronal cell death follows, which is uniformly fatal (Charles et al., 1995, Ryzhikov et al., 1991, Ryzhikov et al., 1995). Infection in the brain is associated with the development of an inflammatory response in the immunocompetent host, with perivascular and vascular infiltration of mononuclear cells, edema and micro-hemorrhages. The pathology associated with infection is disseminated throughout the brain, with the exception of a more prominent focal response in the cortex. Immunohistochemical analysis of the distribution of viral antigen in the CNS indicates staining in neurons and in the neuropil, as well as in astrocytes (Anishchenko et al., 2006, Paessler et al., 2006b).
VEEV is a potential biothreat agent; thus, vaccines to limit infection and/or fatal encephalitis are currently under development using a variety of approaches (Charles et al., 1997, Davis et al., 1995, Paessler et al., 2006b). The live attenuated vaccine strain, TC-83, was developed over four decades ago by serial passaging of the Trinidad Donkey (TrD) VEEV strain in guinea pig myocytes and remains the only available vaccine for humans. However, residual pathogenicity has been a concern with this strain (Anishchenko et al., 2006, Jahrling and Scherer, 1973, Paessler et al., 2006b). Recent new generation live-attenuated vaccines that incorporate the use of alphavirus vectors to express VEEV proteins have been shown to be both safe and effective in protecting mice and hamsters from lethal disease (Paessler et al., 2003, Paessler et al., 2006a). Using a relatively rigorous challenge with the VEEV ZPC738 strain via the intracerebral or intranasal routes, mice were protected from lethal encephalitis following vaccination. Vaccinated mice did not develop any overt signs of disease and had reduced levels of viral replication. Moreover, vaccinated NIH Swiss (outbred) mice developed a strong anti-VEEV neuroinflammatory response upon challenge with wild type virus and had high titers of serum-neutralizing antibody (Paessler et al., 2003, Paessler et al., 2006a). Despite the initial characterization of these responses in vaccinated mice protected from a challenge dose that would be uniformly lethal in unvaccinated animals, the precise basis of the adaptive immune response stimulated by the Sindbis/VEEV chimera that ultimately leads to this protection has not been fully characterized.
To evaluate (1) the safety of the recently described, live-attenuated vaccine candidate and (2) the cellular basis of protection following vaccination, which protects immunocompetent, wild type mice against acute, lethal encephalitis (Paessler et al., 2003, Paessler et al., 2006a), we utilized inbred mice with selective immunodeficiencies in the T cell compartments (αβ TCR-deficient or γδ TCR-deficient mice) (Itohara et al., 1993, Mombaerts et al., 1992), in the B cell compartment (μMT-deficient mice) (Kitamura et al., 1991), or in their ability to respond to the cytokine interferon gamma (IFN-γR-deficient mice) (Huang et al., 1993) in a series of studies. In preliminary VEEV infection studies, these immunodeficient mouse strains succumbed to disease between 6 and 8 days following ZPC738 infection, as did immunocompetent (wild type) mice (data not shown). This indicates that these immunodeficient mice do not differ significantly in their susceptibility to VEEV, and therefore, can be used in this immunization/challenge model to examine different immune effector mechanisms.
Interferon-γ is linked to activation of antigen-presenting cells in the CNS and arming of effector activities of both the innate and adaptive effector anti-viral pathways (Binder and Griffin, 2003, Burdeinick-Kerr and Griffin, 2005, Owens et al., 2005). Alpha-beta (αβ) T cells represent the majority of peptide-specific T cells, whereas γδ T cells have been hypothesized to form a link between innate and adaptive immunity; however, many of the functions of γδ T cells are unknown, particularly in the central nervous system (Born et al., 2006). Protection from lethal encephalitis was dependent on the presence of αβ T cell receptor (TCR)-bearing (CD3+) cells, but not on γδ TCR-bearing cells. Remarkably, γδ TCR-deficient mice were protected from lethal viral challenge by intranasal inoculation with VEEV but a continued persistence of variants of the ZPC738 challenge strain was found up to 28 days following challenge. Our data unambiguously demonstrate distinct roles for murine αβ and γδ T cells in the vaccine and challenge response to neurovirulent VEEV.
Results
Safety of a Sindbis-VEEV vaccine candidate in mice
Chimeric virus is attenuated in mice with selective immune deficiencies
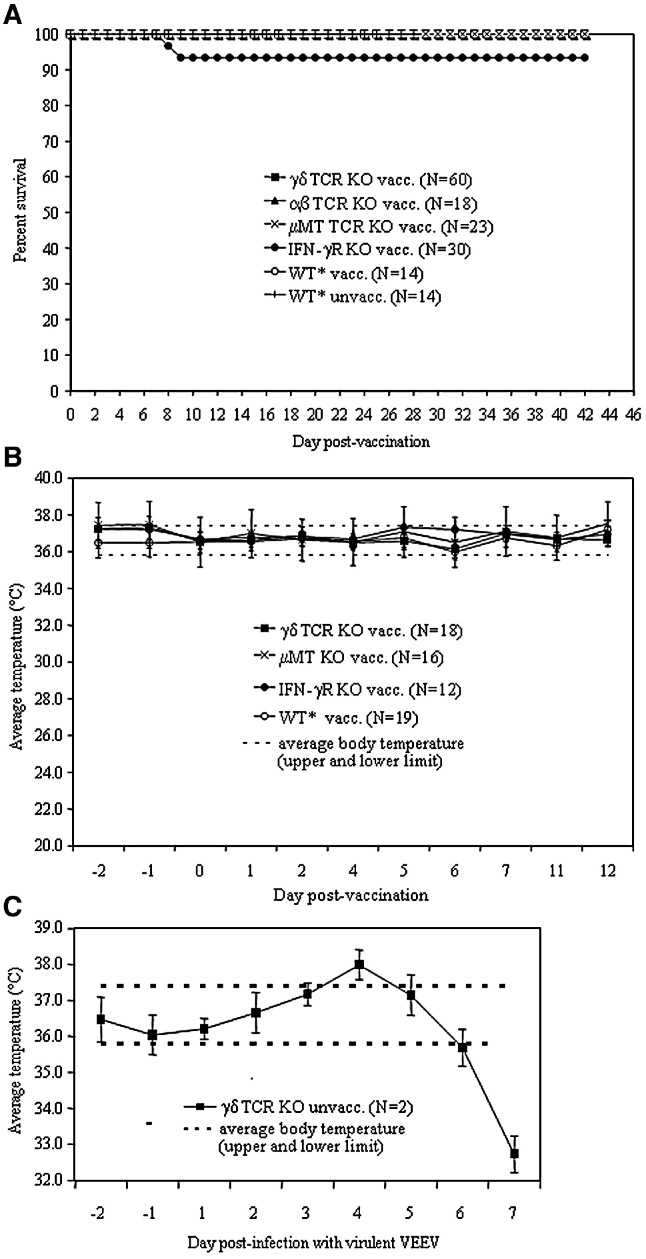
All αβ TCR-, γδ TCR-, and μMT-deficient mice survived vaccination and none of these animals showed any signs of disease during a period of 14 days following vaccination. 93% of IFN-γR-deficient mice survived vaccination; in one of the three replicate experiments, two mice suddenly died without any previous signs typical of VEE (anorexia and/or paralysis), while all other animals appeared healthy throughout the vaccination period (Fig. 1A). We were unable to recover any infectious virus from the brains of the two animals that died and do not know the precise cause of death. However, we have observed that the health status of the IFN-γR-deficient mice prior to any manipulations is not equivalent to the other immunodeficient strains, and therefore, may explain this result.
Fig. 1.
Vaccine safety studies in mice. Survival and average body temperature of wild type and immunodeficient mice vaccinated with live-attenuated chimeric alphavirus vaccine (SIN/ZPC). Panels A and B show the survival and average body temperature of parental C57BL/6 (wild type, WT) and immunodeficient mice inoculated with live-attenuated chimeric alphavirus vaccine (SIN/ZPC). Panel C shows the average body temperature following VEEV infection of unvaccinated mice. The total percentage of surviving mice (A) and the average body temperature (B) of SIN/ZPC vaccinated IFNγR KO, γδ TCR KO, μMT KO and WT mice before and after vaccination on day 0 were evaluated, as follows. Vaccination: eleven to 18-week-old, female mice were inoculated on day 0 and day 34 subcutaneously into the medial thigh with the chimeric SIN/ZPC virus at a dose of 5 × 105 plaque forming units in a total volume of 100 μl. Mock-vaccinated mice (indicated as “WT* unvacc”) were inoculated subcutaneously with 100 μl of PBS. Virus challenge: SIN/ZPC or mock-vaccinated mice were inoculated on day 0 intranasally with an identical dose of virulent VEEV (ZPC738) in 40 μl of PBS and deaths were recorded. Daily telemetric monitoring of body temperature was performed for a total period of 14 days, beginning 2 days prior to the first vaccination on day 0. Asterisk (⁎) indicates parental C57BL/6 mice (wild type, WT). The characteristics of the parental WT and immunodeficient mice are described in Materials and methods (Table 1). (C) Positive control for telemetric monitoring. The body temperature of unvaccinated γδ TCR KO mice 2 days before (day − 1 and − 2) and 7 days after (day + 1 through + 7) infection with virulent VEEV (ZPC738) on day 0.
Vaccination does not influence body temperature or white blood cell counts
In the majority of mice, no febrile or hypothermic response to vaccination was evident in daily telemetric body measurement of vaccinated animals (Fig. 1B). As a positive control for VEEV-infection-induced changes in body temperature and leukopenia, we infected unvaccinated γδ TCR-deficient mice with VEEV. In these naïve mice infected with virulent VEEV (ZPC738), febrile responses occurred on day 4 after infection and were followed by the development of hypothermia by day 7. Among the μMT-deficient mice, a short period of increased body temperature occurred on day 4 in some animals. Additionally, hematological evaluation indicated that no changes in white-blood-cell counts occurred in vaccinated animals, while in naïve VEEV-infected mice, leukopenia did occur on day 4 after infection (data not shown).
Protection studies
Sindbis-VEEV vaccine candidate induces protection against disease
Animals with selective immunodeficiencies, with the exception of IFNγR-deficient mice, did not develop any detectable disease after vaccination indicating that this recombinant genetics approach offers a highly attenuated vaccine candidate in the mouse model. In addition, we previously demonstrated that the attenuated vaccine candidate was highly immunogenic (Paessler et al., 2006a). The ZPC738 strain of VEEV is uniformly lethal in naive mice (NIH Swiss strain) by 7 days after inoculation of 2 × 105 plaque forming units (PFU) (10,000 lethal dose (LD)50) via intranasal (i.n.) route. In subsequent vaccination studies utilizing the chimeric SIN/ZPC virus, mice survive this lethal challenge regimen, unlike naïve, mock vaccinated control mice (Paessler et al., 2006a). This prompted us to further examine the effector mechanism(s) that this chimeric alphavirus vaccine elicits to provide protection against lethal encephalitis caused by VEEV.
In this study, we utilized a similar vaccination/challenge regimen to immunize naïve parental (wild type, WT) and immunodeficient C57BL/6 mice with chimeric alphavirus-VEE virus (SIN/ZPC), challenged these mice with virulent VEEV (ca. 5000 LD50) and assessed survival over a 28-day period. In addition, we evaluated the histopathology, inflammatory response, and viral replication in the brain and peripheral tissues (lung, liver, spleen) of vaccinated mice following ZPC738 challenge.
Survival proportions
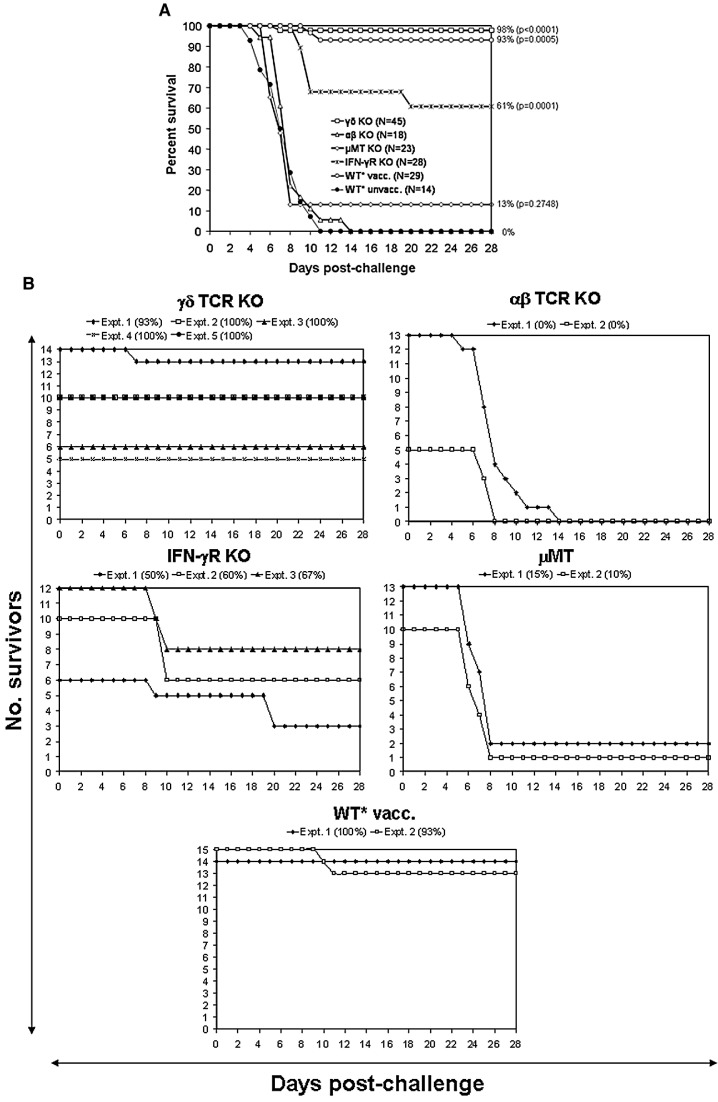
Survival curves after two immunizations with SIN/ZPC and subsequent VEEV challenge are presented in Fig. 2 . The proportion of αβ TCR-deficient mice that survived the 28-day observation period (0 of 18; 2 replicates; 0%) was identical to that of mock-vaccinated WT mice (0 of 14; 1 replicate; 0%). In contrast, the proportion of survivors among vaccinated γδ TCR-deficient mice was high (44 of 45; 5 independent replicates; 98%) and was significantly greater (p = 0.0001) than for vaccinated WT mice (27 of 29; 2 independent replicates; 93%). Survival of IFN-γR KO mice was reduced (17 of 28; 3 independent replicates; 61%) compared to vaccinated WT and γδ TCR KO mice. The majority of μMT-deficient mice did not survive challenge (3 of 23; 2 independent replicates; 13%); there was no statistically significant difference between the survival proportion of vaccinated μMT-deficient mice and mock vaccinated WT mice (p = 0.2748).
Fig. 2.
Vaccine efficacy in the mouse model. Survival of SIN/ZPC double-vaccinated wild type and immunodeficient mice following challenge with virulent VEEV. Six-week-old, female parental C57BL/6 (wild type, WT) and immunodeficient mice were vaccinated on day − 42 and day − 14 subcutaneously into the medial thigh with the chimeric SIN/ZPC vaccine at a dose of 5 × 105 plaque forming units (PFU) in a total volume of 100 μl of PBS or with PBS alone (WT⁎ unvacc). Deaths were recorded following intranasal challenge with virulent VEEV (ZPC738) at a dose of 2 × 105 PFU per animal (ca. 1000 LD50) in 20 μl of PBS at 6 weeks following initial vaccination. Asterisk (⁎) indicates parental C57BL/6 mice (wild type, WT) and the characteristics of immunodeficient mice are described in Materials and methods (Table 1). (A) The total percentage of surviving mice. (B) Number of surviving mice in replicate experiments. Comparison of survival curves using the logrank test (Altman, 1991) indicates that there is a statistically significant difference in survival overall between the experimental groups over the 28-day monitoring period (logrank, p < 0.0001). Fisher's exact test was used to compare the survival proportion of each vaccinated strain to the mock vaccinated WT, and the individual p-values are indicated on the survival curve.
Kinetics of death
The median survival time following challenge for the vaccinated αβ T cell deficient and vaccinated μMT mice was similar to that of mock-vaccinated WT mice: 8, 7 and 7.5 days, respectively); however, none of the vaccinated αβ TCR deficient mice survived beyond 14 days post-infection (Fig. 2). IFN-γR KO mice also survived for a longer period of time, with deaths occurring at day 9 as compared to day 5 and day 7 for mock-vaccinated wild type and αβ TCR-deficient mice, respectively.
Level of infectious virus in organs
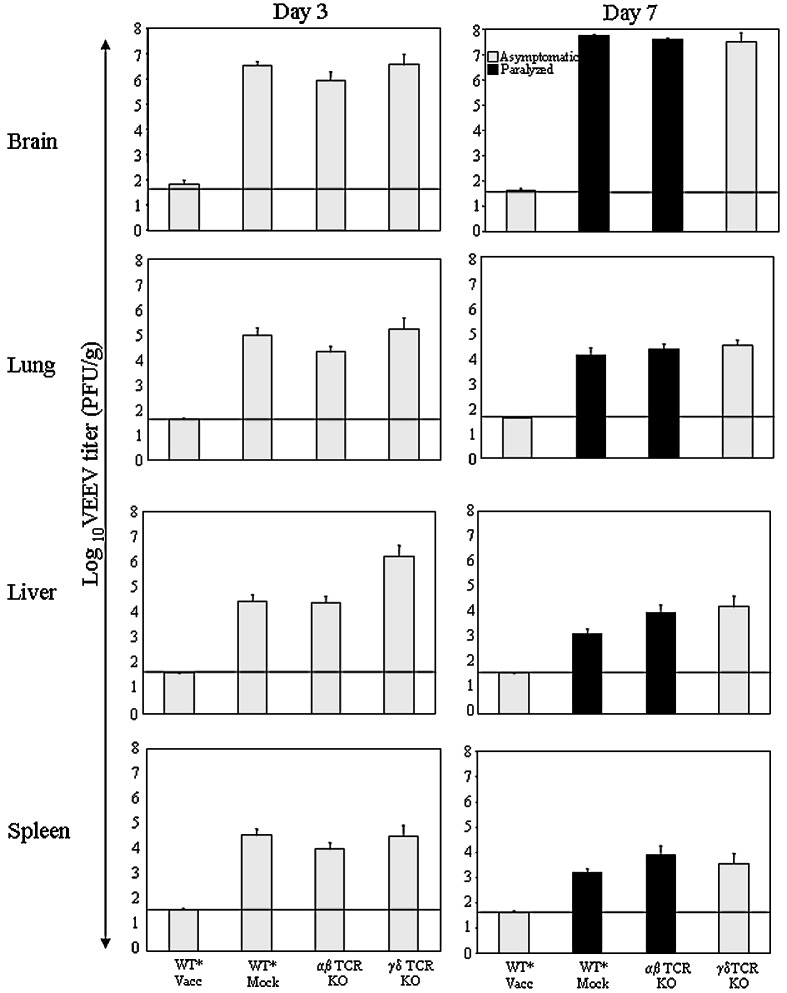
Virus titers were evaluated in the brain and peripheral organs of vaccinated, challenged immunodeficient mice (Fig. 3 ). At day 3 post-challenge, viral titers in the brain and peripheral tissues of wild type vaccinated mice were uniformly low (< 102 PFU/g) and at day 7 post-challenge, were undetectable, in contrast to mock-vaccinated wild type mice, in which high titers were detected (> 105 PFU/g, Fig. 3). For animals with selective immune deficiencies that were vaccinated, high levels of infectious virus were detected in all tissues at either day 3 or day 7, with the highest titers in the brain at day 7 post-challenge (Fig. 3). All the parental WT and αβ TCR-deficient mice developed hind limb paralysis prior to death (as indicated by the black bar in Fig. 3), which is a hallmark of disease development in VEEV-infected mice. Statistical analysis comparing the differences in viral titer between groups indicates that, despite the appearance of a higher average viral titer in the liver at 3 dpi for the γδ TCR-deficient mice, this difference is not statistically significant (Kruskal–Wallis test, p = 0.3916) (Table 1 ).
Fig. 3.
Vaccine efficacy in the mouse model. Mean VEEV titer in organs of SIN/ZPC vaccinated wild type and immunodeficient mice at 3 and 7 days post-challenge with ZPC738. Mice were immunized on day − 42 and day − 14 with chimeric SIN/ZPC vaccine at a dose of 5 × 105 plaque-forming units (PFU) in 100 μl of PBS or with PBS (WT* Mock) via subcutaneous route and challenged intranasally with 2 × 105 PFU of virulent VEEV (ZPC738) per animal in 20 μl of PBS. Asterisk (⁎) indicates parental C57BL/6 mice (wild type, WT) and the characteristics of immunodeficient mice are described in Materials and methods (Table 1). Animals were sacrificed at (A) 3 days and (B) 7 days post-challenge and organs were harvested for viral titration. The limit of detection was 40 PFU/g, as indicated by the solid line.
Table 1.
Characteristics of immunodeficient mice
| Name | Strain (Stock number)a | Description | Reference |
|---|---|---|---|
| C57BL/6 | B6 (#000664) | Parental strain (wild type) | (Waterston et al., 2002) |
| αβ TCR KO | B6.129P2-Tcrbtm1Mom/J (#002118) | Disruption of T-cell receptor beta (Tcrb) chain | (Mombaerts et al., 1992) |
| Deficient in αβ T cells (CD4+CD8+ cells ~ 6% of wild-type) | |||
| γδ TCR KO | B6.129P2-Tcrdtm1Mom/J (#002120) | Disruption of T-cell receptor delta (Tcrd) chain | (Itohara et al., 1993) |
| Deficient in γδ T cells (all adult lymphoid and epithelial organs) | |||
| μMT KO | B6.129S2-Igh-6tm1Cgn/J (#002288) | Disruption in immunoglobulin heavy chain-6 (Igh-6, heavy chain of IgM) | (Kitamura et al., 1991) |
| Deficient in mature B cells | |||
| IFN-γR KO | 129-Ifngr1tm1Agt/J (#002702) | Disruption of interferon gamma receptor 1 (Ifngr1) | (Huang et al., 1993) |
| Deficient in functional IFN-γR |
All mice were purchased from Jackson Laboratories (Bar Harbor, Maine).
Immunodeficient (knockout, KO) mice were developed on the C57BL/6 (B6) background, which are described as parental (wild type, WT) in the text.
Histopathology and distribution of viral antigen in the brain in response to VEEV infection in vaccinated immunodeficient and wild type mice
We also evaluated the relative degree of inflammation, necrosis (Fig. 4 ) and viral antigen level (Fig. 5 ) in the brains of vaccinated, ZPC738 challenged mice at day 3 and day 7 post-infection. Semi-quantitative analysis of the histopathology and immunohistochemistry results is presented in Table 2 . On day 3 post-infection, γδ TCR-deficient mice exhibited an inflammatory response comparable to other groups of vaccinated, immunodeficient mouse strains tested, in addition to slightly more extensive tissue necrosis, which was comparable to that of mock-vaccinated WT control mice. In αβ TCR-deficient mice, the extent of inflammation and necrosis was at an intermediate level relative to mock vaccinated WT and vaccinated γδ TCR-deficient mice (Table 2).
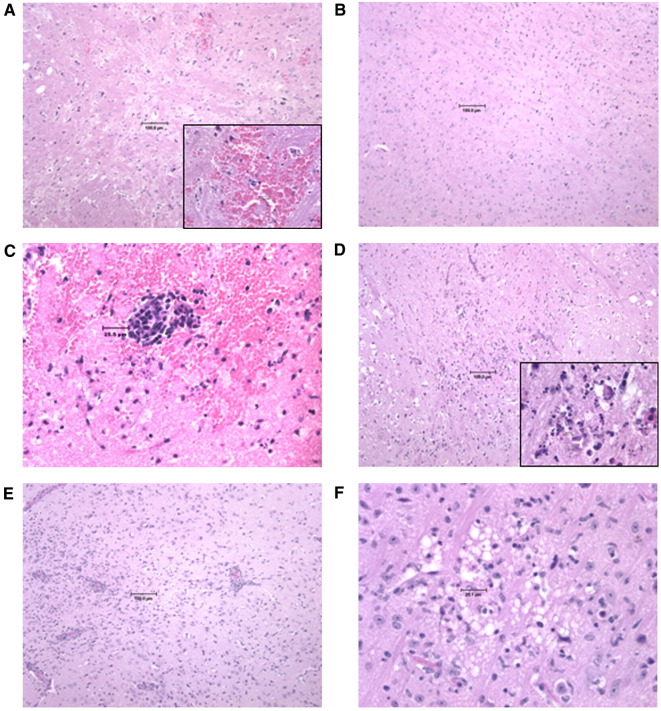
Fig. 4.
Vaccine efficacy in the mouse model. Histopathological analysis of brains from vaccinated mice challenged intranasally with VEEV ZPC738. Brains were harvested from mice obtained following the vaccination and challenge experiments described in Figs. 2 and3. The characteristics of parental C57BL/6 mice (wild type, WT) and immunodeficient mice are described in Materials and methods (Table 1). Photomicrographs of hematoxylin and eosin-stained brains obtained on day 7 from the following mice: (A) WT unvaccinated, (B) WT vaccinated; and the following chimeric SIN/ZPC-vaccinated immunodeficient mice: (C) μMT, (D) αβ TCR KO, and (E) γδ TCR KO mice. (F) A higher magnification image of panel E is shown. Multifocal mononuclear cell infiltrates as well as focal necrosis and neuronal cell death characterized through hyperangulation of hypereosinophilic neurons and loss of neuropil, are prominent in γδ TCR KO and αβ TCR KO mice. μMT KO mice have prominent micro-hemorrhages in addition to inflammation.
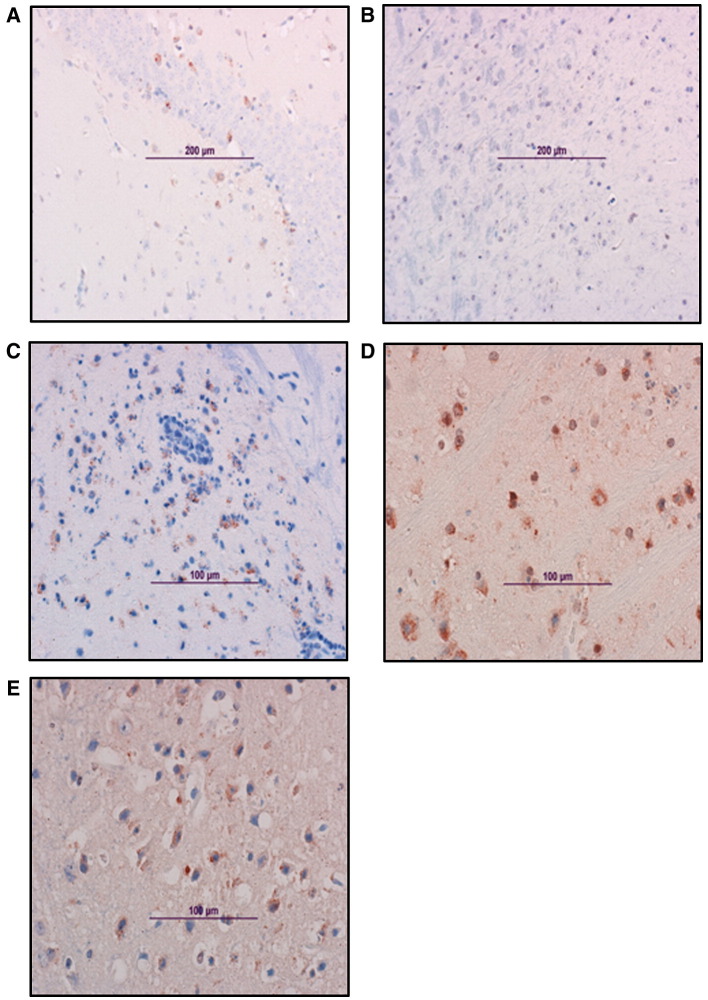
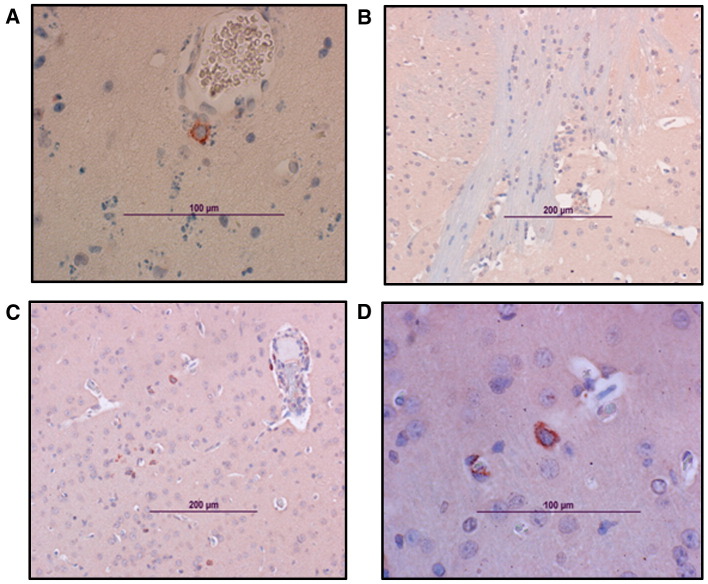
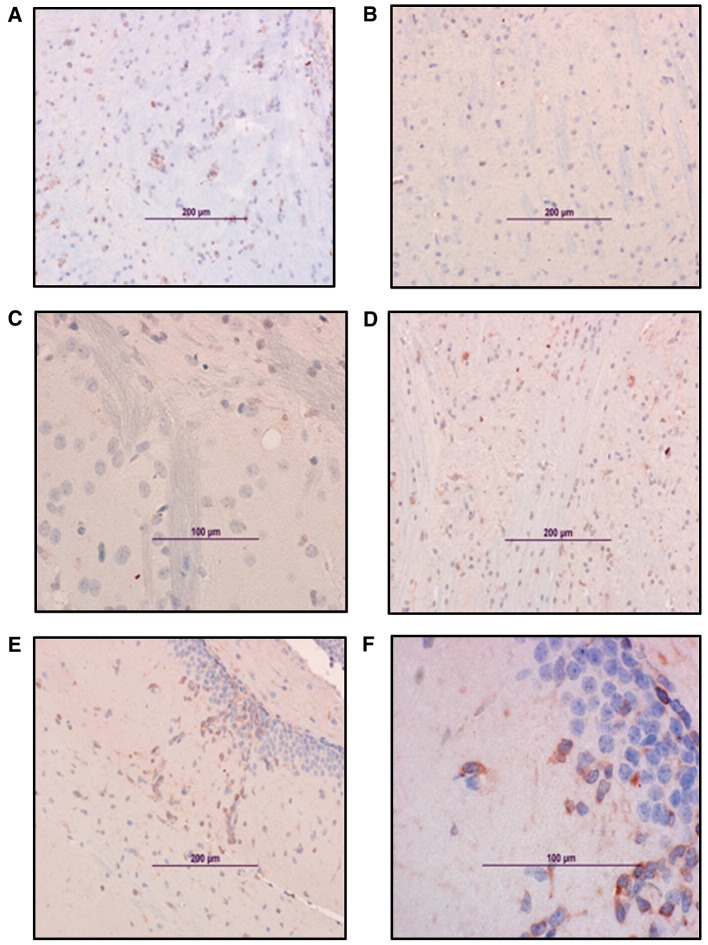
Fig. 5.
Vaccine efficacy in the mouse model. Immunohistochemical analysis of the brain from SIN/ZPC- or mock-vaccinated and VEEV-challenged mice. Viral antigen in the brain. Representative photomicrographs were obtained from immunohistological analysis of brains of 6-day-old female chimeric SIN/ZPC virus- or mock-vaccinated mice euthanized on day 7 following virulent VEEV (ZPC738) challenge infection, as described in Figs. 2 and3. The characteristics of parental C57BL/6 mice (wild type, WT) and immunodeficient mice are described in Materials and methods (Table 1). The localization of viral antigen (reddish-brown) was demonstrated via immunostaining of brain sections using antibody to VEEV (ATCC, cat number: VR1250AF). VEEV antibody was detected using biotinylated secondary antibody, followed by avidin–peroxidase color development. Slides were counter-stained with Mayer's Modified Hematoxylin prior to mounting and microscopy. (A) WT unvaccinated, (B) WT chimeric SIN/ZPC-vaccinated; and the following chimeric SIN/ZPC-vaccinated immunodeficient mice: (C) μMT, (D) αβ TCR KO mice and (E) γδ TCR KO. Viral antigen is detected in association with neuronal cells in the brain stem (C and D), hippocampus (A) and cortex (E).
Table 2.
Semi-quantitative analysis of brain pathology and viral antigen distribution in wild type and immunodeficient mice
| Replicate mice | Day 3 |
Day 7 |
|||||
|---|---|---|---|---|---|---|---|
| Inflammation | Necrosis | Viral staining | Inflammation | Necrosis | Viral staining | ||
| αβ TCR KO | 1 | 2 | 2 | 3 | 4 | 4 | 4 |
| 2 | 2 | 2 | 3 | 4 | 4 | 3 | |
| γδ TCR KO | 1 | 3 | 1 | 3 | 4 | 4 | 2 |
| 2 | 2 | 1 | 3 | 3 | 4 | 2 | |
| μMT KO | 1 | 2 | 1 | 3 | 3 | 3 | 3 |
| 2 | 3 | 2 | 3 | 3 | 4 | 3 | |
| IFN-γR KO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| WT⁎ vaccine | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | |
| WT⁎ mock | 1 | 1 | 1 | 3 | 4 | 4 | 3 |
| 2 | 2 | 2 | 3 | 3 | 4 | 3 | |
Mice were immunized with chimeric SIN/ZPC virus (5 × 105 plaque-forming units (PFU) in 100 μl of PBS) or with PBS alone (mock) on day − 42 and day − 14 via subcutaneous route and challenged on day 0 intranasally with 2 × 105 PFU of ZPC738 per animal in 20 μl of PBS. Animals were sacrificed at 3 and 7 days post-challenge and organs were harvested for histopathological analysis. Histopathology scoring: 1 = normal/negative, 2 = 1–3 focal spots, 3 = disseminated, 4 = most severe. n.d., not done. Asterisk (⁎) indicates parental C57BL/6 mice (wild type, WT) and the characteristics of immunodeficient mice are described in Materials and methods (Table 1).
In agreement with the viral titers presented in Fig. 3, the relative levels and localization of viral antigen in various areas of the brain that was identified by immunohistochemistry did not differ extensively among the treatment groups (Fig. 5, Table 2). Early in infection (day 4 post challenge), viral antigen was detectable in the olfactory bulb and brain stem in all of the animals, with the exception of previously vaccinated WT mice (Fig. 5). By day 7 post challenge, almost all of the parts of the brain were positive for virus antigen in immunodeficient mice and in mock vaccinated, WT mice. Strong staining of viral antigen was detected in the hippocampus, the brain stem as well as in the cortex (Fig. 5) and the cerebellum.
B- and T-cells actively migrate into VEEV-infected brains
We performed histopathology and immunohistochemistry studies (Fig. 5, Fig. 6, Fig. 7 ) to determine whether a correlation existed between the presence of viral antigen, the type of lymphocytes entering the brain and protection of vaccinated animals. On day 7 post-challenge, we detected histopathological encephalitis and focal infiltration of B220 positive (B220+) cells in αβ TCR-deficient mice (Fig. 6A) and in γδ TCR-deficient mice (Fig. 6B), while detecting a relatively high proportion of B220+ cells in brains of γδ TCR-deficient mice on day 12 post-challenge (Figs. 6C–D). In contrast, immunocompetent mice had few B220+ cells in their brains, while in μMT-deficient mice, no B220+ cells could be detected at any time (data not shown). In vaccinated αβ TCR-deficient mice, encephalitis was evident in histology sections of the brains on day 7 post-challenge (Fig. 4D), in the absence of CD3 positive (CD3+) cells (Fig. 7C), while CD3+ cells were abundant in brains of γδ TCR-deficient mice (Figs. 7E–F). A similar level of CD3+ cells was demonstrable on day 12 in asymptomatic γδ TCR-deficient mice (Figs. 7C–D), while the level was strongly reduced on day 28 (data not shown). WT mice that were previously vaccinated developed a relatively modest inflammatory response (Fig. 7A), in contrast to mock-vaccinated, encephalitic animals from which brain sections stained intensely for CD3+ cells on day 7 post-infection (Fig. 7B).
Fig. 6.
Vaccine efficacy in the mouse model. Immunohistochemical analysis of the brain from SIN/ZPC- and mock-vaccinated and VEEV-challenged mice. B cells in the brain. Brains were harvested from mice obtained following the vaccination and challenge experiments described in Figs. 2 and3. The characteristics of parental C57BL/6 mice (wild type, WT) and immunodeficient mice are described in Materials and methods (Table 1). B cells were detected by staining with antibody to the B cell marker, CD45R/B220 and counter-stained with Mayer's Modified Hematoxylin prior to mounting and microscopy, as described in Materials and methods. CD45R/B220-positive cells (reddish-brown) in brains of chimeric SIN/ZPC-vaccinated, virulent VEEV (ZPC738) challenged animals from the following immunodeficient mice are shown: (A) αβ TCR KO, 7 days post-infection (dpi), (B) γδ TCR KO mice, 7 dpi, (C) γδ TCR KO, 12 dpi, and (D) a higher magnification image of panel C.
Fig. 7.
Vaccine efficacy in the mouse model. Immunohistochemical analysis of the brain from SIN/ZPC- and mock-vaccinated and VEEV-challenged mice. T cells in the brain. Brains were harvested from mice obtained following the vaccination and challenge experiments described in Figs. 2 and3. The characteristics of parental C57BL/6 mice (wild type, WT) and immunodeficient mice are described in Materials and methods (Table 1). T cells were detected by staining with antibody to the T cell marker, CD3, and counter-stained with Mayer's Modified Hematoxylin prior to mounting and microscopy, as described in Materials and methods. CD3-positive cells (reddish-brown) are shown in the brains of the following animals: (A) WT mock-vaccinated; 7 days post-infection (dpi), (B) WT chimeric SIN/ZPC-vaccinated; 7 dpi, and for the following vaccinated immunodeficient mice: (C) αβ TCR KO at 7 dpi, (D) γδ TCR KO at 7 dpi, (E) γδ TCR KO at 12 dpi. (F) A higher magnification image of panel E is shown.
Serum neutralizing antibody responses to vaccination and challenge with VEEV in immunodeficient mice
The humoral immune response was assessed using plaque reduction neutralization test (PRNT, 80% reduction) to determine the levels of VEEV-neutralizing antibody in serum of vaccinated mice (parental WT and immunodeficient) pre- and post- challenge (Table 3 ). The PRNT titers of SIN/ZPC vaccinated WT C57BL/6 mice were > 80 (14/14; 100% positive). The majority of the vaccinated γδ TCR-deficient mice (27/33; 82% negative) did not develop detectable levels of neutralizing antibodies by the end of the study (28 days after challenge). Overall, the titers were slightly reduced in the majority of the positive serum samples obtained from γδ TCR-deficient mice relative to WT mice, and ranged from 20 to 320. Neutralizing antibody titers in αβ TCR-deficient mice (0/20; 0% positive) were uniformly below the limit of detection (titer < 20). A minor proportion of IFN-γR KO mice developed detectable levels of neutralizing antibodies (2/8; 25% positive).
Table 3.
Production of VEEV-neutralizing antibody in wild type and immunodeficient mice
| Animal strain | 80% PRNT |
|||
|---|---|---|---|---|
| Pre-challengea |
Post-challengeb |
|||
| No. positive/total testedc | Percent positived | No. positive/total testedc | Percent positived | |
| αβ TCR KO | 0/20 | 0% | 0/0 | – |
| γδ TCR KO | 5/28 | 3.6% | 6/33 | 18.2% |
| μMT KO | NT | N/A | 0/3 | 0% |
| IFN-γR KO | 1/3 | 33% | 2/8 | 25% |
| WT⁎ vaccine | 29/29 | 100% | 14/14 | 100% |
| WT⁎ mock | 0/0 | – | 0/0 | – |
Mice were immunized with chimeric SIN/ZPC virus (5 × 105 plaque-forming units (PFU) in 100 μl of PBS) or with PBS alone (mock) on day − 42 and day − 14 via subcutaneous route and challenged on day 0 intranasally with 2 × 105 PFU of ZPC738 per animal in 20 μl of PBS (Bellavia et al.). Plaque reduction neutralization test (PRNT) was performed at day 0a and at day 28b following challenge with VEEV ZPC738. cThe proportion of mice that tested positive at a dilution ≥ 1:20 is reported. dPercent positive was calculated as the percentage that tested positive at a dilution ≥ 1:20, as for column referenced in c. For mice that did not survive challenge, 0/0 is reported. NT, not tested; N/A, not applicable. Asterisk (⁎) indicates parental C57BL/6 mice (wild type, WT) and the characteristics of immunodeficient mice are described in Materials and methods (Table 1).
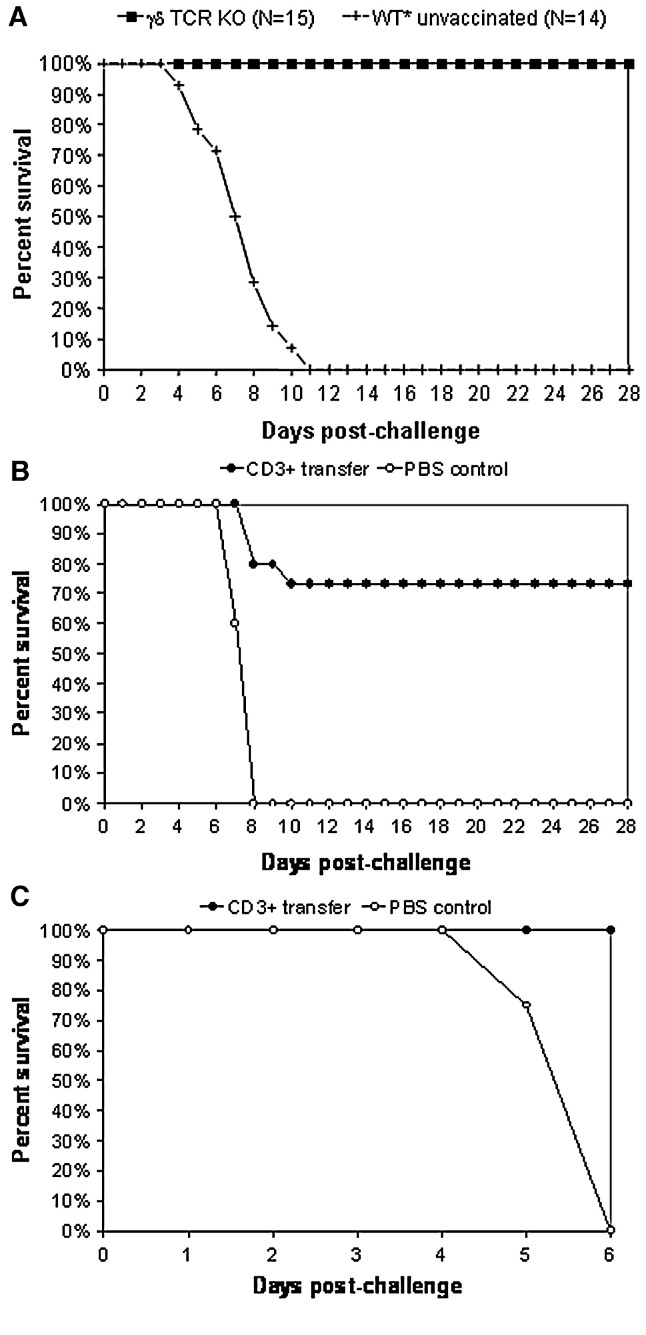
Reconstitution of protection in αβ TCR-deficient mice
To exclude the possibility that the persistence of effector cells is responsible for this protection in γδ TCR-deficient mice, we performed an additional experiment in which mice were challenged 1 month after a single vaccination (Fig. 8 ); these mice were fully protected from lethal outcome (15/15; 100% survival). Further, to determine if αβ T cells directly mediate protection, adoptive transfer experiments were performed using VEEV-specific T cells obtained from vaccinated donor mice. Experiments were designed to test restoration of protection by adoptive transfer of CD3+ T cells from spleen of WT C57BL/6 donor mice vaccinated with chimeric SIN/ZPC (Figs. 8B–C). The purity of isolated T cells, as assessed via flow cytometric analysis of cells immunostained with CD3 antibody, was 90% for the adoptive transfer experiments shown in Figs. 8B and C (data not shown). Transfer of CD3+ splenic T cells from vaccinated donors into αβ TCR-deficient mice resulted in restoration of protection from lethal encephalitis, while control (mock) mice were not protected. This result suggests that the primary protective cell type elicited by vaccination with the chimeric SIN/ZPC experimental vaccine resides within the CD3+ αβ T cell population.
Fig. 8.
Vaccine efficacy in a single immunization model and protection mediated by passive cell transfer. (A) Six-week-old, female γδ TCR-deficient mice were vaccinated on day − 42 into the medial thigh with the chimeric SIN/ZPC vaccine at a dose of 5 × 105 plaque forming units (PFU) in a total volume of 100 μl of PBS or with PBS alone (WT⁎ mock). Deaths were recorded following intranasal challenge with virulent VEEV (ZPC738) at a dose of 2 × 105 PFU per animal (ca. 1000 LD50) in 20 μl of PBS at 6 weeks following initial vaccination. Asterisk (⁎) indicates parental C57BL/6 mice (wild type, WT) and the characteristics of immunodeficient mice are described in Materials and methods (Table 1). (B–C) Naïve (recipient) αβ TCR-deficient mice (N = 15) were injected on day − 28 via intraperitoneal route with 5 × 107 CD3+ T cells (isolated as described in Materials and methods) suspended in 100 μl of sterile phosphate-buffered saline (PBS). As controls for reconstitution experiments, five mice were inoculated with PBS alone. Recipient mice were immunized with chimeric SIN/ZPC 2 days after T cell transfer and challenged with virulent VEEV (ZPC738, 2 × 105 PFU/animal) at 28 days following immunization. Animals were monitored for death, as indicated. Due to Hurricane Rita evacuation, experiment shown in 2A was prematurely terminated at 6 days post-challenge.
Infectious VEEV is present in the brains of γδ TCR-deficient mice
Infectious virus could be recovered from the brains of persistently infected γδ-TCR deficient mice either via cell culture inoculation with brain homogenates or, alternatively, by intracranial infection of newborn mice (Aaronson et al., 1982). The latter proved to be a more sensitive method of isolation of live virus from the brain tissue, with 3/33 (9%) versus 9/12 (75%) positive by cell culture or by newborn mice inoculation, respectively (Table 4 ). Thus, VEEV, which is considered to be a highly cytopathic virus, and which is uniformly lethal to susceptible (unvaccinated) mice, can persist in the brains of infected animals without causing clinical encephalitis or mortality up to 28 days following challenge infection.
Table 4.
Persistence of infectious virus in the brains of SIN/ZPC-vaccinated, VEEV-challenged mice
| Plaque positive samples | ||
|---|---|---|
| No. positive/total tested (% positive) |
||
| Cell culturea | Suckling miceb | |
| γδ TCR KO | 3/33 (9%) | 9/12 (75%) |
| αβ TCR KO | N/A | N/A |
| μMT KO | 0/3 (0%) | 0/3 (0%) |
| IFN-γR KO | 0/8 (0%) | 0/8 (0%) |
| WT (C57BL/6) vaccine | 0/22 (0%) | 0/9 (0%) |
| NIH Swiss vaccine | 0/12 (0%) | 0/12 (0%) |
| WT⁎ mock vaccine | N/A | N/A |
C57BL/6 mice and immunodeficient strains were vaccinated with SIN/ZPC and subsequently challenged with ZPC738, as described in Materials and methods. NIH Swiss mice were vaccinated as described previously (Paessler et al., 2003, 2006a). Brains were harvested at 0 and 28 days post-challenge time point and sagitally sectioned in half. After 48 h, brains were collected and plaque assay performed, as for the vaccinated/challenged animals. The proportion of plaque positive samples is reported. N/A, not available. Asterisk (⁎) indicates parental C57BL/6 mice (wild type, WT) and the characteristics of immunodeficient mice are described in Materials and methods (Table 1).
One half of the brain was suspended in 500 μl of media and plaque assay was performed using 50 μl of the suspension.
Suckling mice were inoculated with 20 μl of brain suspension via intracranial route.
Discussion
Safety of the chimeric alphavirus in immunodeficient mice
Chimeric alphavirus-based vaccines represent a critical advance in vaccine development for a number of infectious pathogens, as Sindbis replicon particles have been shown to be safe and effective for the induction of both cell-mediated and humoral immune responses (Davis et al., 2000). Our results in this and previously published studies (Paessler et al., 2003, Paessler et al., 2006a) confirm the safety of Sindbis/VEE viruses in outbred 6-day-old and inbred adult mice (NIH Swiss and C57BL/6). The vast majority of adult immunodeficient mice (100% survival of αβ TCR-, γδ TCR- and μMT-deficient mice; 93% survival of IFNγR KO mice) tested survived vaccination and did not develop leukopenia, fever or hypothermia.
Our results challenge the prevailing paradigm that the penetration of the CNS and high-level replication by VEEV in the brain leads to lethal, virus-induced encephalitis in the mouse model. The results presented here indicate that pre-immunized, VEEV-infected animals can tolerate relatively high levels of viral replication in the brain without apparent clinical disease, e.g., encephalitis or paralysis, indicating that other mechanism(s) such as the host response to the infection may, in addition, play a pivotal role.
The role of neutralizing antibodies and IFN-γ in protection against lethal VEEV
Previous studies have suggested that production of neutralizing antibody is critical to protection from encephalitis caused by alphaviruses (Griffin et al., 1997). This is likely due to the potential to augment clearance of extracellular virus. In TC-83 vaccinated mice, T helper cells (Th1-type) cells were shown to play an important role in protection (Bennett et al., 1998). We report here that neutralizing antibody is elicited by vaccination with the attenuated Sindbis/VEE virus in animals depending, in part, on loss of an adaptive immune function. However, detectable levels of serum neutralizing antibody are not required for survival, as demonstrated using γδ TCR-deficient mice, IFNγR-deficient mice and μMT mice, although neutralizing antibody promotes protection from lethal encephalitis.
IFN-γ is an essential switch factor for IgG2a and it is anticipated that the IgG isotype is predominantly induced as a result of vaccination with the Sindbis/VEEV chimera (Snapper and Paul, 1987). Prior studies in VSV have shown that neutralization of IFN-γ reduces neutralizing IgG antibodies (Maloy et al., 1998). In this same series of studies, αβ TCR-deficient mice produced IgG neutralizing antibodies as a result of compensation from γδ T cell-derived IFN-γ. A similar mechanism may be initiated in response to VEEV in the CNS and could account for the reduction in neutralizing antibody in both γδ TCR-deficient mice and IFNγR-deficient mice. A minor proportion of μMT mice survived infection following vaccination whereas vaccinated αβ TCR-deficient mice uniformly died. Adoptive transfer of CD4+ T cells from vaccinated WT donor mice results in reconstitution of protection in αβ TCR-deficient mice, suggesting a predominant role for this cell type (S. Paessler, unpublished observations).
This study does not address the role of non-neutralizing antibodies that may be produced as a result of vaccination and/or viral challenge. Previously, it has been shown that the prophylactic, passive transfer of non-neutralizing, monoclonal antibody into animals infected with neuroadapted Sindbis virus, which causes encephalitis under experimental conditions, can prolong survival or protect mice completely (Schmaljohn et al., 1982). However, the efficacy of different non-neutralizing antibody described by Schmaljohn et al. correlated with the attenuation of CNS infection that was reflected by dramatically (100-fold to a million-fold) reduced viral titers in the brains of infected and treated mice in comparison to infected, but untreated mice. This led to the hypothesis that host cell infection was blocked via either of two alternative mechanisms: (i) antibody-dependent cytolysis of infected cells, or (ii) hindrance of virus budding from infected cells by binding to the viral proteins embedded in the membranes of infected cells (Schmaljohn et al., 1982). Similar to results from experimental work with Sindbis virus, Hunt et al. have demonstrated the protection of non-neutralizing antibody to the amino terminus of E2 of Trinidad Donkey strain of VEEV, which was based on its ability to limit virus replication in the brains of infected mice, and, thereby, reducing the viral load in the brain (Hunt et al., 1990, Hunt et al., 1991). In our evaluation of the brains in this study, the viral loads are reduced only in vaccinated WT mice, while other strains with selective immune deficiencies are equally susceptible to VEEV replication in the brain, regardless of the outcome of infection. Based on our data, in which seroconversion was measured pre- and post-challenge, we believe that the humoral immune response was highly limited in mice with selective immune deficiencies, while it was robust in WT mice. Nevertheless, we can only speculate about the importance of non-neutralizing antibody in our model and subsequent studies may be needed to address this issue.
Role of T cells in protection from lethal encephalitis
These studies demonstrate that protection, as measured by survival of vaccinated mice relative to mock-vaccinated controls, may not completely depend on the presence of detectable levels of virus neutralizing antibody in the serum. The role of virus-specific T cells was confirmed by restoration of protection using adoptive transfer of CD3+ donor T cells from vaccinated WT donor mice into αβ TCR-deficient recipients. Furthermore, the relative degree of immune-mediated inflammation in αβ TCR-versus γδ TCR-deficient mice did not fully correlate with viral clearance. Remarkably, vaccinated γδ TCR-deficient mice were protected from lethal intranasal viral challenge relative to WT mice, but VEEV persisted in the brain for up to 28 days following inoculation. Virus clearance was not affected in WT inbred or outbred mice or in surviving animals lacking functional IFN-γ receptor, as demonstrated by a lack of detectable infectious virus in the brains of animals that survived 28 days after infection. Our data demonstrate distinct differences in the vaccine and challenge (adaptive) response to neurovirulent VEEV in αβ vs. γδ T-cell deficient mice. Thus, while αβ TCR-bearing T cells appear to be strictly required for protection, γδ TCR-bearing T cells may not; it is possible that persistence is an indirect effect of a deficit in T cell help for B cells in the latter mice. Future studies will examine the cell types that are responsible for protection.
The role of cytolytic CD8+ T cells in the response to VEEV infection has not been clearly defined, as previous studies demonstrated a significant degree of variability, depending upon the virus and/or mouse strain tested (Jones et al., 2003). As neurons are not highly proliferative and are not believed to regenerate, the mechanism of viral elimination by neurons remains under intensive study, with focus on non-lytic and lytic mechanisms of viral clearance. As CD4+ and γδ T cells have been shown to perform redundant functions, the extent of their involvement in the immune response may be highly dependent upon which cell type is recruited to sites of viral replication in the CNS and the periphery.
As noted, protection from lethal VEEV infection in infected mice does not entirely correlate with the clearance of infectious virus from the brain. However, protection is positively correlated with the accumulation of cellular infiltrates in and around the vasculature in the brain. We routinely observed lower levels of cellular infiltrates (morphological differences in inflammatory foci) in the brains of αβ TCR-deficient mice. Thus, survival appears to be enhanced by increased level of inflammation in the brain early during the course of infection. These results support the further study of T cell subsets, e.g., CD4+ and CD8+ T cells, and clearance mechanisms, to enable us to refine our understanding of the relationship between encephalitic VEEV invasion and replication in the brain and the clinical outcomes of vaccination/challenge studies.
γδ T cells in VEEV immunity and protection against lethal encephalitis
A surprising result of this study was that γδ T cell deficient mice tolerated: (i) high levels of viral replication in the first 7 days of infection (acute phase), which was indistinguishable from those observed in unvaccinated mice that became paralyzed and died, and (ii) lower level virus persistence over an extended period of time (asymptomatic, persistent phase), which was not observed in any other group. Infectious virus persisted in the brain, despite a moderate level of inflammation and cellular infiltration at the site of infection, which was observed in histopathological evaluation of brain tissues. The persistence of infectious VEEV has not heretofore been described, although viral RNA has been detected in the brains of mice that survived acute Sindbis virus infection (Levine and Griffin, 1992). The nature of persistence of VEEV in these γδ T cell deficient mice, which occurs in the absence of clinical disease, is an interesting finding that warrants further molecular pathogenesis studies. These results suggest the potential for a novel role for γδ T cells in the CNS. TCR αβ-deficiency has been shown to impact γδ T cell development (Pennington et al., 2003). However, in our studies, γδ TCR-deficient mice survive a lethal VEEV challenge dose.
Immune effector roles of γδ T cells in other viral infections
In general, γδ TCR-deficient mice have increased susceptibility to tumors and exaggerated immunopathology in bacterial infection models (Mombaerts et al., 1993, Mukasa et al., 1995). Functionally, IFN-γ producing γδ T cells have been shown to play a role in the production of neutralizing antibody responses in TCRα- and TCRβ-deficient mice to vesicular stomatitis virus (VSV) (Maloy et al., 1998). In response to vaccination and challenge with VEEV, the majority of γδ TCR-deficient mice did not mount detectable neutralizing antibody responses. The immunoregulatory potential of γδ TCR-bearing cells is diverse with the potential to regulate cellular infiltration and retention at sites of inflammation, positively or negatively regulate effector T cell function including cytotoxicity, directly lyse antigen-presenting cells and block the influx of neutrophils (Hayday and Tigelaar, 2003). Further, in experimental models of autoimmune encephalomyelitis, γδ T cells have been shown to promote downmodulation of the inflammatory response by inducing turnover of encephalitogenic T cells via a CD95L-dependent mechanism (Ponomarev and Dittel, 2005). Interestingly, this regulation appears to occur late in the disease process and may involve cross-talk with αβ TCR-bearing cells. The precise basis of the specific host–pathogen-interaction(s) responsible for clearance of infectious virus or for selection of viral variants is unclear, but occurs in a microenvironment of a more extensive inflammatory and necrotic response in this particular model. These results are in contrast to previously published role for αβ TCR-bearing T cells in the encephalitic Sindbis virus model. Animals deficient in αβ, but not γδ T cells had lower mortality rates when infected with neuroadapted Sindbis virus, indicating different contributions to the outcome of the brain infection (Rowell and Griffin, 2002). Recent studies for orthopox virus infections have shown a key role for γδ T cell-derived IFN-γ in innate immunity to infection (Agrati et al., 2006). In coronavirus infection, human Vγ9Vδ2 T cells display an interferon-γ-dependent anti-SARS-CoV activity and are able to directly kill SARS-CoV-infected target cells (Poccia et al., 2006). Gamma-delta T cells also mediate demyelination in athymic mice infected with the neurotropic coronavirus mouse hepatitis virus (Dandekar et al., 2005). This process has been shown to be dependent on IFN-γ production. These results contrast with alphavirus-mediated immunity to VEEV, which appears to be partially independent of IFN-γR signaling and partially independent of γδ T cells in early antiviral response.
Conclusions
Our data indicate that αβ TCR-bearing T cells may be stringently required for protection; currently, additional passive transfer studies are ongoing to further define the role of the specific cell types in this protection. Sequencing of persistent viruses isolated from vaccinated and challenged γδ TCR-deficient mice has revealed several genetic changes that are present, irrespective of the isolation method. Studies aimed at the characterization of the genotype and phenotypes of persistent viruses are ongoing. We believe that the observations described herein provide the opportunity to dissect potential intervention points and could enable us to design additional studies to identify prognostic markers that correlate with protection against encephalitis in vaccinated mice.
Materials and methods
Animals
The following mice used in this study (Table 1) were purchased from Jackson Laboratories (Bar Harbor, Maine): C57BL/6 (wild type, WT) (Waterston et al., 2002), αβ TCR KO (strain B6.129P2-Tcrb tm1Mom/J, C57BL/6 mice deficient in αβ T cells) (Mombaerts et al., 1992), γδ TCR KO (strain B6.129P2-Tcrd tm1Mom/J, C57BL/6 mice deficient in γδ T cells) (Itohara et al., 1993), μMT (strain B6.129S2-Igh-6 tm1Cgn/J, mature B cell-deficient) (Kitamura et al., 1991) mice and IFN-γR KO (strain 129-Ifngr1 tm1Agt/J, homozygous interferon-gamma receptor alpha chain (R1)-chain-disrupted mice) (Huang et al., 1993). Mice and Syrian golden hamsters (Mesocricetus auratus purchased from Harlan, Indianapolis, Indiana) were housed in a specific pathogen-free environment for a minimum of 7 days until immunized. Immunization studies were carried out in ABSL-2 and challenge studies in ABSL-3. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and carried out according to NIH guidelines.
Telemetry
For measurement of body temperature, animals were anaesthetized with isoflurane (5%) and implanted subcutaneously with BMDS IPTT-300 transponders (chip), purchased from Bio Medic Data Systems, Inc. (BMDS, Seaford, Delaware), using a trocar needle assembly. Animals were monitored for signs of infection or migration of transponder prior to temperature recording. Chips were scanned using a DAS-6007 transponder reader (BMDS). Downloading of digital temperature data was performed in accordance with the manufacturer's protocol.
Generation of chimeric SIN/VEE virus
The chimeric alphavirus SIN/ZPC construct encoding the replicative machinery from Sindbis (SIN) virus, and the structural genes from virulent VEEV strains were described previously (Paessler et al., 2006a). Infectious virus for immunization of mice was obtained by electroporation of BHK-21 cells with viral RNA transcripts obtained from SP6-promoter driven in vitro transcription reactions utilizing SP6 RNA polymerase (Invitrogen). Virus was harvested after development of cytopathic effects (CPE), usually at 24 h following electroporation (Bredenbeek et al., 1993).
Immunization and challenge with virulent VEEV strain, ZPC738
Immunization of mice
For safety studies, eleven to eighteen-week-old, female mice were inoculated on day 0 and day 34 subcutaneously (s.c.) into the medial thigh with the chimeric SIN/ZPC virus at a dose of 5 × 105 PFU in a total volume of 100 μl or intranasally with an identical dose of virulent VEEV (ZPC738) in 40 μl of PBS or with PBS alone (WT⁎ mock) and deaths were recorded. Daily telemetric monitoring of body temperature was performed for a period of 14 days beginning 2 days prior to the first vaccination on day 0.
Immunization and challenge of mice
For efficacy studies, immunization and challenge with virulent VEEV (ZPC738) were performed according to previously established protocols, as follows. Mice were inoculated on day − 42 subcutaneously (s.c.) into the medial thigh with chimeric SIN/ZPC virus at a dose of 5 × 105 plaque-forming units (PFU) in total volume of 100 μl of PBS. All animals received an additional booster on day − 14, unless stated differently, using the identical route and dosage as the first immunization. Animals were bled on days 0 and 28 after initial immunization. Serum samples were collected by centrifugation, heat-inactivated at 56 °C for 30 min and stored at − 70 °C until serological tests were performed. At day 0 (14 days following second immunization), mice were challenged intranasally (i.n.) with 2 × 105 PFU of virulent VEEV (ZPC738) per animal in 20 μl of PBS. Mice were observed twice daily for a period of 28 days for clinical illness (anorexia and/or paralysis) and/or death. For chimeric SIN/ZPC vaccinated mice, three animals per experimental group were sacrificed on days 3 and 7 and the brains and peripheral tissues (lungs, livers, spleens, and kidneys) were collected for viral titration or for histological examinations and immunohistochemistry staining. On day 28 post-challenge, all animals were sacrificed and organs were collected. For mock-vaccinated animals, two (day 7) or three (day 3) mice were sacrificed for viral titration.
Statistical analysis
Statistical analysis of survival for all groups over the 28-day period was performed using logrank test at a significant level of α < 0.05 in GraphPad Prism (San Diego, CA). Statistical comparison of vaccinated mice for each strain in comparison to the mock vaccinated WT mice was performed using Fisher's Exact Test at a significant level of α < 0.05 in GraphPad® Prism.
T cell subset isolation
CD3+ T cells were isolated from immunized donor C57BL/6 mice (Lambert et al., 2005) approximately 7 days after the last immunization with chimeric SIN/ZPC. Briefly, thirty donor spleens were removed and single cells suspensions generated by mechanical disruption. Splenocytes were fractionated over Lympholyte-M according to the manufacturer's protocol (Accurate Chemicals, Westbury, NY). The cells were washed three times in MACS buffer (phosphate buffered saline, pH 7.4 supplemented with 0.5% bovine serum albumin (BSA), 2 mM EDTA) and CD3+ T cells were separated by magnetic sorting-based negative selection according to the manufacturer's instructions (AutoMacs, Miltenyi Biotec; Auburn, CA). Purity was confirmed by cell surface staining and flow cytometric analysis prior to adoptive transfer.
Flow cytometric analysis
Cells (2.5–5 × 105/tube) were incubated for 10 min on ice in 50 μL of blocking buffer (PBS/0.5% BSA) and then incubated for 30 min with hamster anti-mouse CD3 antibody (Pharmingen, San Jose, CA). Cells were washed and PE-Cy5-conjugated hamster anti-mouse antibody (BD Pharmingen, San Jose, CA) was added (1:100) and cells incubated for 30 min. Cells were washed in FACS buffer and fixed in PBS/2% formaldehyde for fluorescence-activated cell sorting (FACS) analysis. Acquisition of data was performed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Data were collected and analyzed with CellQuest software (Becton Dickinson, San Jose, CA). T-lymphocyte populations were gated based on forward and side scatter and expression of CD3. At least 10,000 CD3+ lymphocytes were acquired for analysis.
Adoptive transfer
Alpha-beta TCR-deficient mice (recipients, N = 15) were injected on day − 28 via intraperitoneal route with 5 × 107 CD3+ T cells (obtained from SIN/ZPC immunized WT C57BL/6 donors, as described in T cell subset isolation), suspended in 100 μl of sterile phosphate-buffered saline (PBS). As controls for reconstitution experiments, five mice were inoculated with PBS alone. Recipient mice were immunized with chimeric SIN/ZPC 2 days after T cell transfer and challenged with virulent VEEV (ZPC738) at 28 days following immunization, as described in Immunization and challenge of mice.
Viral replication in the tissues
Viral replication in the brains and peripheral organs was assessed by plaque assay. Mouse organs, e.g., brain, lungs, liver, spleen and kidneys, were collected at 3 and 7 days post-challenge and sagittally sectioned in half. One half of each brain was homogenized in MEM containing 10% FBS, and a 10% suspension was made. The organ suspension was maintained at − 80 °C until further processing. For days 3 and 7 organs, the titer of infectious virus was calculated using a plaque assay, as previously described (Paessler et al., 2003). For all animals that survived challenge to 28 days, qualitative assay of infectious virus was performed.
Histopathology
Histopathology analysis of brain sections was performed by a neuropathologist blinded to the sample identification and scoring was determined as indicated in Table 1. Brains were fixed in 10% buffered formalin for 48 h, and stored in 70% ethanol for 12 h. The samples were then embedded in paraffin, sectioned (5 μm), mounted on slides and standard hematoxylin and eosin (H&E) staining was performed.
Viral antigen staining
Mouse VEEV-specific hyperimmune serum (HIS) raised against Everglades virus (a member of the VEE complex) (ATCC, cat number: VR1250AF) was applied in a 1:200 dilution in PBS to brain sections for 60 min. Normal mouse serum (Ig-Ready-to-Use-Kit, DakoCytomation, Cat. No. N1698) was used as a negative control antibody on brains from infected mice. Brain sections from age-matched, uninfected mice were used as a negative control tissue for immunostaining. The Histomouse-SP Kit (Zymed, Cat. No. 95-9541) was used to detect mouse antibody on mouse tissue. Slides were counter-stained with Mayer's Modified Hematoxylin for microscopy.
Plaque reduction neutralization test (PRNT)
The baby hamster kidney (BHK-21) cell line (kindly provided by Dr. Paul Olivo, Washington University, St. Louis, MO) was maintained at 37 °C in alpha minimum essential medium supplemented with 10% fetal bovine serum (FBS) and vitamins. Plaque reduction neutralization tests (PRNT) were performed on BHK-21 monolayers. Briefly, a stock of chimeric SIN/ZPC virus was incubated for 1 h at 37 °C with different dilutions of sera taken individually from infected mice. Subsequently, cell monolayers were incubated with virus/serum mixtures for 1 h at 37 °C, overlaid with 0.5% agarose, maintained for 36 h at 37 °C and stained with crystal violet (Beaty et al., 1989). The serum dilution corresponding to an endpoint of 80% plaque reduction was determined.
Acknowledgments
This work was supported by a grant from the NIAID through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research, NIH grant no. U54 AI057156. SP was supported by NIH K08 grant no. AI059491. Excellent technical assistance was provided by Dr. Haolin Ni, Seth Linde and Melinda Kelley. We thank Jenna Linde and Nikki Ward for assisting in preparation of the manuscript figures. Mardelle Susman provided editorial/proofreading expertise.
References
- Aaronson R.P., Young J.F., Palese P. Oligonucleotide mapping: evaluation of its sensitivity by computer-simulation. Nucleic Acids Res. 1982;10:237–246. doi: 10.1093/nar/10.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrati C., Castilletti C., De Santis R., Cimini E., Bordi L., Malkovsky M., Poccia F., Capobianchi M.R. Interferon- gamma -mediated antiviral immunity against orthopoxvirus infection is provided by gamma delta T cells. J. Infect. Dis. 2006;193(11):1606–1607. doi: 10.1086/503438. [DOI] [PubMed] [Google Scholar]
- Altman D.G. Practical Statistics for Medical Research. Chapman and Hall; London: 1991. Analysis of survival times. [Google Scholar]
- Anishchenko M., Bowen R.A., Paessler S., Austgen L., Greene I.P., Weaver S.C. Venezuelan encephalitis emergence mediated by a phylogenetically predicted viral mutation. Proc. Natl. Acad. Sci. U.S.A. 2006;103(13):4994–4999. doi: 10.1073/pnas.0509961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty B.J., Calisher C.H., Shope R.E. Arboviruses. In: Schmidt N.J., Emmons R.W., editors. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. 6th edition. American Public Health Association; Washington, DC: 1989. pp. 797–855. [Google Scholar]
- Bennett A.M., Lescott T., Phillpotts R.J. Improved protection against Venezuelan equine encephalitis by genetic engineering of a recombinant vaccinia virus. Viral Immunol. 1998;11(3):109–117. doi: 10.1089/vim.1998.11.109. [DOI] [PubMed] [Google Scholar]
- Binder G.K., Griffin D.E. Immune-mediated clearance of virus from the central nervous system. Microbes Infect. 2003;5(5):439–448. doi: 10.1016/s1286-4579(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Born W.K., Reardon C.L., O'Brien R.L. The function of gammadelta T cells in innate immunity. Curr. Opin. Immunol. 2006;18(1):31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Bredenbeek P.J., Frolov I., Rice C.M., Schlesinger S. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J. Virol. 1993;67(11):6439–6446. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdeinick-Kerr R., Griffin D.E. Gamma interferon-dependent, noncytolytic clearance of sindbis virus infection from neurons in vitro. J. Virol. 2005;79(9):5374–5385. doi: 10.1128/JVI.79.9.5374-5385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P.C., Walters E., Margolis F., Johnston R.E. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology. 1995;208(2):662–671. doi: 10.1006/viro.1995.1197. [DOI] [PubMed] [Google Scholar]
- Charles P.C., Brown K.W., Davis N.L., Hart M.K., Johnston R.E. Mucosal immunity induced by parenteral immunization with a live attenuated Venezuelan equine encephalitis virus vaccine candidate. Virology. 1997;228(2):153–160. doi: 10.1006/viro.1996.8381. [DOI] [PubMed] [Google Scholar]
- Dandekar A.A., O'Malley K., Perlman S. Important roles for gamma interferon and NKG2D in gammadelta T-cell-induced demyelination in T-cell receptor beta-deficient mice infected with a coronavirus. J. Virol. 2005;79(15):9388–9396. doi: 10.1128/JVI.79.15.9388-9396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N.L., Brown K.W., Greenwald G.F., Zajac A.J., Zacny V.L., Smith J.F., Johnston R.E. Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology. 1995;212(1):102–110. doi: 10.1006/viro.1995.1458. [DOI] [PubMed] [Google Scholar]
- Davis N.L., Caley I.J., Brown K.W., Betts M.R., Irlbeck D.M., McGrath K.M., Connell M.J., Montefiori D.C., Frelinger J.A., Swanstrom R., Johnson P.R., Johnston R.E. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles [In Process Citation] J. Virol. 2000;74(1):371–378. doi: 10.1128/jvi.74.1.371-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S., Castro F., Bonilla N.J., Gaskin de Urdaneta A., Hutchins G.M. The systemic pathology of Venezuelan equine encephalitis virus infection in humans. Am. J. Trop. Med. Hyg. 1985;34(1):194–202. doi: 10.4269/ajtmh.1985.34.194. [DOI] [PubMed] [Google Scholar]
- Griffin D., Levine B., Tyor W., Ubol S., Despres P. The role of antibody in recovery from alphavirus encephalitis. Immunol. Rev. 1997;159:155–161. doi: 10.1111/j.1600-065x.1997.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Hayday A., Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat. Rev., Immunol. 2003;3(3):233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R.M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Hunt A.R., Johnson A.J., Roehrig J.T. Synthetic peptides of Venezuelan equine encephalomyelitis virus E2 glycoprotein: I. Immunogenic analysis and identification of a protective peptide. Virology. 1990;179(2):701–711. doi: 10.1016/0042-6822(90)90137-g. [DOI] [PubMed] [Google Scholar]
- Hunt A.R., Short W.A., Johnson A.J., Bolin R.A., Roehrig J.T. Synthetic peptides of the E2 glycoprotein of Venezuelan equine encephalomyelitis virus: II. Antibody to the amino terminus protects animals by limiting viral replication. Virology. 1991;185(1):281–290. doi: 10.1016/0042-6822(91)90775-7. [DOI] [PubMed] [Google Scholar]
- Itohara S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A.R., Hooper M.L., Farr A., Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72(3):337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Jahrling P.B., Scherer F. Histopathology and distribution of viral antigens in hamsters infected with virulent and benign Venezuelan encephalitis viruses. Am. J. Pathol. 1973;72(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- Jones L.D., Bennett A.M., Moss S.R., Gould E.A., Phillpotts R.J. Cytotoxic T-cell activity is not detectable in Venezuelan equine encephalitis virus-infected mice. Virus Res. 2003;91(2):255–259. doi: 10.1016/s0168-1702(02)00275-7. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kuhn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Lambert K.C., Curran E.M., Judy B.M., Milligan G.N., Lubahn D.B., Estes D.M. Estrogen receptor {alpha} (ER{alpha}) deficiency in macrophages results in increased stimulation of CD4+ T Cells while 17{beta}-estradiol acts through ER{alpha} to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J. Immunol. 2005;175(9):5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- Levine B., Griffin D.E. Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J. Virol. 1992;66(11):6429–6435. doi: 10.1128/jvi.66.11.6429-6435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy K.J., Odermatt B., Hengartner H., Zinkernagel R.M. Interferon gamma-producing gammadelta T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc. Natl. Acad. Sci. U.S.A. 1998;95(3):1160–1165. doi: 10.1073/pnas.95.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A.R., Rudnicki M.A., Iacomini J., Itohara S., Lafaille J.J., Wang L., Ichikawa Y., Jaenisch R., Hooper M.L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Arnoldi J., Russ F., Tonegawa S., Kaufmann S.H. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365(6441):53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- Mukasa A., Hiromatsu K., Matsuzaki G., O'Brien R., Born W., Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J. Immunol. 1995;155(4):2047–2056. [PubMed] [Google Scholar]
- Owens T., Babcock A.A., Millward J.M., Toft-Hansen H. Cytokine and chemokine inter-regulation in the inflamed or injured CNS. Brain Res. Brain Res. Rev. 2005;48(2):178–184. doi: 10.1016/j.brainresrev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Paessler S., Fayzulin R.Z., Anishchenko M., Greene I.P., Weaver S.C., Frolov I. Recombinant sindbis/Venezuelan equine encephalitis virus is highly attenuated and immunogenic. J. Virol. 2003;77(17):9278–9286. doi: 10.1128/JVI.77.17.9278-9286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paessler S., Ni H., Petrakova O., Fayzulin R.Z., Yun N., Anishchenko M., Weaver S.C., Frolov I. Replication and clearance of Venezuelan equine encephalitis virus from the brains of animals vaccinated with chimeric SIN/VEE viruses. J. Virol. 2006;80(6) doi: 10.1128/JVI.80.6.2784-2796.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paessler S., Ni H., Petrakova O., Fayzulin R.Z., Yun N., Anishchenko M., Weaver S.C., Frolov I. Replication and clearance of Venezuelan equine encephalitis virus from the brains of animals vaccinated with chimeric SIN/VEE viruses. J. Virol. 2006;80(6):2784–2796. doi: 10.1128/JVI.80.6.2784-2796.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington D.J., Silva-Santos B., Shires J., Theodoridis E., Pollitt C., Wise E.L., Tigelaar R.E., Owen M.J., Hayday A.C. The inter-relatedness and interdependence of mouse T cell receptor gammadelta+ and alphabeta+ cells. Nat. Immunol. 2003;4(10):991–998. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- Poccia F., Agrati C., Castilletti C., Bordi L., Gioia C., Horejsh D., Ippolito G., Chan P.K., Hui D.S., Sung J.J., Capobianchi M.R., Malkovsky M. Anti-severe acute respiratory syndrome coronavirus immune responses: the role played by V gamma 9V delta 2 T cells. J. Infect. Dis. 2006;193(9):1244–1249. doi: 10.1086/502975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev E.D., Dittel B.N. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J. Immunol. 2005;174(8):4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- Rowell J.F., Griffin D.E. Contribution of T cells to mortality in neurovirulent Sindbis virus encephalomyelitis. J. Neuroimmunol. 2002;127(1–2):106–114. doi: 10.1016/s0165-5728(02)00108-x. [DOI] [PubMed] [Google Scholar]
- Ryzhikov A.B., Tkacheva N.V., Sergeev A.N., Ryabchikova E.I. Venezuelan equine encephalitis virus propagation in the olfactory tract of normal and immunized mice. Biomed. Sci. 1991;2(6):607–614. [PubMed] [Google Scholar]
- Ryzhikov A.B., Ryabchikova E.I., Sergeev A.N., Tkacheva N.V. Spread of Venezuelan equine encephalitis virus in mice olfactory tract. Arch. Virol. 1995;140(12):2243–2254. doi: 10.1007/BF01323243. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A.L., Johnson E.D., Dalrymple J.M., Cole G.A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Snapper C.M., Paul W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Waterston R.H., Lindblad-Toh K., Birney E., Rogers J., Abril J.F., Agarwal P., Agarwala R., Ainscough R., Alexandersson M., An P., Antonarakis S.E., Attwood J., Baertsch R., Bailey J., Barlow K., Beck S., Berry E., Birren B., Bloom T., Bork P., Botcherby M., Bray N., Brent M.R., Brown D.G., Brown S.D., Bult C., Burton J., Butler J., Campbell R.D., Carninci P., Cawley S., Chiaromonte F., Chinwalla A.T., Church D.M., Clamp M., Clee C., Collins F.S., Cook L.L., Copley R.R., Coulson A., Couronne O., Cuff J., Curwen V., Cutts T., Daly M., David R., Davies J., Delehaunty K.D., Deri J., Dermitzakis E.T., Dewey C., Dickens N.J., Diekhans M., Dodge S., Dubchak I., Dunn D.M., Eddy S.R., Elnitski L., Emes R.D., Eswara P., Eyras E., Felsenfeld A., Fewell G.A., Flicek P., Foley K., Frankel W.N., Fulton L.A., Fulton R.S., Furey T.S., Gage D., Gibbs R.A., Glusman G., Gnerre S., Goldman N., Goodstadt L., Grafham D., Graves T.A., Green E.D., Gregory S., Guigo R., Guyer M., Hardison R.C., Haussler D., Hayashizaki Y., Hillier L.W., Hinrichs A., Hlavina W., Holzer T., Hsu F., Hua A., Hubbard T., Hunt A., Jackson I., Jaffe D.B., Johnson L.S., Jones M., Jones T.A., Joy A., Kamal M., Karlsson E.K., Karolchik D., Kasprzyk A., Kawai J., Keibler E., Kells C., Kent W.J., Kirby A., Kolbe D.L., Korf I., Kucherlapati R.S., Kulbokas E.J., Kulp D., Landers T., Leger J.P., Leonard S., Letunic I., Levine R., Li J., Li M., Lloyd C., Lucas S., Ma B., Maglott D.R., Mardis E.R., Matthews L., Mauceli E., Mayer J.H., McCarthy M., McCombie W.R., McLaren S., McLay K., McPherson J.D., Meldrim J., Meredith B., Mesirov J.P., Miller W., Miner T.L., Mongin E., Montgomery K.T., Morgan M., Mott R., Mullikin J.C., Muzny D.M., Nash W.E., Nelson J.O., Nhan M.N., Nicol R., Ning Z., Nusbaum C., O'Connor M.J., Okazaki Y., Oliver K., Overton-Larty E., Pachter L., Parra G., Pepin K.H., Peterson J., Pevzner P., Plumb R., Pohl C.S., Poliakov A., Ponce T.C., Ponting C.P., Potter S., Quail M., Reymond A., Roe B.A., Roskin K.M., Rubin E.M., Rust A.G., Santos R., Sapojnikov V., Schultz B., Schultz J., Schwartz M.S., Schwartz S., Scott C., Seaman S., Searle S., Sharpe T., Sheridan A., Shownkeen R., Sims S., Singer J.B., Slater G., Smit A., Smith D.R., Spencer B., Stabenau A., Stange-Thomann N., Sugnet C., Suyama M., Tesler G., Thompson J., Torrents D., Trevaskis E., Tromp J., Ucla C., Ureta-Vidal A., Vinson J.P., Von Niederhausern A.C., Wade C.M., Wall M., Weber R.J., Weiss R.B., Wendl M.C., West A.P., Wetterstrand K., Wheeler R., Whelan S., Wierzbowski J., Willey D., Williams S., Wilson R.K., Winter E., Worley K.C., Wyman D., Yang S., Yang S.P., Zdobnov E.M., Zody M.C., Lander E.S. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Weaver S.C. Host range, amplification and arboviral disease emergence. Arch. Virol., Suppl. 2005;19:33–44. doi: 10.1007/3-211-29981-5_4. [DOI] [PubMed] [Google Scholar]
- Weaver S.C., Salas R., Rico-Hesse R., Ludwig G.V., Oberste M.S., Boshell J., Tesh R.B. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. Lancet. 1996;348:436–440. doi: 10.1016/s0140-6736(96)02275-1. [DOI] [PubMed] [Google Scholar]
- Weaver S.C., Ferro C., Barrera R., Boshell J., Navarro J.C. Venezuelan equine encephalitis⁎. Annu. Rev. Entomol. 2004;49:141–174. doi: 10.1146/annurev.ento.49.061802.123422. [DOI] [PubMed] [Google Scholar]