Abstract
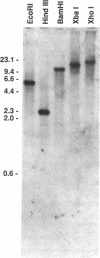
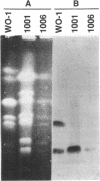
Candida albicans produces large amounts of the pentitol D-arabitol in culture and in infected mammalian hosts, but the functional and pathogenic significance of D-arabitol in C. albicans is not known. In this study, we sought to elucidate the pathway by which C. albicans synthesizes D-arabitol and to identify and characterize key enzymes in this pathway. C. albicans B311 produced D-[14C-1]arabitol from [14C-2]glucose; this finding implies on structural grounds that D-ribulose-5-PO4 from the pentose pathway is the major metabolic precursor of D-arabitol. NAD- or NADP-dependent pentitol dehydrogenases catalyze the final steps in D-arabitol biosynthesis in other fungi; therefore, lysates of C. albicans B311 were tested for enzymes of this class and were found to contain a previously unknown NAD-dependent D-arabitol dehydrogenase (ArDH). The ArDH structural gene was cloned by constructing a new D-arabitol utilization pathway in Escherichia coli. The C. albicans ArDH gene expressed in E. coli and Saccharomyces cerevisiae an enzyme that catalyzes the reaction D-arabitol + NAD <-->D-ribulose + NADH; this gene was present as a single copy per haploid genome, and its deduced peptide sequence was homologous with sequences of several members of the short-chain dehydrogenase family of enzymes. These results suggest that (i) C. albicans synthesizes D-arabitol by dephosphorylating and reducing the pentose pathway intermediate D-ribulose-5-PO4 and (ii) ArDH catalyzes the final step in this pathway.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAKLEY E. R., SPENCER J. F. Studies on the formation of D-arabitol by osmophilic yeasts. Can J Biochem Physiol. 1962 Dec;40:1737–1748. [PubMed] [Google Scholar]
- Bernard E. M., Wong B., Armstrong D. Stereoisomeric configuration of arabinitol in serum, urine, and tissues in invasive candidiasis. J Infect Dis. 1985 Apr;151(4):711–715. doi: 10.1093/infdis/151.4.711. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. D-Arabitol catabolic pathway in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):170–175. doi: 10.1128/jb.119.1.170-175.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. Ribitol catabolic pathway in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):162–169. doi: 10.1128/jb.119.1.162-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattaway F. W., Odds F. C., Barlow A. J. An examination of the production of hydrolytic enzymes and toxins by pathogenic strains of Candida albicans. J Gen Microbiol. 1971 Aug;67(3):255–263. doi: 10.1099/00221287-67-3-255. [DOI] [PubMed] [Google Scholar]
- Deacon A. G. Estimations of serum arabinitol for diagnosing invasive candidosis. J Clin Pathol. 1986 Aug;39(8):842–850. doi: 10.1136/jcp.39.8.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H., LAMPEN J. O. Fermentation of 1-C14-d-xylose by Lactobacillus pentosus. J Biol Chem. 1952 Feb;194(2):555–562. [PubMed] [Google Scholar]
- Gold J. W., Wong B., Bernard E. M., Kiehn T. E., Armstrong D. Serum arabinitol concentrations and arabinitol/creatinine ratios in invasive candidiasis. J Infect Dis. 1983 Mar;147(3):504–513. doi: 10.1093/infdis/147.3.504. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hult K., Gatenbeck S. Production of NADPH in the mannitol cycle and its relation to polyketide formation in Alternaria alternata. Eur J Biochem. 1978 Aug 1;88(2):607–612. doi: 10.1111/j.1432-1033.1978.tb12487.x. [DOI] [PubMed] [Google Scholar]
- INGRAM J. M., WOOD W. A. ENZYMATIC BASIS FOR D-ARBITOL PRODUCTION BY SACCHAROMYCES ROUXII. J Bacteriol. 1965 May;89:1186–1194. doi: 10.1128/jb.89.5.1186-1194.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings D. H. Polyol metabolism in fungi. Adv Microb Physiol. 1984;25:149–193. doi: 10.1016/s0065-2911(08)60292-1. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., von Bahr-Lindström H., Jany K. D., Ulmer W., Fröschle M. Extended superfamily of short alcohol-polyol-sugar dehydrogenases: structural similarities between glucose and ribitol dehydrogenases. FEBS Lett. 1984 Jan 9;165(2):190–196. doi: 10.1016/0014-5793(84)80167-2. [DOI] [PubMed] [Google Scholar]
- Kiehn T. E., Bernard E. M., Gold J. W., Armstrong D. Candidiasis: detection by gas-liquid chromatography of D-arabinitol, a fungal metabolite, in human serum. Science. 1979 Nov 2;206(4418):577–580. doi: 10.1126/science.493963. [DOI] [PubMed] [Google Scholar]
- Kurtz M. B., Marrinan J. Isolation of hem3 mutants from Candida albicans by sequential gene disruption. Mol Gen Genet. 1989 May;217(1):47–52. doi: 10.1007/BF00330941. [DOI] [PubMed] [Google Scholar]
- Leblanc D. J., Mortlock R. P. The metabolism of D-arabinose: alternate kinases for the phosphorylation of D-ribulose in Escherichia coli and Aerobacter aerogenes. Arch Biochem Biophys. 1972 Jun;150(2):774–781. doi: 10.1016/0003-9861(72)90097-5. [DOI] [PubMed] [Google Scholar]
- Lee N., Bendet I. Crystalline L-ribulokinase from Escherichia coli. J Biol Chem. 1967 May 10;242(9):2043–2050. [PubMed] [Google Scholar]
- Magee B. B., Koltin Y., Gorman J. A., Magee P. T. Assignment of cloned genes to the seven electrophoretically separated Candida albicans chromosomes. Mol Cell Biol. 1988 Nov;8(11):4721–4726. doi: 10.1128/mcb.8.11.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Rikkerink E. H., Magee B. B. Methods for the genetics and molecular biology of Candida albicans. Anal Biochem. 1988 Dec;175(2):361–372. doi: 10.1016/0003-2697(88)90559-3. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B., Krook M., Jörnvall H. Characteristics of short-chain alcohol dehydrogenases and related enzymes. Eur J Biochem. 1991 Sep 1;200(2):537–543. doi: 10.1111/j.1432-1033.1991.tb16215.x. [DOI] [PubMed] [Google Scholar]
- RAO G. R., RAMAKRISHNAN T., SIRSI M. Enzymes in Candida albicans. I. Pathways of glucose dissimilation. J Bacteriol. 1960 Nov;80:654–658. doi: 10.1128/jb.80.5.654-658.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Genes for ribitol and D-arabitol catabolism in Escherichia coli: their loci in C strains and absence in K-12 and B strains. J Bacteriol. 1975 Aug;123(2):530–536. doi: 10.1128/jb.123.2.530-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roboz J., Suzuki R., Holland J. F. Quantification of arabinitol in serum by selected ion monitoring as a diagnostic technique in invasive candidiasis. J Clin Microbiol. 1980 Oct;12(4):594–601. doi: 10.1128/jcm.12.4.594-601.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos G. A., Reiner A. M. Ribitol and D-arabitol catabolism in Escherichia coli. J Bacteriol. 1978 May;134(2):492–500. doi: 10.1128/jb.134.2.492-500.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Magee P. T. Genetics of Candida albicans. Microbiol Rev. 1990 Sep;54(3):226–241. doi: 10.1128/mr.54.3.226-241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyama K., Ono E. Improved procedure for determining serum D-arabinitol by resazurin-coupled method. Clin Chim Acta. 1987 Sep 30;168(2):259–260. doi: 10.1016/0009-8981(87)90297-x. [DOI] [PubMed] [Google Scholar]
- WEIMBERG R. Mode of formation of D-arabitol by Saccharomyces mellis. Biochem Biophys Res Commun. 1962 Aug 31;8:442–445. doi: 10.1016/0006-291x(62)90293-0. [DOI] [PubMed] [Google Scholar]
- Whimbey E., Wong B., Kiehn T. E., Armstrong D. Clinical correlations of serial quantitative blood cultures determined by lysis-centrifugation in patients with persistent septicemia. J Clin Microbiol. 1984 Jun;19(6):766–771. doi: 10.1128/jcm.19.6.766-771.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. L., Mortlock R. P. Regulation of D-xylose and D-arabitol catabolism by Aerobacter aerogenes. J Bacteriol. 1973 Mar;113(3):1404–1411. doi: 10.1128/jb.113.3.1404-1411.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B., Bernard E. M., Gold J. W., Fong D., Silber A., Armstrong D. Increased arabinitol levels in experimental candidiasis in rats: arabinitol appearance rates, arabinitol/creatinine ratios, and severity of infection. J Infect Dis. 1982 Sep;146(3):346–352. doi: 10.1093/infdis/146.3.346. [DOI] [PubMed] [Google Scholar]
- Wong B., Brauer K. L. Enantioselective measurement of fungal D-arabinitol in the sera of normal adults and patients with candidiasis. J Clin Microbiol. 1988 Sep;26(9):1670–1674. doi: 10.1128/jcm.26.9.1670-1674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B., Castellanos M. Enantioselective measurement of the Candida metabolite D-arabinitol in human serum using multi-dimensional gas chromatography and a new chiral phase. J Chromatogr. 1989 Oct 27;495:21–30. doi: 10.1016/s0378-4347(00)82606-7. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]