Abstract
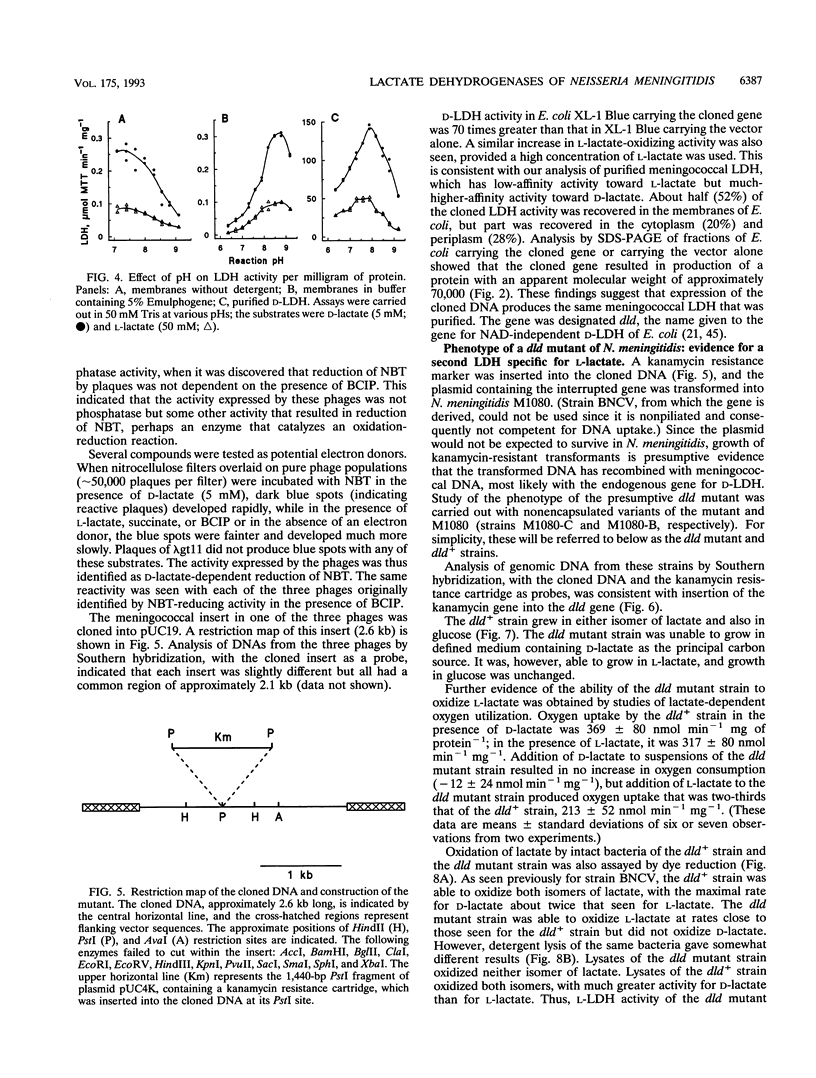
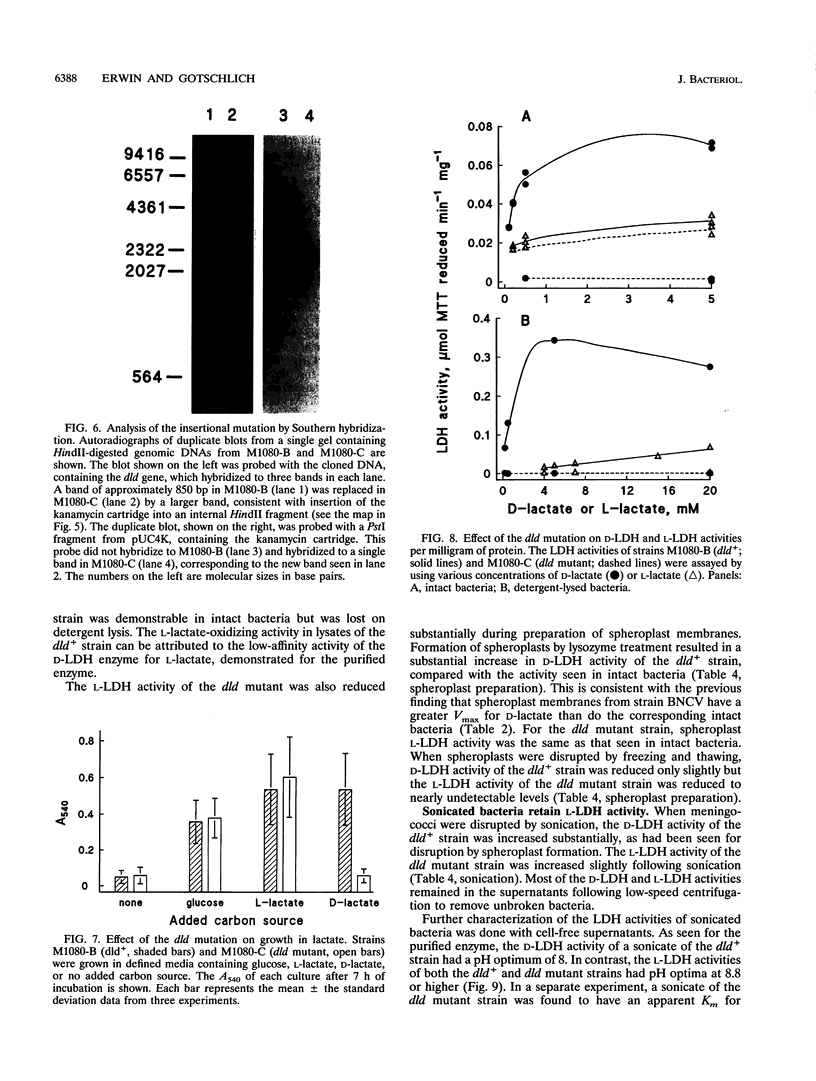
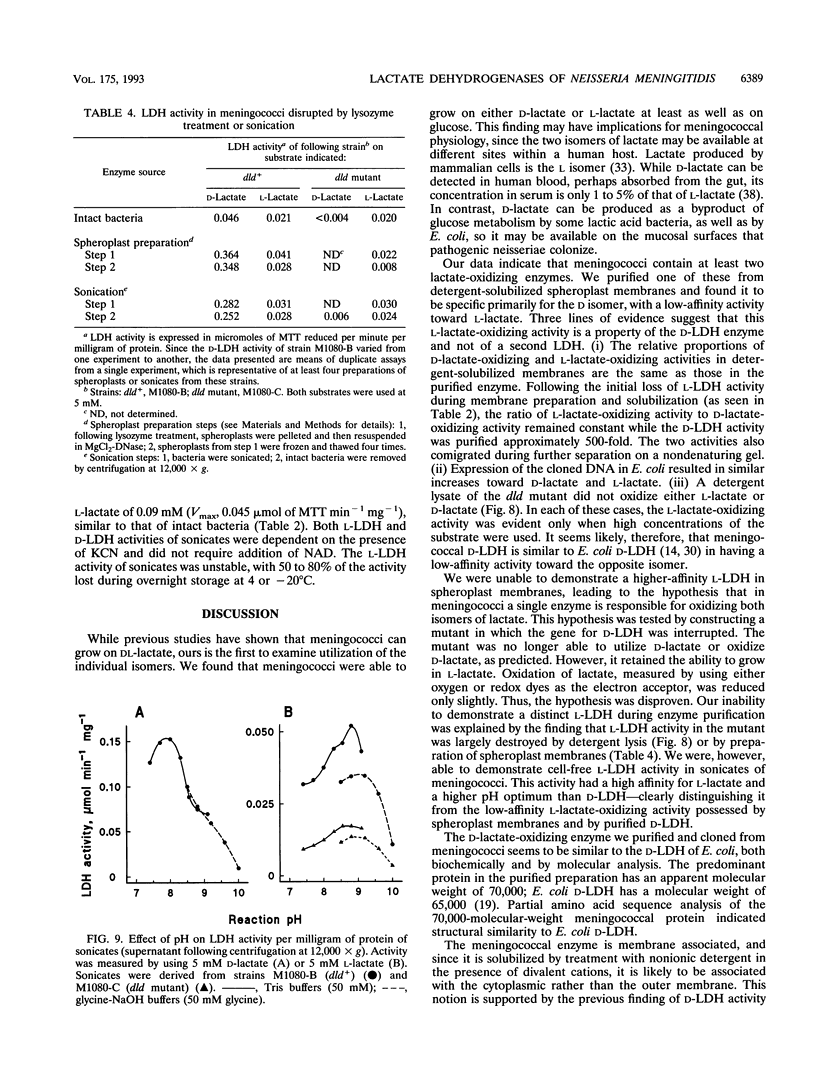
Neisseria meningitidis was found to contain at least two lactate-oxidizing enzymes. One of these was purified 460-fold from spheroplast membranes and found to be specific primarily for D-lactate, with low-affinity activity for L-lactate. The gene for this enzyme (dld) was cloned, and a dld mutant was constructed by insertional inactivation of the gene. The mutant was unable to grow on D-lactate but retained the ability to grow on L-lactate, providing evidence for a second lactate-oxidizing enzyme with specificity for L-lactate. High-affinity L-lactate-oxidizing activity was detected in intact bacteria of both the dld+ and dld mutant strains. This L-lactate-oxidizing activity was also seen in sonicated bacteria but was reduced substantially on detergent solubilization or on preparation of spheroplast membranes.
Full text
PDF




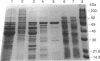
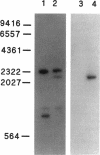
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. Z., Glode M., Schneerson R., Robbins J. B. Identification of immunoglobulin heavy-chain isotypes of specific antibodies of horse 46 group B meningococcal antiserum. J Clin Microbiol. 1982 Feb;15(2):324–329. doi: 10.1128/jcm.15.2.324-329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison N., O'Donnell M. J., Fewson C. A. Membrane-bound lactate dehydrogenases and mandelate dehydrogenases of Acinetobacter calcoaceticus. Purification and properties. Biochem J. 1985 Oct 15;231(2):407–416. doi: 10.1042/bj2310407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R. K., Hendry A. T., Shanmugam K. T., Jensen R. A. The broad-specificity, membrane-bound lactate dehydrogenase of Neisseria gonorrhoeae: ties to aromatic metabolism. J Gen Microbiol. 1989 Feb;135(Pt 2):353–360. doi: 10.1099/00221287-135-2-353. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Klapper D., Svendsen T., Cohen M. S. Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. An unrecognized aspect of the oxygen metabolism of phagocytosis. J Clin Invest. 1988 Feb;81(2):318–324. doi: 10.1172/JCI113323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATLIN B. W., SCHLOER G. M. A defined agar medium for genetic transformation of Neisseria meningitidis. J Bacteriol. 1962 Mar;83:470–474. doi: 10.1128/jb.83.3.470-474.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Genetic studies of sulfadiazine-resistant and methionine-requiring Neisseria isolated from clinical material. J Bacteriol. 1967 Sep;94(3):719–733. doi: 10.1128/jb.94.3.719-733.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson V. L. Steady-state kinetic analysis of the quinoprotein methylamine dehydrogenase from Paracoccus denitrificans. Biochem J. 1989 Jul 1;261(1):107–111. doi: 10.1042/bj2610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J., DeMott M., Atherton D., Mische S. M. Internal protein sequence analysis: enzymatic digestion for less than 10 micrograms of protein bound to polyvinylidene difluoride or nitrocellulose membranes. Anal Biochem. 1992 Mar;201(2):255–264. doi: 10.1016/0003-2697(92)90336-6. [DOI] [PubMed] [Google Scholar]
- Frosch M., Schultz E., Glenn-Calvo E., Meyer T. F. Generation of capsule-deficient Neisseria meningitidis strains by homologous recombination. Mol Microbiol. 1990 Jul;4(7):1215–1218. doi: 10.1111/j.1365-2958.1990.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Fu H. S., Hassett D. J., Cohen M. S. Oxidant stress in Neisseria gonorrhoeae: adaptation and effects on L-(+)-lactate dehydrogenase activity. Infect Immun. 1989 Jul;57(7):2173–2178. doi: 10.1128/iai.57.7.2173-2178.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Kimura H. Inducible membrane-bound L-lactate dehydrogenase from Escherichia coli. Purification and properties. J Biol Chem. 1977 Aug 25;252(16):5820–5827. [PubMed] [Google Scholar]
- Futai M. Membrane D-lactate dehydrogenase from Escherichia coli. Purification and properties. Biochemistry. 1973 Jun 19;12(13):2468–2474. doi: 10.1021/bi00737a016. [DOI] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossowicz N. Growth Requirements and Metabolism of Neisseria intracellularis. J Bacteriol. 1945 Jul;50(1):109–115. doi: 10.1128/jb.50.1.109-115.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry A. T., Stewart I. O. Auxanographic grouping and typing of Neisseria gonorrhoeae. Can J Microbiol. 1979 Apr;25(4):512–521. doi: 10.1139/m79-075. [DOI] [PubMed] [Google Scholar]
- Ho C., Pratt E. A., Rule G. S. Membrane-bound D-lactate dehydrogenase of Escherichia coli: a model for protein interactions in membranes. Biochim Biophys Acta. 1989 May 9;988(2):173–184. doi: 10.1016/0304-4157(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Holten E., Jyssum K. Activities of some enzymes concerning pyruvate metabolism in Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):843–848. doi: 10.1111/j.1699-0463.1974.tb02382.x. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Kaback H. R. Mutants of Salmonella typhimurium and Escherichia coli pleiotropically defective in active transport. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3336–3340. doi: 10.1073/pnas.69.11.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino E., Araya A. Lactate degradation by polysaccharide-producing Neisseria isolated from human dental plaque. Arch Oral Biol. 1980;25(3):211–212. doi: 10.1016/0003-9969(80)90023-0. [DOI] [PubMed] [Google Scholar]
- Hoshino E., Karino H., Yamada T. Lactate metabolism by human dental plaque and Veillonella under aerobic and anaerobic conditions. Arch Oral Biol. 1981;26(1):17–22. doi: 10.1016/0003-9969(81)90066-2. [DOI] [PubMed] [Google Scholar]
- Hoshino E. L-serine enhances the anaerobic lactate metabolism of Veillonella dispar ATCC 17745. J Dent Res. 1987 Jun;66(6):1162–1165. doi: 10.1177/00220345870660061401. [DOI] [PubMed] [Google Scholar]
- Hoshino E., Yamada T., Araya S. Lactate degradation by a strain of Neisseria isolated from human dental plaque. Arch Oral Biol. 1976;21(11):677–683. doi: 10.1016/0003-9969(76)90142-4. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JYSSUM K. Intermediate reactions of the tricarboxylic acid cycle in meningococci. Acta Pathol Microbiol Scand. 1960;48:121–132. doi: 10.1111/j.1699-0463.1960.tb04748.x. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E., Heym G. A. Studies of some naturally occurring auxotrophs of Neisseria gonorrhoeae. J Gen Microbiol. 1980 Nov;121(1):85–92. doi: 10.1099/00221287-121-1-85. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XV. Purification and properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1973 Oct 25;248(20):7012–7017. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long G. L., Kaplan N. O. D-lactate specific pyridine nucleotide lactate dehydrogenase in animals. Science. 1968 Nov 8;162(3854):685–686. doi: 10.1126/science.162.3854.685. [DOI] [PubMed] [Google Scholar]
- Lysko P. G., Morse S. A. Effects of steroid hormones on Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Aug;18(2):281–288. doi: 10.1128/aac.18.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Massa E. M., Leiva M. A., Farías R. N. Study of a time lag in the assay of Escherichia coli membrane-bound dehydrogenases based on tetrazolium salt reduction. Biochim Biophys Acta. 1985 Feb 4;827(2):150–156. doi: 10.1016/0167-4838(85)90084-6. [DOI] [PubMed] [Google Scholar]
- McGadey J. A tetrazolium method for non-specific alkaline phosphatase. Histochemie. 1970;23(2):180–184. doi: 10.1007/BF00305851. [DOI] [PubMed] [Google Scholar]
- McLellan A. C., Phillips S. A., Thornalley P. J. Fluorimetric assay of D-lactate. Anal Biochem. 1992 Oct;206(1):12–16. doi: 10.1016/s0003-2697(05)80004-1. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Cacciapuoti A. F., Lysko P. G. Physiology of Neisseria gonorrhoeae. Adv Microb Physiol. 1979;20:251–320. doi: 10.1016/s0065-2911(08)60209-x. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Fitzgerald T. J. Effect of progesterone on Neisseria gonorrhoeae. Infect Immun. 1974 Dec;10(6):1370–1377. doi: 10.1128/iai.10.6.1370-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Gotschlich E. C., Seiff M. E. Cloning and characterization of the structural gene for the class 2 protein of Neisseria meningitidis. Infect Immun. 1989 Aug;57(8):2318–2323. doi: 10.1128/iai.57.8.2318-2323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt E. A., Fung L. W., Flowers J. A., Ho C. Membrane-bound D-lactate dehydrogenase from Escherichia coli: purification and properties. Biochemistry. 1979 Jan 23;18(2):312–316. doi: 10.1021/bi00569a013. [DOI] [PubMed] [Google Scholar]
- Rule G. S., Pratt E. A., Chin C. C., Wold F., Ho C. Overproduction and nucleotide sequence of the respiratory D-lactate dehydrogenase of Escherichia coli. J Bacteriol. 1985 Mar;161(3):1059–1068. doi: 10.1128/jb.161.3.1059-1068.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Kung H., Young I. G., Kaback H. R. In vitro synthesis of the membrane-bound D-lactate dehydrogenase of Escherichia coli. Biochemistry. 1982 Apr 27;21(9):2085–2091. doi: 10.1021/bi00538a016. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley C. R., Manjula B. N., Gotschlich E. C. Purification and characterization of polyphosphate kinase from Neisseria meningitidis. Infect Immun. 1993 Sep;61(9):3703–3710. doi: 10.1128/iai.61.9.3703-3710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wetzler L. M., Gotschlich E. C., Blake M. S., Koomey J. M. The construction and characterization of Neisseria gonorrhoeae lacking protein III in its outer membrane. J Exp Med. 1989 Jun 1;169(6):2199–2209. doi: 10.1084/jem.169.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D. B., Morse S. A. Physiology and metabolism of pathogenic Neisseria: partial characterization of the respiratory chain of Neisseria gonorrhoeae. J Bacteriol. 1975 Aug;123(2):631–636. doi: 10.1128/jb.123.2.631-636.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. P., Shockley R. K., Johnston K. H. WSJM, a simple chemically defined medium for growth of Neisseria gonorrhoeae. J Clin Microbiol. 1980 Apr;11(4):363–369. doi: 10.1128/jcm.11.4.363-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]