Abstract
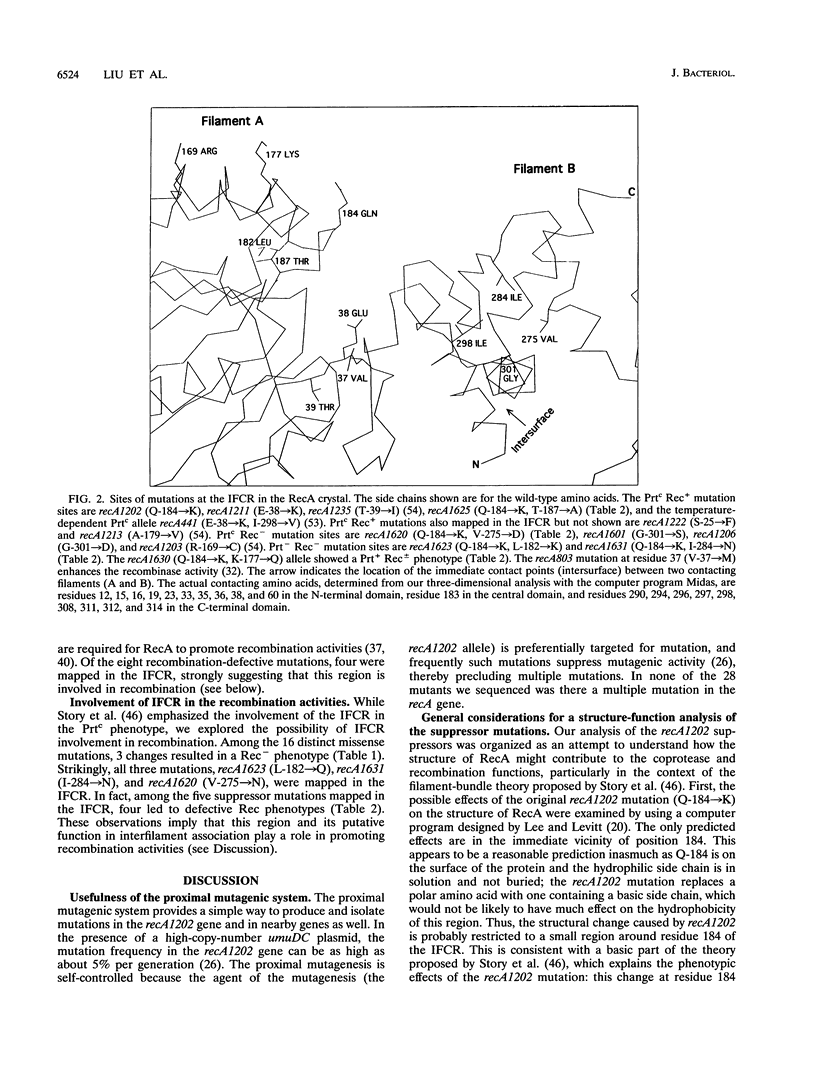
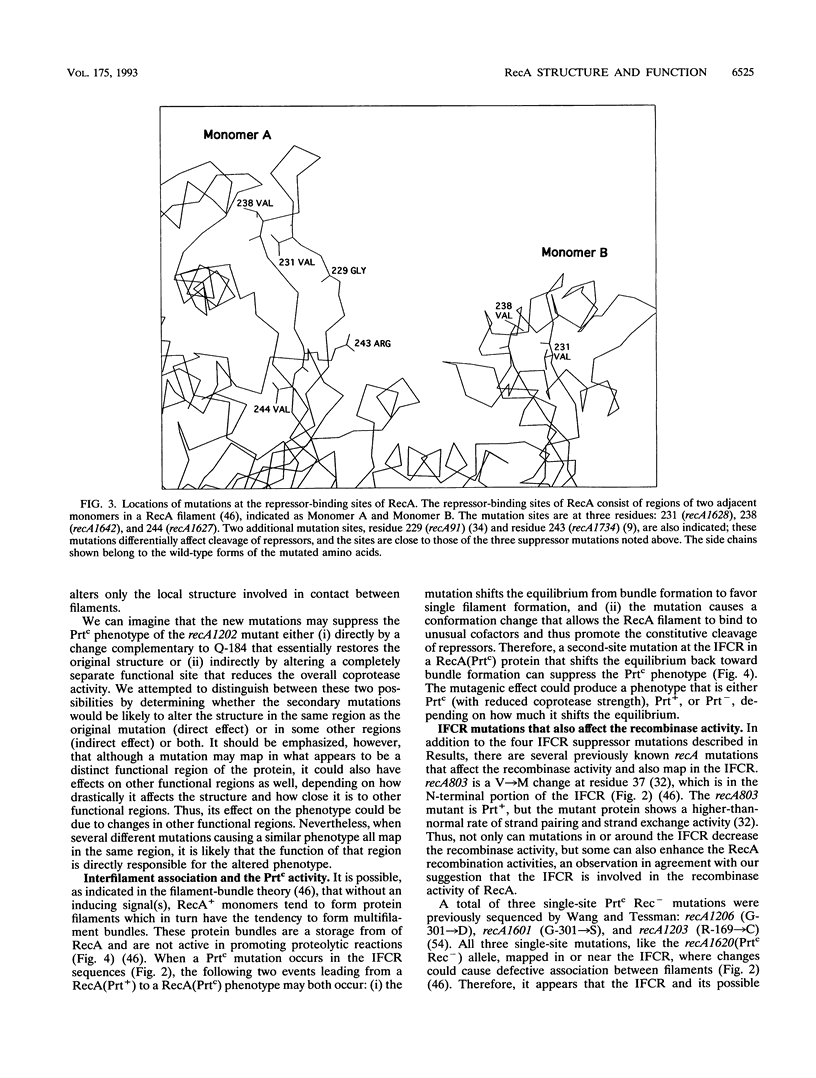
Twenty-eight recA mutants, isolated after spontaneous mutagenesis generated by the combined action of RecA1202(Prtc) and UmuDC proteins, were characterized and sequenced. The mutations are intragenic suppressors of the recA1202 allele and were detected by the reduced coprotease activity of the gene product. Twenty distinct mutation sites were found, among which two mutations, recA1620 (V-275-->D) and recA1631 (I-284-->N), were mapped in the C-terminal portion of the interfilament contact region (IFCR) in the RecA crystal. An interaction of this region with the part of the IFCR in which the recA1202 mutation (Q-184-->K) is mapped could occur only intermolecularly. Thus, altered IFCR and the likely resulting change in interfilament association appear to be important aspects of the formation of a constitutively active RecA coprotease. This observation is consistent with the filament-bundle theory (R. M. Story, I. T. Weber, and T. A. Steitz, Nature (London) 335:318-325, 1992). Furthermore, we found that among the 20 suppressor mutations, 3 missense mutations that lead to recombination-defective (Rec-) phenotypes also mapped in the IFCR, suggesting that the IFCR, with its putative function in interfilament association, is required for the recombinase activity of RecA. We propose that RecA-DNA complexes may form bundles analogous to the RecA bundles (lacking DNA) described by Story et al. and that these RecA-DNA bundles play a role in homologous recombination.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Kenyon C. J., Walker G. C. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5749–5753. doi: 10.1073/pnas.78.9.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R. C., Kowalczykowski S. C. Increase of the DNA strand assimilation activity of recA protein by removal of the C terminus and structure-function studies of the resulting protein fragment. J Biol Chem. 1988 Oct 25;263(30):15513–15520. [PubMed] [Google Scholar]
- Brenner S. L., Zlotnick A., Griffith J. D. RecA protein self-assembly. Multiple discrete aggregation states. J Mol Biol. 1988 Dec 20;204(4):959–972. doi: 10.1016/0022-2836(88)90055-1. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. The lexA gene product represses its own promoter. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1932–1936. doi: 10.1073/pnas.77.4.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt S. E., Woodgate R., Scheuermann R. H., Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- Dutreix M., Moreau P. L., Bailone A., Galibert F., Battista J. R., Walker G. C., Devoret R. New recA mutations that dissociate the various RecA protein activities in Escherichia coli provide evidence for an additional role for RecA protein in UV mutagenesis. J Bacteriol. 1989 May;171(5):2415–2423. doi: 10.1128/jb.171.5.2415-2423.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelman E. H., Stasiak A. Structure of helical RecA-DNA complexes. Complexes formed in the presence of ATP-gamma-S or ATP. J Mol Biol. 1986 Oct 20;191(4):677–697. doi: 10.1016/0022-2836(86)90453-5. [DOI] [PubMed] [Google Scholar]
- Egelman E. H., Stasiak A. Structure of helical RecA-DNA complexes. II. Local conformational changes visualized in bundles of RecA-ATP gamma S filaments. J Mol Biol. 1988 Mar 20;200(2):329–349. doi: 10.1016/0022-2836(88)90245-8. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- Gardner R. C., Howarth A. J., Hahn P., Brown-Luedi M., Shepherd R. J., Messing J. The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acids Res. 1981 Jun 25;9(12):2871–2888. doi: 10.1093/nar/9.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda D. K., Radding C. M. The mechanism of the search for homology promoted by recA protein. Facilitated diffusion within nucleoprotein networks. J Biol Chem. 1986 Oct 5;261(28):13087–13096. [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larminat F., Defais M. Modulation of the SOS response by truncated RecA proteins. Mol Gen Genet. 1989 Mar;216(1):106–112. doi: 10.1007/BF00332237. [DOI] [PubMed] [Google Scholar]
- Lee C., Levitt M. Accurate prediction of the stability and activity effects of site-directed mutagenesis on a protein core. Nature. 1991 Aug 1;352(6334):448–451. doi: 10.1038/352448a0. [DOI] [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. Control of the SOS regulatory system by the level of RecA protease. Biochimie. 1982 Aug-Sep;64(8-9):585–589. doi: 10.1016/s0300-9084(82)80092-8. [DOI] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. Mechanism of specific LexA cleavage: autodigestion and the role of RecA coprotease. Biochimie. 1991 Apr;73(4):411–421. doi: 10.1016/0300-9084(91)90108-d. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W., Yanisch-Perron C. R. Purified lexA protein is a repressor of the recA and lexA genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4199–4203. doi: 10.1073/pnas.78.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. K., Tessman I. Mutagenesis by proximity to the recA gene of Escherichia coli. J Mol Biol. 1990 Jan 20;211(2):351–358. doi: 10.1016/0022-2836(90)90356-Q. [DOI] [PubMed] [Google Scholar]
- Liu S. K., Tessman I. groE genes affect SOS repair in Escherichia coli. J Bacteriol. 1990 Oct;172(10):6135–6138. doi: 10.1128/jb.172.10.6135-6138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Echols H. RecA protein and SOS. Correlation of mutagenesis phenotype with binding of mutant RecA proteins to duplex DNA and LexA cleavage. J Mol Biol. 1987 Aug 5;196(3):497–504. doi: 10.1016/0022-2836(87)90027-1. [DOI] [PubMed] [Google Scholar]
- Madiraju M. V., Templin A., Clark A. J. Properties of a mutant recA-encoded protein reveal a possible role for Escherichia coli recF-encoded protein in genetic recombination. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6592–6596. doi: 10.1073/pnas.85.18.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M. tif-1 mutation alters polynucleotide recognition by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6061–6065. doi: 10.1073/pnas.78.10.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi T., Battista J. R., Dodson L. A., Walker G. C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Ogawa T. General recombination: functions and structure of RecA protein. Adv Biophys. 1986;21:135–148. doi: 10.1016/0065-227x(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Peterson K. R., Ossanna N., Thliveris A. T., Ennis D. G., Mount D. W. Derepression of specific genes promotes DNA repair and mutagenesis in Escherichia coli. J Bacteriol. 1988 Jan;170(1):1–4. doi: 10.1128/jb.170.1.1-4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L., Phizicky E. M. Activity of the Escherichia coli recA-gene product. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):917–920. doi: 10.1101/sqb.1979.043.01.100. [DOI] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster H., Beyersmann D., Mikolajczyk M., Schlicht M. Prophage induction by high temperature in thermosensitive dna mutants lysogenic for bacteriophage lambda. J Virol. 1973 Jun;11(6):879–885. doi: 10.1128/jvi.11.6.879-885.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H., Iwasaki H., Kato T., Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slilaty S. N., Little J. W. Lysine-156 and serine-119 are required for LexA repressor cleavage: a possible mechanism. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3987–3991. doi: 10.1073/pnas.84.12.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story R. M., Steitz T. A. Structure of the recA protein-ADP complex. Nature. 1992 Jan 23;355(6358):374–376. doi: 10.1038/355374a0. [DOI] [PubMed] [Google Scholar]
- Story R. M., Weber I. T., Steitz T. A. The structure of the E. coli recA protein monomer and polymer. Nature. 1992 Jan 23;355(6358):318–325. doi: 10.1038/355318a0. [DOI] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. Isolation of protease-proficient, recombinase-deficient recA mutants of Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):688–695. doi: 10.1128/jb.163.2.688-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. Plaque color method for rapid isolation of novel recA mutants of Escherichia coli K-12: new classes of protease-constitutive recA mutants. J Bacteriol. 1985 Aug;163(2):677–687. doi: 10.1128/jb.163.2.677-687.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman E. S., Tessman I., Peterson P. K., Forestal J. D. Roles of RecA protease and recombinase activities of Escherichia coli in spontaneous and UV-induced mutagenesis and in Weigle repair. J Bacteriol. 1986 Dec;168(3):1159–1164. doi: 10.1128/jb.168.3.1159-1164.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang S. S., Chow S. A., Radding C. M. Networks of DNA and RecA protein are intermediates in homologous pairing. Biochemistry. 1985 Jun 18;24(13):3226–3232. doi: 10.1021/bi00334a023. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. B., Sassanfar M., Tessman I., Roberts J. W., Tessman E. S. Activation of protease-constitutive recA proteins of Escherichia coli by all of the common nucleoside triphosphates. J Bacteriol. 1988 Oct;170(10):4816–4822. doi: 10.1128/jb.170.10.4816-4822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. B., Tessman E. S. Evidence that the recA441 (tif-1) mutant of Escherichia coli K-12 contains a thermosensitive intragenic suppressor of RecA constitutive protease activity. J Bacteriol. 1985 Jul;163(1):407–409. doi: 10.1128/jb.163.1.407-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. B., Tessman E. S. Location of functional regions of the Escherichia coli RecA protein by DNA sequence analysis of RecA protease-constitutive mutants. J Bacteriol. 1986 Nov;168(2):901–910. doi: 10.1128/jb.168.2.901-910.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. B., Tessman E. S., Tessman I. Activation of protease-constitutive recA proteins of Escherichia coli by rRNA and tRNA. J Bacteriol. 1988 Oct;170(10):4823–4827. doi: 10.1128/jb.170.10.4823-4827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C. Protein-DNA interactions in genetic recombination. Trends Genet. 1988 Jan;4(1):8–13. doi: 10.1016/0168-9525(88)90121-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. H., Benight A. S. Kinetic analysis of the pre-equilibrium steps in the self-assembly of RecA protein from Escherichia coli. J Biol Chem. 1990 May 5;265(13):7351–7359. [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Egelman E. H. Removal of the RecA C-terminus results in a conformational change in the RecA-DNA filament. J Struct Biol. 1991 Jun;106(3):243–254. doi: 10.1016/1047-8477(91)90074-7. [DOI] [PubMed] [Google Scholar]
- Yu X., Egelman E. H. The LexA repressor binds within the deep helical groove of the activated RecA filament. J Mol Biol. 1993 May 5;231(1):29–40. doi: 10.1006/jmbi.1993.1254. [DOI] [PubMed] [Google Scholar]



