Abstract
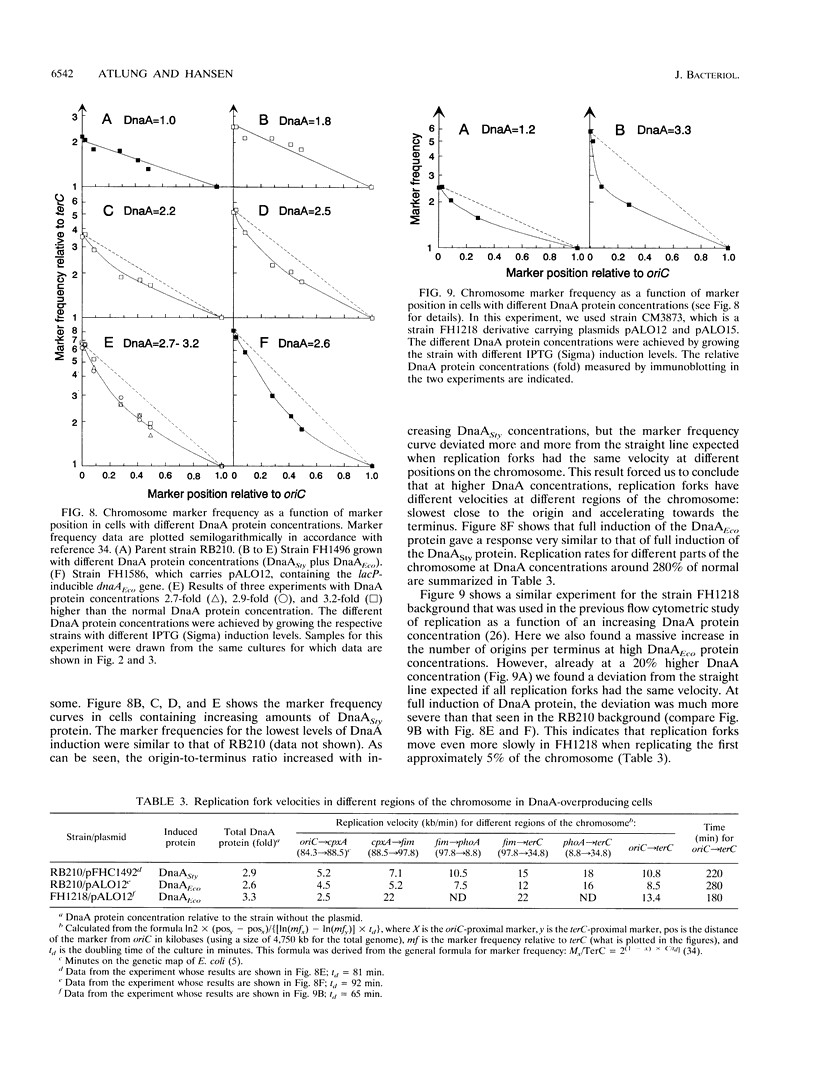
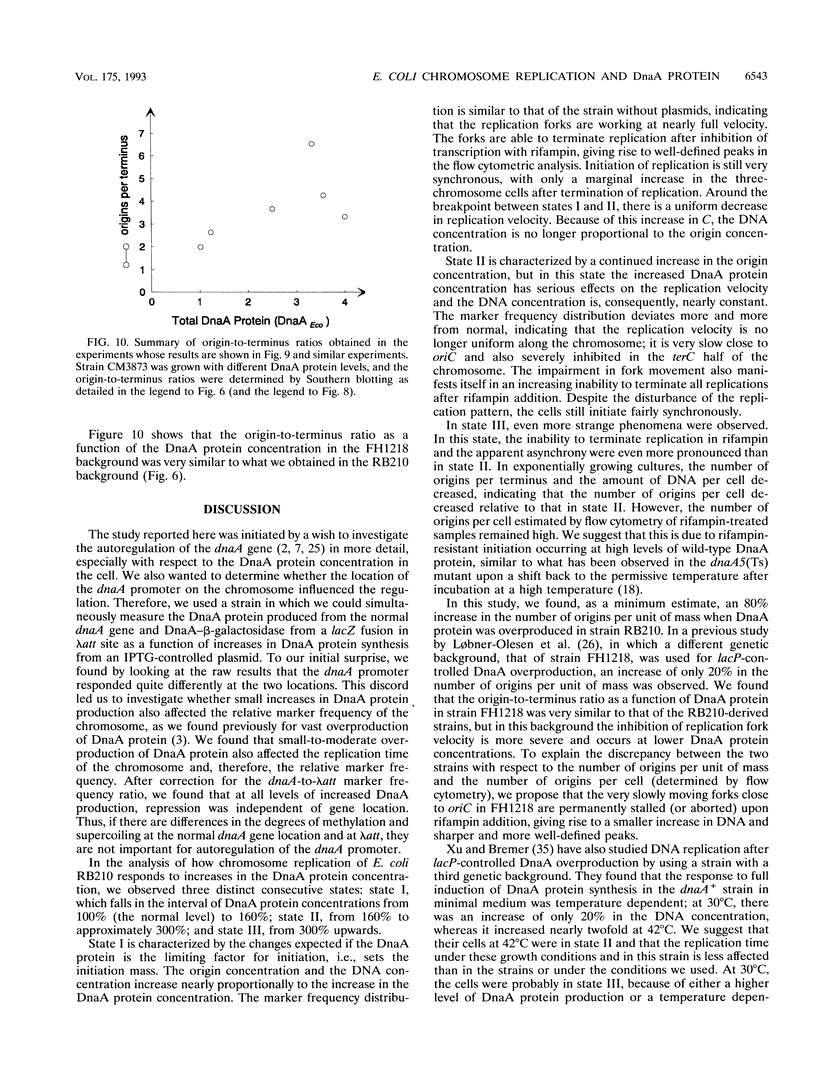
The DnaA protein concentration in Escherichia coli was increased above the wild-type level by inducing a lacP-controlled dnaA gene located on a plasmid. In these cells with different DnaA protein levels, we measured several parameters: dnaA gene expression; cell size, amount of DNA per cell, and number of origins per cell by flow cytometry; and origin-to-terminus ratio and the frequencies of five other markers on the chromosome by Southern hybridization. The response of the cells to higher levels of DnaA protein could be divided into three states. From the normal level to a level 1.5-fold higher, DnaA protein had little effect on dnaA gene expression and the rate of DNA replication but led to nearly proportional increases in DNA and origin concentrations. Between 1.5- and 3-fold, the normal DnaA protein concentration, dnaA gene expression was gradually decreased. In this interval, the origin concentration increased significantly; however, the replication rate was severely affected, becoming slower--especially near the origin--the higher the DnaA protein concentration, and as a result, the DNA concentration was constant. Further increases in the DnaA protein concentration did not lead to an increased origin concentration. Thus, the initiation mass was set by the DnaA protein from the normal level to an at least twofold-increased level, but the increased initiation did not lead to a large increase in the amount of DNA per unit of mass because of the inhibition of replication fork velocity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrup L., Atlung T., Ogasawara N., Yoshikawa H., Hansen F. G. Interaction of the Bacillus subtilis DnaA-like protein with the Escherichia coli DnaA protein. J Bacteriol. 1988 Mar;170(3):1333–1338. doi: 10.1128/jb.170.3.1333-1338.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlung T., Clausen E. S., Hansen F. G. Autoregulation of the dnaA gene of Escherichia coli K12. Mol Gen Genet. 1985;200(3):442–450. doi: 10.1007/BF00425729. [DOI] [PubMed] [Google Scholar]
- Atlung T., Løbner-Olesen A., Hansen F. G. Overproduction of DnaA protein stimulates initiation of chromosome and minichromosome replication in Escherichia coli. Mol Gen Genet. 1987 Jan;206(1):51–59. doi: 10.1007/BF00326535. [DOI] [PubMed] [Google Scholar]
- Atlung T., Nielsen A., Rasmussen L. J., Nellemann L. J., Holm F. A versatile method for integration of genes and gene fusions into the lambda attachment site of Escherichia coli. Gene. 1991 Oct 30;107(1):11–17. doi: 10.1016/0378-1119(91)90291-i. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech F. W., Jørgensen S. T., Diderichsen B., Karlström O. H. Sequence of the relB transcription unit from Escherichia coli and identification of the relB gene. EMBO J. 1985 Apr;4(4):1059–1066. doi: 10.1002/j.1460-2075.1985.tb03739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. E., O'Day K., Wright A. Autoregulation of the DNA replication gene dnaA in E. coli K-12. Cell. 1985 Jan;40(1):159–169. doi: 10.1016/0092-8674(85)90319-8. [DOI] [PubMed] [Google Scholar]
- Braun R. E., Wright A. DNA methylation differentially enhances the expression of one of the two E. coli dnaA promoters in vivo and in vitro. Mol Gen Genet. 1986 Feb;202(2):246–250. doi: 10.1007/BF00331644. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990 Sep 7;62(5):967–979. doi: 10.1016/0092-8674(90)90271-f. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Churchward G., Holmans P., Bremer H. Increased expression of the dnaA gene has no effect on DNA replication in a dnaA+ strain of Escherichia coli. Mol Gen Genet. 1983;192(3):506–508. doi: 10.1007/BF00392197. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Roll J. The requirement of IHF protein for extrachromosomal replication of the Escherichia coli oriC in a mutant deficient in DNA polymerase I activity. New Biol. 1990 Sep;2(9):818–827. [PubMed] [Google Scholar]
- Filutowicz M., Ross W., Wild J., Gourse R. L. Involvement of Fis protein in replication of the Escherichia coli chromosome. J Bacteriol. 1992 Jan;174(2):398–407. doi: 10.1128/jb.174.2.398-407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA region of Micrococcus luteus: conservation and variations among eubacteria. Gene. 1990 Sep 1;93(1):73–78. doi: 10.1016/0378-1119(90)90138-h. [DOI] [PubMed] [Google Scholar]
- Gille H., Egan J. B., Roth A., Messer W. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli. Nucleic Acids Res. 1991 Aug 11;19(15):4167–4172. doi: 10.1093/nar/19.15.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimberg J., Maguire S., Belluscio L. A simple method for the preparation of plasmid and chromosomal E. coli DNA. Nucleic Acids Res. 1989 Nov 11;17(21):8893–8893. doi: 10.1093/nar/17.21.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M. H., Carl P. L. Reinitiation of deoxyribonucleic acid synthesis by deoxyribonucleic acid initiation mutants of Escherichia coli: role of ribonucleic acid synthesis, protein synthesis, and cell division. J Bacteriol. 1975 Jan;121(1):219–226. doi: 10.1128/jb.121.1.219-226.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Atlung T., Braun R. E., Wright A., Hughes P., Kohiyama M. Initiator (DnaA) protein concentration as a function of growth rate in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1991 Aug;173(16):5194–5199. doi: 10.1128/jb.173.16.5194-5199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Christensen B. B., Atlung T. The initiator titration model: computer simulation of chromosome and minichromosome control. Res Microbiol. 1991 Feb-Apr;142(2-3):161–167. doi: 10.1016/0923-2508(91)90025-6. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., Koefoed S., Atlung T. Cloning and nucleotide sequence determination of twelve mutant dnaA genes of Escherichia coli. Mol Gen Genet. 1992 Jul;234(1):14–21. doi: 10.1007/BF00272340. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., Rasmussen K. V. Regulation of the dnaA product in Escherichia coli. Mol Gen Genet. 1977 Oct 20;155(2):219–225. doi: 10.1007/BF00393163. [DOI] [PubMed] [Google Scholar]
- Kücherer C., Lother H., Kölling R., Schauzu M. A., Messer W. Regulation of transcription of the chromosomal dnaA gene of Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):115–121. doi: 10.1007/BF02428040. [DOI] [PubMed] [Google Scholar]
- Løbner-Olesen A., Skarstad K., Hansen F. G., von Meyenburg K., Boye E. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell. 1989 Jun 2;57(5):881–889. doi: 10.1016/0092-8674(89)90802-7. [DOI] [PubMed] [Google Scholar]
- Mahaffy J. M., Zyskind J. W. A model for the initiation of replication in Escherichia coli. J Theor Biol. 1989 Oct 23;140(4):453–477. doi: 10.1016/s0022-5193(89)80109-2. [DOI] [PubMed] [Google Scholar]
- Nellemann L. J., Holm F., Atlung T., Hansen F. G. Cloning and characterization of the Escherichia coli phosphoglycerate kinase (pgk) gene. Gene. 1989 Apr 15;77(1):185–191. doi: 10.1016/0378-1119(89)90373-9. [DOI] [PubMed] [Google Scholar]
- Pato M. L. Alterations of deoxyribonucleoside triphosphate pools in Escherichia coli: effects on deoxyribonucleic acid replication and evidence for compartmentation. J Bacteriol. 1979 Nov;140(2):518–524. doi: 10.1128/jb.140.2.518-524.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaczek P. Bending of the origin of replication of E. coli by binding of IHF at a specific site. New Biol. 1990 Mar;2(3):265–271. [PubMed] [Google Scholar]
- Skovgaard O., Hansen F. G. Comparison of dnaA nucleotide sequences of Escherichia coli, Salmonella typhimurium, and Serratia marcescens. J Bacteriol. 1987 Sep;169(9):3976–3981. doi: 10.1128/jb.169.9.3976-3981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. C., Bremer H. Chromosome replication in Escherichia coli induced by oversupply of DnaA. Mol Gen Genet. 1988 Jan;211(1):138–142. doi: 10.1007/BF00338404. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]