Abstract
A hydrolase constitutively expressed in Pseudomonas aeruginosa which converts carbaryl to 1-naphthol was purified 1,767-fold by using a combination of anion-exchange, hydroxylapatite, gel filtration, and hydrophobic interaction chromatography techniques. The presence of Triton X-100 in buffers was necessary for deaggregation and purification of the hydrolase. This is the first membrane-bound hydrolase involved in the hydrolysis of any methylcarbamate pesticide purified from a bacterial source to date. The enzyme exhibited a unique specificity of hydrolyzing only carbaryl (1-naphthyl N-methylcarbamate) but not any other methylcarbamates. The purified enzyme was a monomer with a molecular mass of 65,000 Da. The pH and temperature optima for the enzyme activity were 8.5 and 45 degrees C, respectively. No cofactor requirement for the hydrolase activity could be demonstrated, and none of the divalent cations studied affected the activity of the enzyme. Also, the enzyme activity was not affected by the thiols: dithioerythritol, dithiothreitol, and 2-mercaptoethanol. The Km and Vmax values for carbaryl were 9 microM and 7.9 mumol/min/mg of protein, respectively.
Full text
PDF

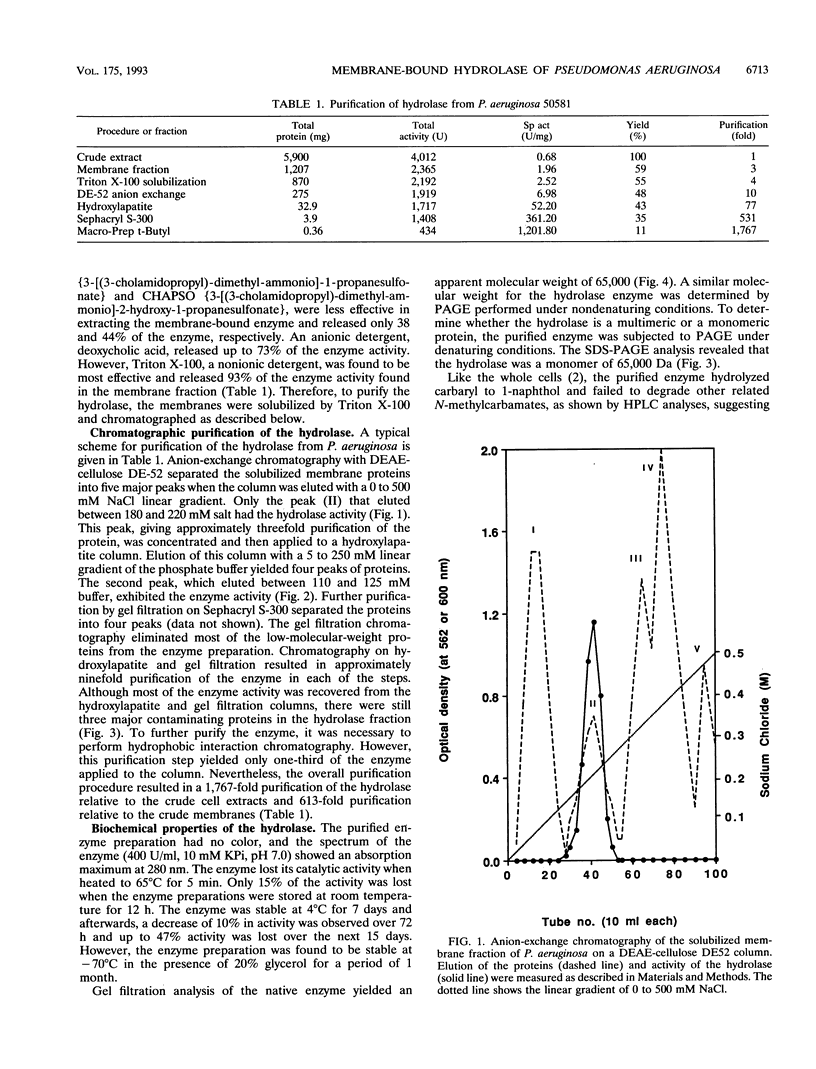
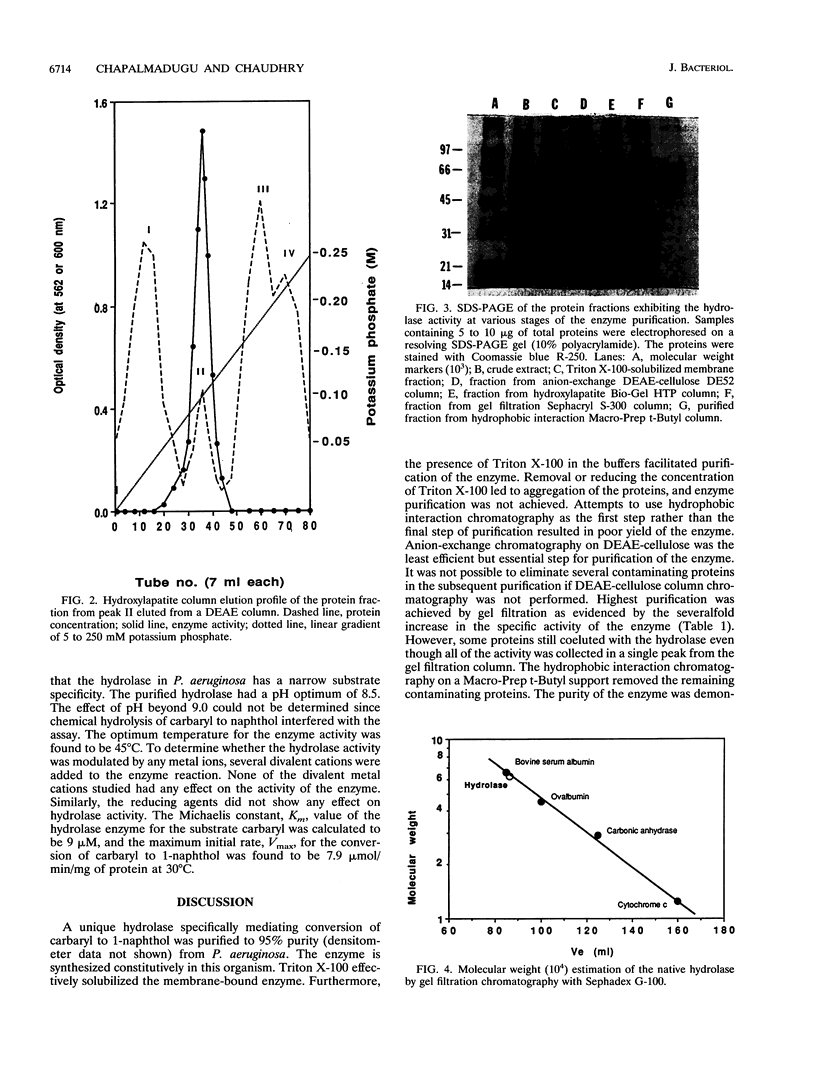


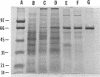
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapalamadugu S., Chaudhry G. R. Hydrolysis of carbaryl by a Pseudomonas sp. and construction of a microbial consortium that completely metabolizes carbaryl. Appl Environ Microbiol. 1991 Mar;57(3):744–750. doi: 10.1128/aem.57.3.744-750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapalamadugu S., Chaudhry G. R. Microbiological and biotechnological aspects of metabolism of carbamates and organophosphates. Crit Rev Biotechnol. 1992;12(5-6):357–389. doi: 10.3109/07388559209114232. [DOI] [PubMed] [Google Scholar]
- Chaudhry G. R., Ali A. N. Bacterial metabolism of carbofuran. Appl Environ Microbiol. 1988 Jun;54(6):1414–1419. doi: 10.1128/aem.54.6.1414-1419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., MacGregor C. H. Cytochrome b from Escherichia coli nitrate reductase. Its properties and association with the enzyme complex. J Biol Chem. 1983 May 10;258(9):5819–5827. [PubMed] [Google Scholar]
- Dumas D. P., Caldwell S. R., Wild J. R., Raushel F. M. Purification and properties of the phosphotriesterase from Pseudomonas diminuta. J Biol Chem. 1989 Nov 25;264(33):19659–19665. [PubMed] [Google Scholar]
- Goldstein R. M., Mallory L. M., Alexander M. Reasons for possible failure of inoculation to enhance biodegradation. Appl Environ Microbiol. 1985 Oct;50(4):977–983. doi: 10.1128/aem.50.4.977-983.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. M., Talbot H. W., Jr Detoxification of pesticides by microbial enzymes. Experientia. 1983 Nov 15;39(11):1236–1246. doi: 10.1007/BF01990361. [DOI] [PubMed] [Google Scholar]
- Klibanov A. M., Tu T. M., Scott K. P. Peroxidase-catalyzed removal of phenols from coal-conversion waste waters. Science. 1983 Jul 15;221(4607):259–261. doi: 10.1126/science.221.4607.259-a. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mulbry W. W., Eaton R. W. Purification and characterization of the N-methylcarbamate hydrolase from Pseudomonas strain CRL-OK. Appl Environ Microbiol. 1991 Dec;57(12):3679–3682. doi: 10.1128/aem.57.12.3679-3682.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulbry W. W., Karns J. S. Purification and characterization of three parathion hydrolases from gram-negative bacterial strains. Appl Environ Microbiol. 1989 Feb;55(2):289–293. doi: 10.1128/aem.55.2.289-293.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnecke D. M. Enzymatic hydrolysis of organophosphate insecticides, a possible pesticide disposal method. Appl Environ Microbiol. 1976 Jul;32(1):7–13. doi: 10.1128/aem.32.1.7-13.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlenz H. D., Boidol W., Schüttke I., Streber W. R. Purification and properties of an Arthrobacter oxydans P52 carbamate hydrolase specific for the herbicide phenmedipham and nucleotide sequence of the corresponding gene. J Bacteriol. 1992 Oct;174(20):6600–6607. doi: 10.1128/jb.174.20.6600-6607.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]