Abstract
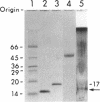
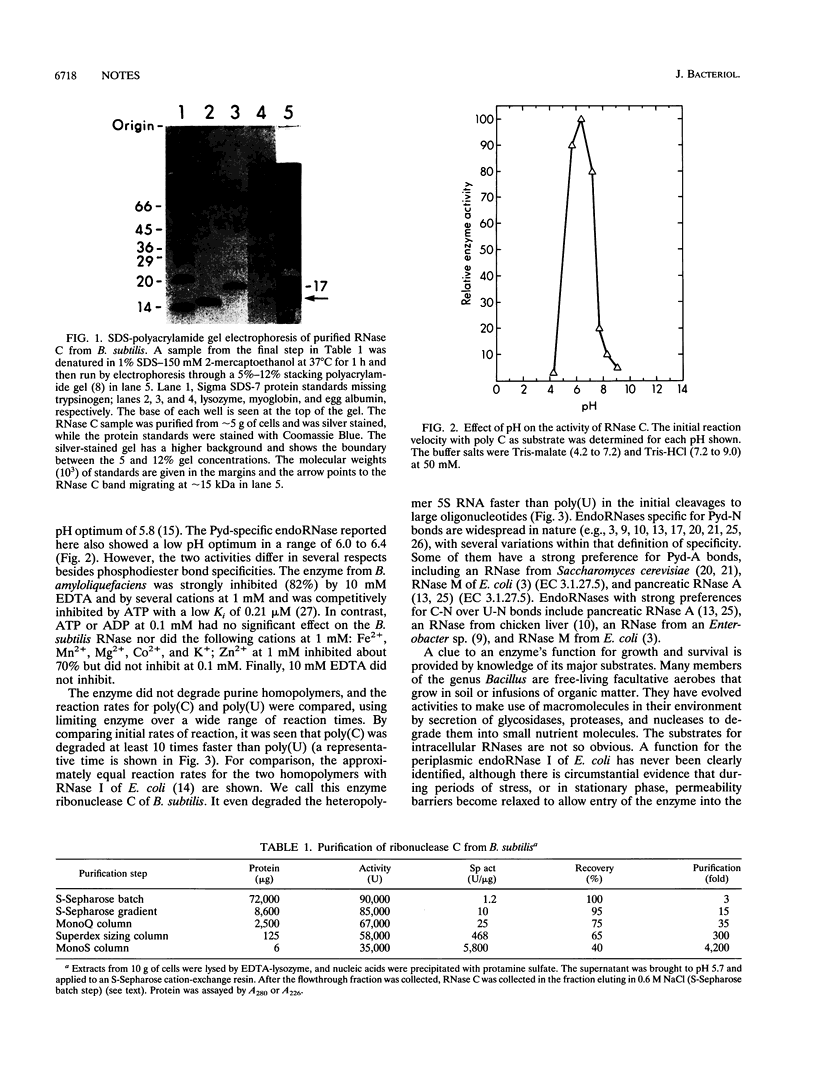
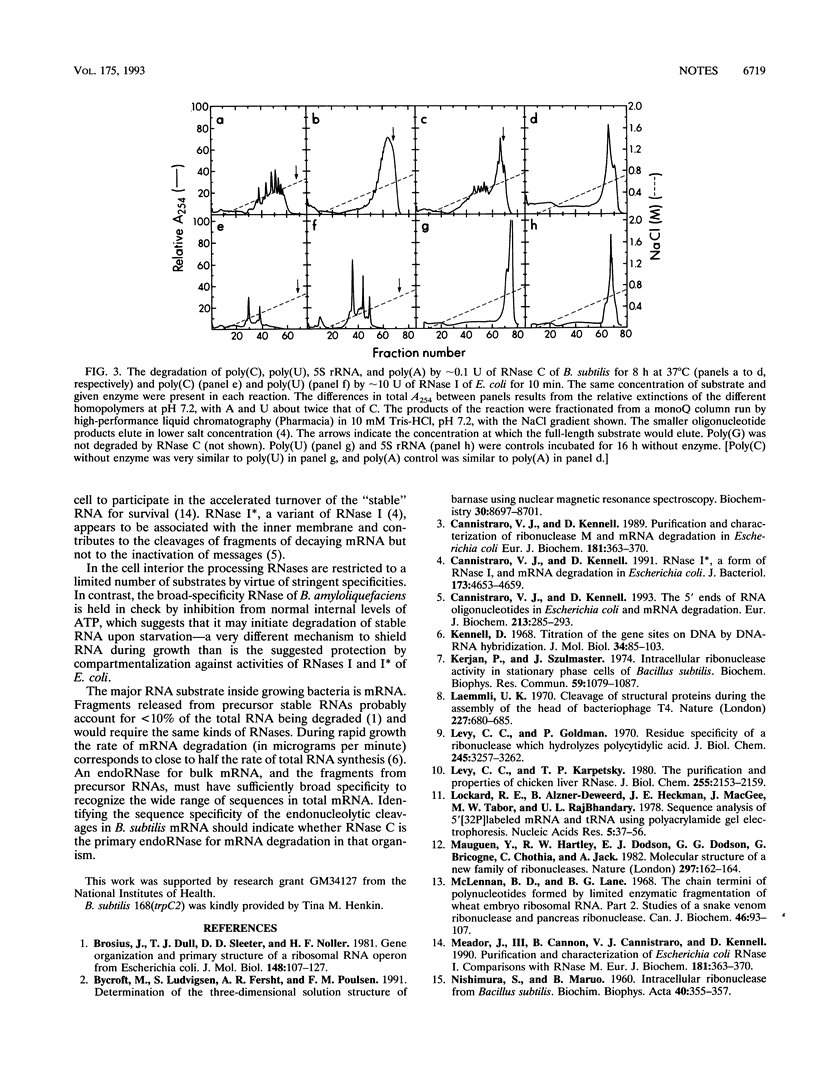
Two intracellular RNases which were easily separated by fractionation on strong anion- or cation-exchange resins were identified from Bacillus subtilis. One cleaved any phosphodiester bond, while the second cleaved only pyrimidine-N bonds. The enzyme with pyrimidine-N specificity was approximately 15 kDa, had a pH optimum of approximately 6.2, degraded C-C bonds approximately 10 times faster than U-U bonds, and was completely inactive against single-stranded DNA. The enzyme is called RNase C and may be the first reported broad-specificity endoribonuclease from B. subtilis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Bycroft M., Ludvigsen S., Fersht A. R., Poulsen F. M. Determination of the three-dimensional solution structure of barnase using nuclear magnetic resonance spectroscopy. Biochemistry. 1991 Sep 3;30(35):8697–8701. doi: 10.1021/bi00099a030. [DOI] [PubMed] [Google Scholar]
- Cannistraro V. J., Kennell D. Purification and characterization of ribonuclease M and mRNA degradation in Escherichia coli. Eur J Biochem. 1989 May 1;181(2):363–370. doi: 10.1111/j.1432-1033.1989.tb14733.x. [DOI] [PubMed] [Google Scholar]
- Cannistraro V. J., Kennell D. RNase I*, a form of RNase I, and mRNA degradation in Escherichia coli. J Bacteriol. 1991 Aug;173(15):4653–4659. doi: 10.1128/jb.173.15.4653-4659.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro V. J., Kennell D. The 5' ends of RNA oligonucleotides in Escherichia coli and mRNA degradation. Eur J Biochem. 1993 Apr 1;213(1):285–293. doi: 10.1111/j.1432-1033.1993.tb17761.x. [DOI] [PubMed] [Google Scholar]
- Kennel D. Titration of the gene sites on DNA by DNA-RNA hybridization. II. The Escherichia coli chromosome. J Mol Biol. 1968 May 28;34(1):85–103. doi: 10.1016/0022-2836(68)90236-2. [DOI] [PubMed] [Google Scholar]
- Kerjan P., Szulmajster J. Intracellular ribonuclease activity in stationary phase cells of Bacillus subtilis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1079–1087. doi: 10.1016/s0006-291x(74)80089-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy C. C., Goldman P. Residue specificity of a ribonuclease which hydrolyzes polycytidylic acid. J Biol Chem. 1970 Jun;245(12):3257–3262. [PubMed] [Google Scholar]
- Levy C. C., Karpetsky T. P. The purification and properties of chicken liver RNase: An enzyme which is useful in distinguishing between cytidylic and uridylic acid residues. J Biol Chem. 1980 Mar 10;255(5):2153–2159. [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauguen Y., Hartley R. W., Dodson E. J., Dodson G. G., Bricogne G., Chothia C., Jack A. Molecular structure of a new family of ribonucleases. Nature. 1982 May 13;297(5862):162–164. doi: 10.1038/297162a0. [DOI] [PubMed] [Google Scholar]
- McLennan B. D., Lane B. G. The chain termini of polynucleotides formed by limited enzymic fragmentation of wheat embryo ribosomal RNA. 2. Studies of a snake venom ribonuclease and pancreas ribonuclease. Can J Biochem. 1968 Jan;46(1):93–107. doi: 10.1139/o68-014. [DOI] [PubMed] [Google Scholar]
- NISHIMURA S., MARUO B. Intracellular ribonuclease from Bacillus subtilis. Biochim Biophys Acta. 1960 May 20;40:355–357. doi: 10.1016/0006-3002(60)91364-0. [DOI] [PubMed] [Google Scholar]
- Ostrowski W., Walczak Z., Stasiuk L., Laskowski M., Sr Properties and specificity of ribonuclease IIA from Thiobacillus thioparus. Acta Biochim Pol. 1970;17(2):73–87. [PubMed] [Google Scholar]
- Paddon C. J., Hartley R. W. Cloning, sequencing and transcription of an inactivated copy of Bacillus amyloliquefaciens extracellular ribonuclease (barnase). Gene. 1985;40(2-3):231–239. doi: 10.1016/0378-1119(85)90045-9. [DOI] [PubMed] [Google Scholar]
- Podder S. K. Synthetic action of ribonuclease T1. Biochim Biophys Acta. 1970;209(2):455–462. doi: 10.1016/0005-2787(70)90742-2. [DOI] [PubMed] [Google Scholar]
- RUSHIZKY G. W., GRECO A. E., HARTLEY R. W., Jr, SOBER H. A. STUDIES ON B. SUBTILIS RIBONUCLEASE. I. CHARACTERIZATION OF ENZYMATIC SPECIFICITY. Biochemistry. 1963 Jul-Aug;2:787–793. doi: 10.1021/bi00904a028. [DOI] [PubMed] [Google Scholar]
- Stevens A. Novel specificity of an endoribonuclease of yeast. FEBS Lett. 1986 Sep 15;205(2):210–214. doi: 10.1016/0014-5793(86)80899-7. [DOI] [PubMed] [Google Scholar]
- Stevens A. Pyrimidine-specific cleavage by an endoribonuclease of Saccharomyces cerevisiae. J Bacteriol. 1985 Oct;164(1):57–62. doi: 10.1128/jb.164.1.57-62.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi M., Yamasaki M., Mizukami T., Tamura G. ATP-sensitive ribonuclease of Bacillus cereus. J Biol Chem. 1980 Feb 10;255(3):943–948. [PubMed] [Google Scholar]
- WITZEL H., BARNARD E. A. Mechanism and binding sites in the ribonuclease reaction. II. Kinetic studies on the first step of the reaction. Biochem Biophys Res Commun. 1962 May 4;7:295–299. doi: 10.1016/0006-291x(62)90194-8. [DOI] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Comparison of the alpha-amylase of Bacillus subtilis and Bacillus amyloliquefaciens. J Bacteriol. 1967 Oct;94(4):1131–1135. doi: 10.1128/jb.94.4.1131-1135.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J Bacteriol. 1967 Oct;94(4):1124–1130. doi: 10.1128/jb.94.4.1124-1130.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroof E. A., Glitz D. G. Purification and characterization of two extracellular ribonucleases from Rhizopus oligosporus. Biochemistry. 1971 Apr 27;10(9):1532–1540. doi: 10.1021/bi00785a004. [DOI] [PubMed] [Google Scholar]
- Yamasaki M., Arima K. ATP-inhibited ribonuclease of Bacillus subtilis. II. Effect of adenosine triphosphate and other compounds. Biochim Biophys Acta. 1970;209(2):475–483. doi: 10.1016/0005-2787(70)90744-6. [DOI] [PubMed] [Google Scholar]
- Yamasaki M., Arima K. Intracellular ribonuclease of Bacillus subtilis; specific inhibition by ATP and dATP. Biochem Biophys Res Commun. 1969 Oct 22;37(3):430–436. doi: 10.1016/0006-291x(69)90933-4. [DOI] [PubMed] [Google Scholar]