Abstract
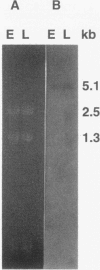
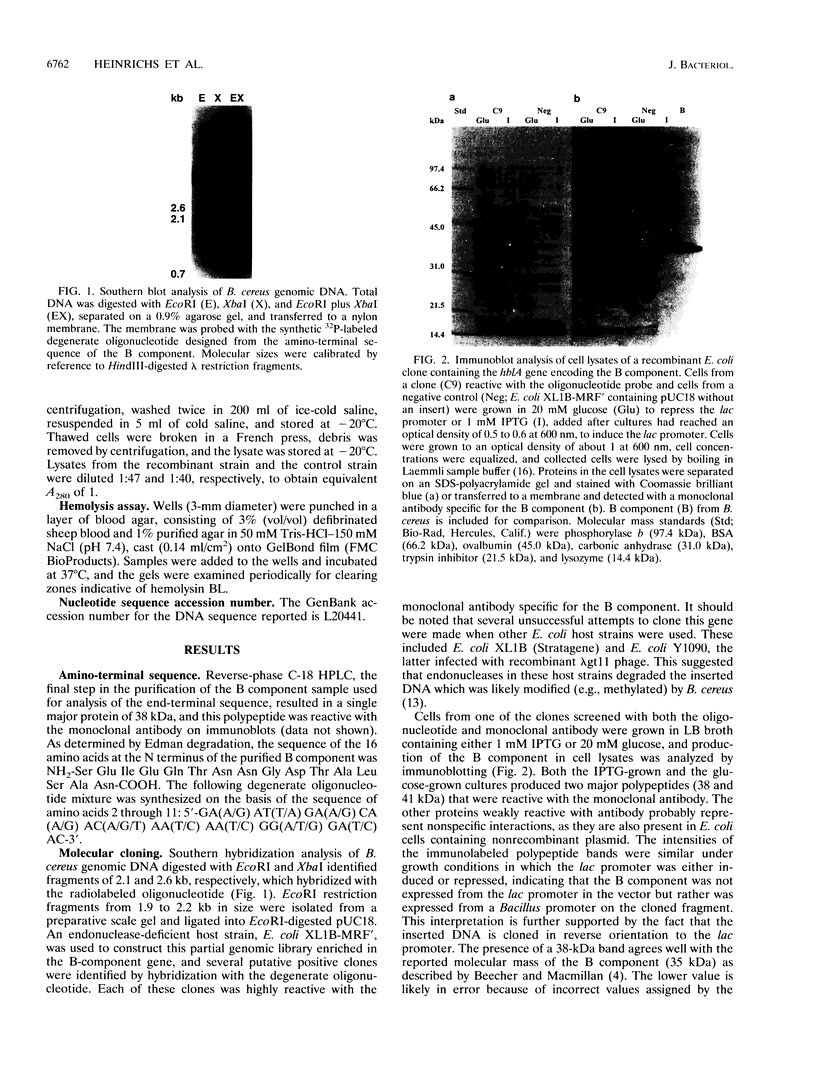
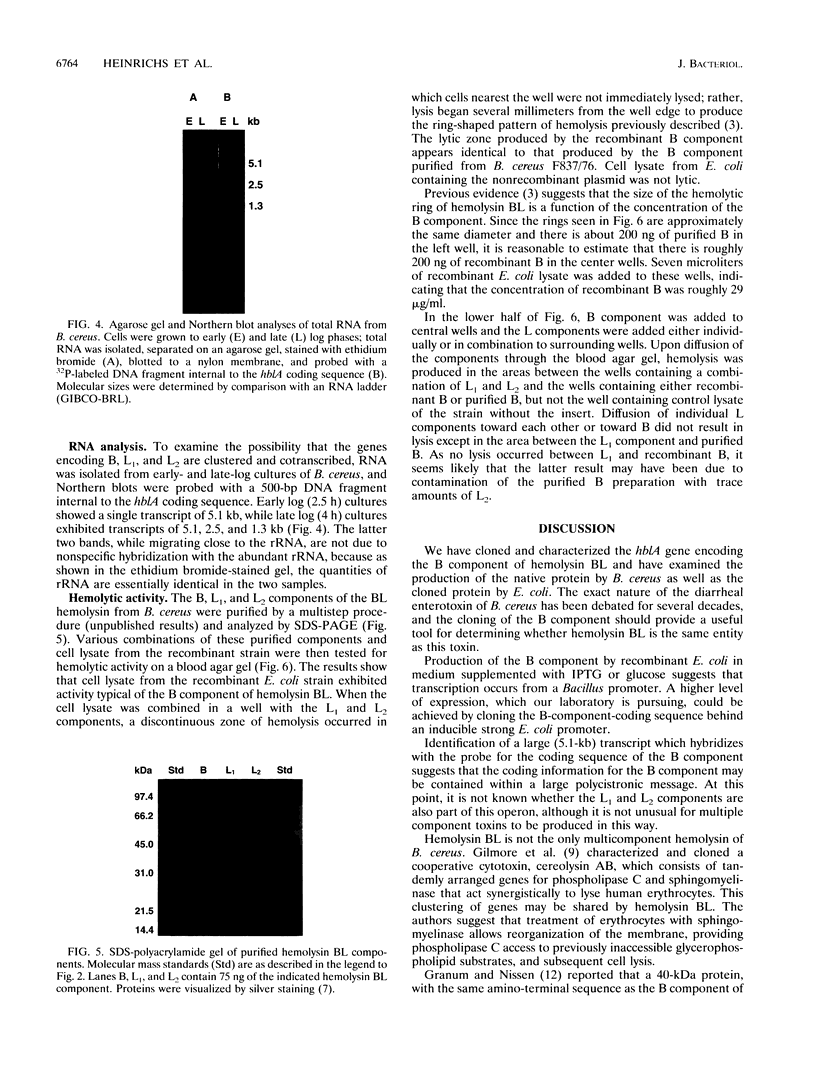
Previous evidence suggests that hemolysin BL, which consists of a binding component, B, and two lytic components, L1 and L2, is the enterotoxin responsible for the diarrheal form of gastroenteritis caused by food-borne strains of Bacillus cereus. To prove that hemolysin BL and the enterotoxin are the same requires large amounts of these components free of other B. cereus proteins. For this purpose, we sought to clone the gene encoding the B component and to express it in Escherichia coli. A partial genomic library was constructed and a 29-base, 1,152-fold-degenerate oligonucleotide probe, designed from the N-terminal amino acid sequence of the B component, was used to identify recombinant clones containing the gene. Detection of gene products reactive with a monoclonal antibody specific for the B component and analysis of the nucleotide sequence confirmed that isolated clones, reactive with the oligonucleotide probe, did contain the gene encoding the B component. The protein, expressed in E. coli, apparently from the B. cereus promoter, produces a ring-shaped zone of hemolysis when combined with purified L components from B. cereus, a reaction typical of hemolysin BL. Northern (RNA) blot analysis of B. cereus RNA showed a large (5.1-kb) transcript which hybridized with a 500-bp probe internal to the B-component-coding sequence, suggesting that the hblA gene encoding the B component may be transcribed as part of a polycistronic message, possibly including the structural genes for the two lytic components. Higher levels of expression and disruption of the hblA gene are being pursued to resolve whether hemolysin BL is indeed the enterotoxin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baddour L. M., Gaia S. M., Griffin R., Hudson R. A hospital cafeteria-related food-borne outbreak due to Bacillus cereus: unique features. Infect Control. 1986 Sep;7(9):462–465. doi: 10.1017/s0195941700064961. [DOI] [PubMed] [Google Scholar]
- Beecher D. J., MacMillan J. D. A novel bicomponent hemolysin from Bacillus cereus. Infect Immun. 1990 Jul;58(7):2220–2227. doi: 10.1128/iai.58.7.2220-2227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher D. J., Macmillan J. D. Characterization of the components of hemolysin BL from Bacillus cereus. Infect Immun. 1991 May;59(5):1778–1784. doi: 10.1128/iai.59.5.1778-1784.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergdoll M. S. Ileal loop fluid accumulation test for diarrheal toxins. Methods Enzymol. 1988;165:306–323. doi: 10.1016/s0076-6879(88)65047-6. [DOI] [PubMed] [Google Scholar]
- Bitsaev A. R., Ezepchuk Iu V. Molekuliarnaia priroda patogennogo deistviia, vyzyvaemogo B. cereus. Mol Gen Mikrobiol Virusol. 1987 Jul;(7):18–23. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Gilmore M. S., Cruz-Rodz A. L., Leimeister-Wächter M., Kreft J., Goebel W. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J Bacteriol. 1989 Feb;171(2):744–753. doi: 10.1128/jb.171.2.744-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz B. A., Goepfert J. M. Defined conditions for synthesis of Bacillus cereus enterotoxin by fermenter-grown cultures. Appl Environ Microbiol. 1976 Sep;32(3):400–404. doi: 10.1128/aem.32.3.400-404.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz B. A., Spira W. M., Goepfert J. M. Alteration of vascular permeability in rabbits by culture filtrates of Bacillus cereus and related species. Infect Immun. 1974 Aug;10(2):299–303. doi: 10.1128/iai.10.2.299-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum P. E., Nissen H. Sphingomyelinase is part of the 'enterotoxin complex' produced by Bacillus cereus. FEMS Microbiol Lett. 1993 Jun 1;110(1):97–100. doi: 10.1111/j.1574-6968.1993.tb06301.x. [DOI] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luby S., Jones J., Dowda H., Kramer J., Horan J. A large outbreak of gastroenteritis caused by diarrheal toxin-producing Bacillus cereus. J Infect Dis. 1993 Jun;167(6):1452–1455. doi: 10.1093/infdis/167.6.1452. [DOI] [PubMed] [Google Scholar]
- Philbrick J. B., Diner B. A., Zilinskas B. A. Construction and characterization of cyanobacterial mutants lacking the manganese-stabilizing polypeptide of photosystem II. J Biol Chem. 1991 Jul 15;266(20):13370–13376. [PubMed] [Google Scholar]
- Rabilloud T., Carpentier G., Tarroux P. Improvement and simplification of low-background silver staining of proteins by using sodium dithionite. Electrophoresis. 1988 Jun;9(6):288–291. doi: 10.1002/elps.1150090608. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa K., Sugiyama J., Terada T., Matsusaka N., Sugii S. Improved methods for purification of an enterotoxin produced by Bacillus cereus. FEMS Microbiol Lett. 1991 May 1;64(1):1–5. doi: 10.1016/0378-1097(91)90199-k. [DOI] [PubMed] [Google Scholar]
- Simonen M., Palva I. Protein secretion in Bacillus species. Microbiol Rev. 1993 Mar;57(1):109–137. doi: 10.1128/mr.57.1.109-137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. E., Ketterhagen M. J., Bergdoll M. S., Schantz E. J. Isolation and some properties of an enterotoxin produced by Bacillus cereus. Infect Immun. 1984 Mar;43(3):887–894. doi: 10.1128/iai.43.3.887-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C. Bacillus cereus toxins. Pharmacol Ther. 1981;13(3):453–505. doi: 10.1016/0163-7258(81)90026-7. [DOI] [PubMed] [Google Scholar]
- Turnbull P. C., Kramer J. M., Jørgensen K., Gilbert R. J., Melling J. Properties and production characteristics of vomiting, diarrheal, and necrotizing toxins of Bacillus cereus. Am J Clin Nutr. 1979 Jan;32(1):219–228. doi: 10.1093/ajcn/32.1.219. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]