Abstract
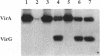
Previous studies have shown that Agrobacterium tumefaciens causes tumors on plants only at temperatures below 32 degrees C, and virulence gene expression is specifically inhibited at temperatures above 32 degrees C. We show here that this effect persists even when the virA and virG loci are expressed under the control of a lac promoter whose activity is temperature independent. This finding suggests that one or more steps in the signal transduction process mediated by the VirA and VirG proteins are temperature sensitive. Both the autophosphorylation of VirA and the subsequent transfer of phosphate to VirG are shown to be sensitive to high temperatures (> 32 degrees C), and this correlates with the reduced vir gene expression observed at these temperatures. At temperatures of 32 degrees C and higher, the VirA molecule undergoes a reversible inactivation while the VirG molecule is not affected. vir gene induction is temperature sensitive in an acetosyringone-independent virA mutant background but not in a virG constitutive mutant which is virA and acetosyringone independent. These observations all support the notion that the VirA protein is responsible for the thermosensitivity of vir gene expression. However, an Agrobacterium strain containing a constitutive virG locus still cannot cause tumors on Kalanchoe plants at 32 degrees C. This strain induces normal-size tumors at temperatures up to 30 degrees C, whereas the wild-type Agrobacterium strain produces almost no tumors at 30 degrees C. These results suggest that at temperatures above 32 degrees C, the plant becomes more resistant to infection by A. tumefaciens and/or functions of some other vir gene products are lost in spite of their normal levels of expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Klee H., Amasino R. M., Nester E. W., Gordon M. P. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbauer R. G., Best E. A., Palanca C. A., Nester E. W. Mutants of the Agrobacterium tumefaciens virA gene exhibiting acetosyringone-independent expression of the vir regulon. Mol Plant Microbe Interact. 1991 Jul-Aug;4(4):400–406. doi: 10.1094/mpmi-4-400. [DOI] [PubMed] [Google Scholar]
- Cangelosi G. A., Ankenbauer R. G., Nester E. W. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6708–6712. doi: 10.1073/pnas.87.17.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Winans S. C. Functional roles assigned to the periplasmic, linker, and receiver domains of the Agrobacterium tumefaciens VirA protein. J Bacteriol. 1992 Nov;174(21):7033–7039. doi: 10.1128/jb.174.21.7033-7039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Davison J., Heusterspreute M., Chevalier N., Ha-Thi V., Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51(2-3):275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. H., Fall M. Z. The loss of tumor-initiating ability in Agrobacterium tumefaciens by incubation at high temperature. Experientia. 1971 Feb 15;27(2):229–230. doi: 10.1007/BF02145913. [DOI] [PubMed] [Google Scholar]
- Han D. C., Chen C. Y., Chen Y. F., Winans S. C. Altered-function mutations of the transcriptional regulatory gene virG of Agrobacterium tumefaciens. J Bacteriol. 1992 Nov;174(21):7040–7043. doi: 10.1128/jb.174.21.7040-7043.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., Schilperoort R. A. Agrobacterium and plant genetic engineering. Plant Mol Biol. 1992 May;19(1):15–38. doi: 10.1007/BF00015604. [DOI] [PubMed] [Google Scholar]
- Huang Y., Morel P., Powell B., Kado C. I. VirA, a coregulator of Ti-specified virulence genes, is phosphorylated in vitro. J Bacteriol. 1990 Feb;172(2):1142–1144. doi: 10.1128/jb.172.2.1142-1144.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. G., Komari T., Gordon M. P., Nester E. W. Genes responsible for the supervirulence phenotype of Agrobacterium tumefaciens A281. J Bacteriol. 1987 Oct;169(10):4417–4425. doi: 10.1128/jb.169.10.4417-4425.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. G., Prusti R. K., Roitsch T., Ankenbauer R. G., Nester E. W. Phosphorylation of the VirG protein of Agrobacterium tumefaciens by the autophosphorylated VirA protein: essential role in biological activity of VirG. J Bacteriol. 1990 Sep;172(9):4945–4950. doi: 10.1128/jb.172.9.4945-4950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. G., Roitsch T., Christie P. J., Nester E. W. The regulatory VirG protein specifically binds to a cis-acting regulatory sequence involved in transcriptional activation of Agrobacterium tumefaciens virulence genes. J Bacteriol. 1990 Feb;172(2):531–537. doi: 10.1128/jb.172.2.531-537.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Roitsch T., Ankenbauer R. G., Gordon M. P., Nester E. W. The VirA protein of Agrobacterium tumefaciens is autophosphorylated and is essential for vir gene regulation. J Bacteriol. 1990 Feb;172(2):525–530. doi: 10.1128/jb.172.2.525-530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Song Y., Pan S. Q., Nester E. W. Characterization of a virG mutation that confers constitutive virulence gene expression in Agrobacterium. Mol Microbiol. 1993 Feb;7(4):555–562. doi: 10.1111/j.1365-2958.1993.tb01146.x. [DOI] [PubMed] [Google Scholar]
- Kanemoto R. H., Powell A. T., Akiyoshi D. E., Regier D. A., Kerstetter R. A., Nester E. W., Hawes M. C., Gordon M. P. Nucleotide sequence and analysis of the plant-inducible locus pinF from Agrobacterium tumefaciens. J Bacteriol. 1989 May;171(5):2506–2512. doi: 10.1128/jb.171.5.2506-2512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Berger D. K., Kustu S. Activity of purified NIFA, a transcriptional activator of nitrogen fixation genes. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2266–2270. doi: 10.1073/pnas.90.6.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux B., Yanofsky M. F., Winans S. C., Ward J. E., Ziegler S. F., Nester E. W. Characterization of the virA locus of Agrobacterium tumefaciens: a transcriptional regulator and host range determinant. EMBO J. 1987 Apr;6(4):849–856. doi: 10.1002/j.1460-2075.1987.tb04830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers L. S., Maroney M. J., den Dulk-Ras A., Thompson D. V., van Vuuren H. A., Schilperoort R. A., Hooykaas P. J. Octopine and nopaline strains of Agrobacterium tumefaciens differ in virulence; molecular characterization of the virF locus. Plant Mol Biol. 1990 Feb;14(2):249–259. doi: 10.1007/BF00018565. [DOI] [PubMed] [Google Scholar]
- Melchers L. S., Regensburg-Tuïnk T. J., Bourret R. B., Sedee N. J., Schilperoort R. A., Hooykaas P. J. Membrane topology and functional analysis of the sensory protein VirA of Agrobacterium tumefaciens. EMBO J. 1989 Jul;8(7):1919–1925. doi: 10.1002/j.1460-2075.1989.tb03595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara T., Lee L., Imae Y. Thermosensing ability of Trg and Tap chemoreceptors in Escherichia coli. J Bacteriol. 1991 Feb;173(3):1120–1124. doi: 10.1128/jb.173.3.1120-1124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ougham H. J., Howarth C. J. Temperature shock proteins in plants. Symp Soc Exp Biol. 1988;42:259–280. [PubMed] [Google Scholar]
- Pazour G. J., Das A. Characterization of the VirG binding site of Agrobacterium tumefaciens. Nucleic Acids Res. 1990 Dec 11;18(23):6909–6913. doi: 10.1093/nar/18.23.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Das A. virG, an Agrobacterium tumefaciens transcriptional activator, initiates translation at a UUG codon and is a sequence-specific DNA-binding protein. J Bacteriol. 1990 Mar;172(3):1241–1249. doi: 10.1128/jb.172.3.1241-1249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Ta C. N., Das A. Constitutive mutations of Agrobacterium tumefaciens transcriptional activator virG. J Bacteriol. 1992 Jun;174(12):4169–4174. doi: 10.1128/jb.174.12.4169-4174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Ta C. N., Das A. Mutants of Agrobacterium tumefaciens with elevated vir gene expression. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6941–6945. doi: 10.1073/pnas.88.16.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986 Jul;5(7):1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986 Aug 1;46(3):325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- Steck T. R., Morel P., Kado C. I. Vir box sequences in Agrobacterium tumefaciens pTiC58 and A6. Nucleic Acids Res. 1988 Sep 12;16(17):8736–8736. doi: 10.1093/nar/16.17.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempé J., Petit A., Holsters M., Montagu M., Schell J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: Possible relation to transformation in crown gall. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Hugly S., Buchholz W. G., Thomashow L. S. Molecular basis for the auxin-independent phenotype of crown gall tumor tissues. Science. 1986 Feb 7;231(4738):616–618. doi: 10.1126/science.3511528. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Ebert P. R., Stachel S. E., Gordon M. P., Nester E. W. A gene essential for Agrobacterium virulence is homologous to a family of positive regulatory loci. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8278–8282. doi: 10.1073/pnas.83.21.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Kerstetter R. A., Nester E. W. Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens. J Bacteriol. 1988 Sep;170(9):4047–4054. doi: 10.1128/jb.170.9.4047-4054.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Kerstetter R. A., Ward J. E., Nester E. W. A protein required for transcriptional regulation of Agrobacterium virulence genes spans the cytoplasmic membrane. J Bacteriol. 1989 Mar;171(3):1616–1622. doi: 10.1128/jb.171.3.1616-1622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C. Two-way chemical signaling in Agrobacterium-plant interactions. Microbiol Rev. 1992 Mar;56(1):12–31. doi: 10.1128/mr.56.1.12-31.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]