Abstract
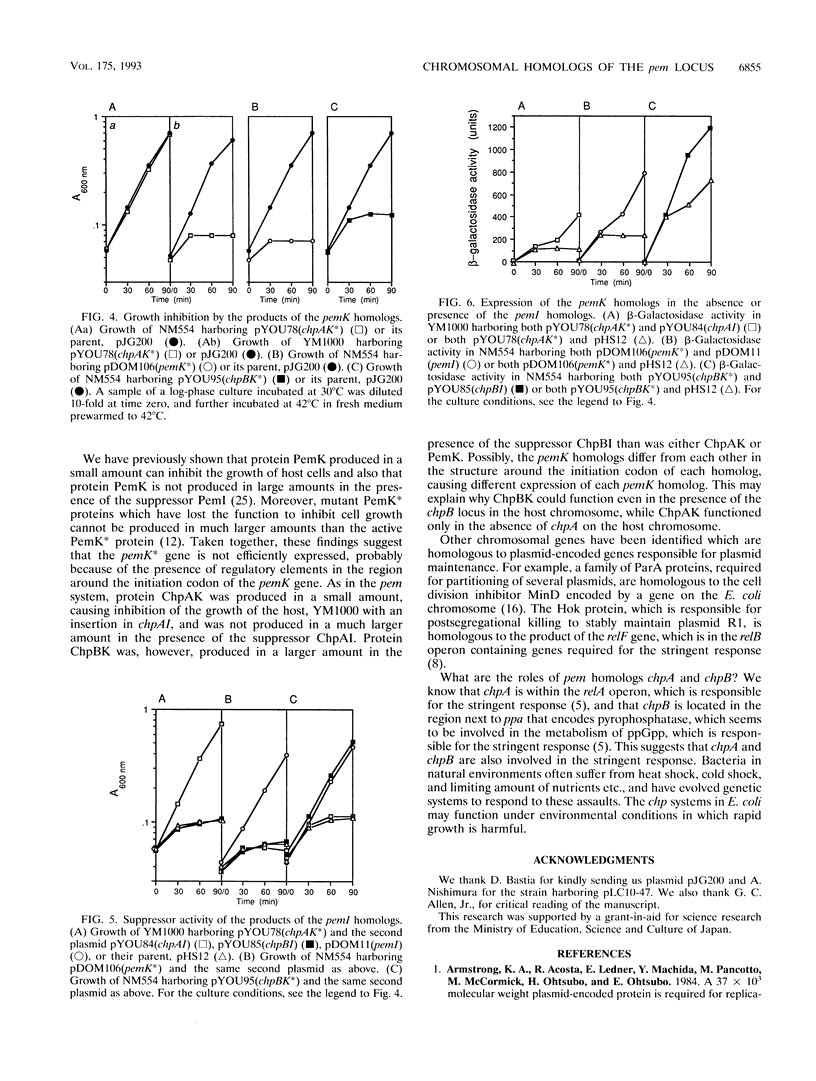
The pem locus is responsible for stable maintenance of plasmid R100 and consists of two genes, pemI and pemK. The pemK gene product is a growth inhibitor, while the pemI gene product is a suppressor of this inhibitory function. We found that the PemI amino acid sequence is homologous to two open reading frames from Escherichia coli called mazE and orf-83, which are located at 60 and 100 min on the chromosome, respectively. We cloned and sequenced these loci and found additional open reading frames, one downstream of each pemI homolog, both of which encode proteins homologous to PemK. The pem locus homolog at 60 min was named chpA and consists of two genes, chpAI and chpAK; the other, at 100 min, was named chpB and consists of two genes, chpBI and chpBK. The distal portion of chpBK was found to be adjacent to the ppa gene that encodes pyrophosphatase, whose map position had not been previously determined. We then demonstrated that the chpAK and chpBK genes encode growth inhibitors, while the chpAI and chpBI genes encode suppressors for the inhibitory function of the ChpAK and ChpBK proteins, respectively. These E. coli pem locus homologs may be involved in regulation of cell growth.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bravo A., de Torrontegui G., Díaz R. Identification of components of a new stability system of plasmid R1, ParD, that is close to the origin of replication of this plasmid. Mol Gen Genet. 1987 Nov;210(1):101–110. doi: 10.1007/BF00337764. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes K., Bech F. W., Jørgensen S. T., Løbner-Olesen A., Rasmussen P. B., Atlung T., Boe L., Karlstrom O., Molin S., von Meyenburg K. Mechanism of postsegregational killing by the hok gene product of the parB system of plasmid R1 and its homology with the relF gene product of the E. coli relB operon. EMBO J. 1986 Aug;5(8):2023–2029. doi: 10.1002/j.1460-2075.1986.tb04459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germino J., Bastia D. Rapid purification of a cloned gene product by genetic fusion and site-specific proteolysis. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4692–4696. doi: 10.1073/pnas.81.15.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Wilde A., Kaderbhai M. A. A simple single-step procedure for small-scale preparation of Escherichia coli plasmids. Nucleic Acids Res. 1990 Mar 25;18(6):1660–1660. doi: 10.1093/nar/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti R., Pitkäranta T., Valve E., Ilta I., Kukko-Kalske E., Heinonen J. Cloning and characterization of the gene encoding inorganic pyrophosphatase of Escherichia coli K-12. J Bacteriol. 1988 Dec;170(12):5901–5907. doi: 10.1128/jb.170.12.5901-5907.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger S., Dror I. B., Aizenman E., Schreiber G., Toone M., Friesen J. D., Cashel M., Glaser G. The nucleotide sequence and characterization of the relA gene of Escherichia coli. J Biol Chem. 1988 Oct 25;263(30):15699–15704. [PubMed] [Google Scholar]
- Motallebi-Veshareh M., Rouch D. A., Thomas C. M. A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol Microbiol. 1990 Sep;4(9):1455–1463. doi: 10.1111/j.1365-2958.1990.tb02056.x. [DOI] [PubMed] [Google Scholar]
- Nishimura A., Akiyama K., Kohara Y., Horiuchi K. Correlation of a subset of the pLC plasmids to the physical map of Escherichia coli K-12. Microbiol Rev. 1992 Mar;56(1):137–151. doi: 10.1128/mr.56.1.137-151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Ryder T. B., Maeda Y., Armstrong K., Ohtsubo E. DNA replication of the resistance plasmid R100 and its control. Adv Biophys. 1986;21:115–133. doi: 10.1016/0065-227x(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh E. A., Murray N. E., Revel H., Blumenthal R. M., Westaway D., Reith A. D., Rigby P. W., Elhai J., Hanahan D. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 1988 Feb 25;16(4):1563–1575. doi: 10.1093/nar/16.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., Kobayashi I., Thaler D., Stahl M. M. Direction of travel of RecBC recombinase through bacteriophage lambda DNA. Genetics. 1986 Jun;113(2):215–227. doi: 10.1093/genetics/113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimoto S., Nishimura Y., Ohtsubo E. The stable maintenance system pem of plasmid R100: degradation of PemI protein may allow PemK protein to inhibit cell growth. J Bacteriol. 1992 Jul;174(13):4205–4211. doi: 10.1128/jb.174.13.4205-4211.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimoto S., Ohtsubo E. Autoregulation by cooperative binding of the PemI and PemK proteins to the promoter region of the pem operon. Mol Gen Genet. 1993 Feb;237(1-2):81–88. doi: 10.1007/BF00282787. [DOI] [PubMed] [Google Scholar]
- Tsuchimoto S., Ohtsubo E. Effect of the pem system on stable maintenance of plasmid R100 in various Escherichia coli hosts. Mol Gen Genet. 1989 Feb;215(3):463–468. doi: 10.1007/BF00427044. [DOI] [PubMed] [Google Scholar]
- Tsuchimoto S., Ohtsubo H., Ohtsubo E. Two genes, pemK and pemI, responsible for stable maintenance of resistance plasmid R100. J Bacteriol. 1988 Apr;170(4):1461–1466. doi: 10.1128/jb.170.4.1461-1466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Weiss D. L., Johnson D. I., Weith H. L., Somerville R. L. Structural analysis of the ileR locus of Escherichia coli K12. J Biol Chem. 1986 Jul 25;261(21):9966–9971. [PubMed] [Google Scholar]
- Yoshioka Y., Ohtsubo H., Ohtsubo E. Repressor gene finO in plasmids R100 and F: constitutive transfer of plasmid F is caused by insertion of IS3 into F finO. J Bacteriol. 1987 Feb;169(2):619–623. doi: 10.1128/jb.169.2.619-623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


