Abstract
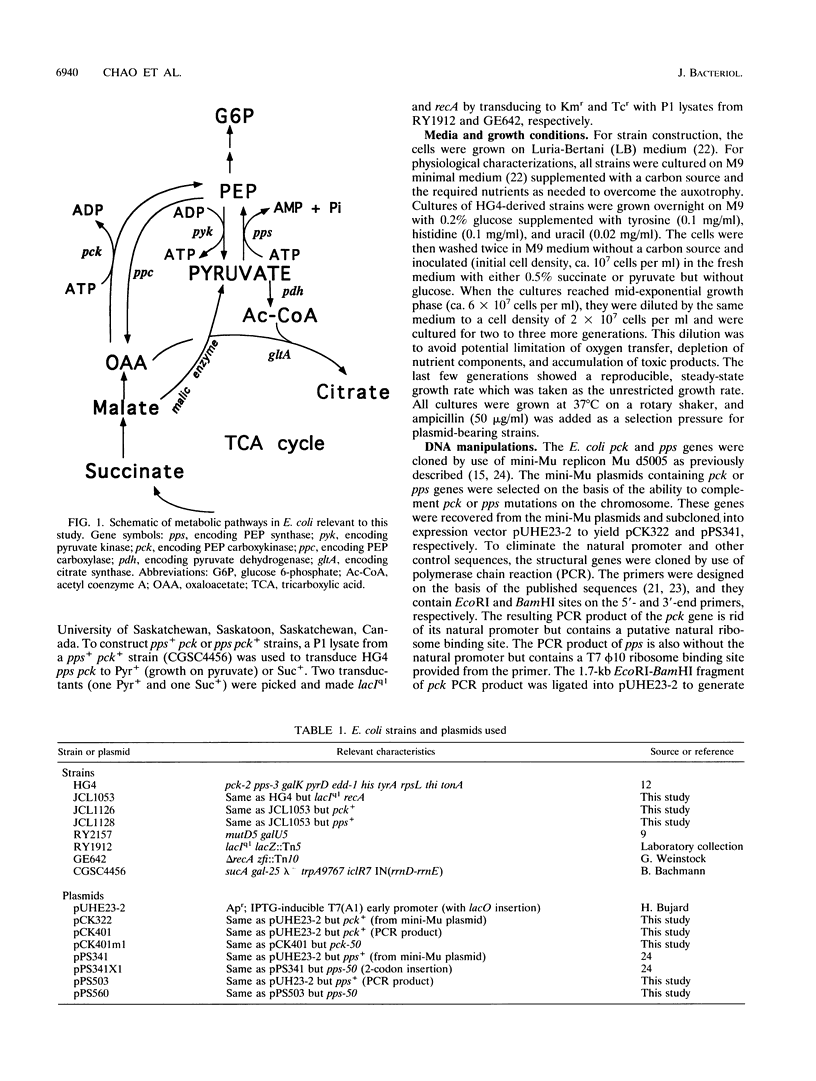
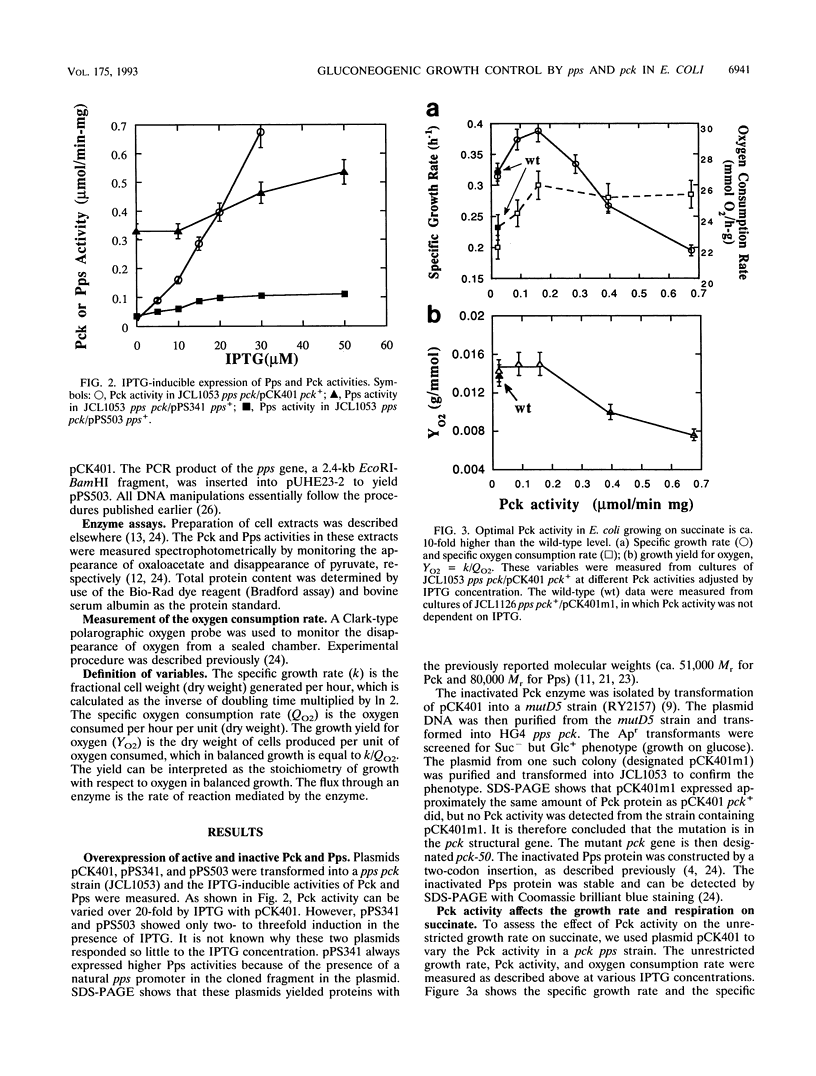
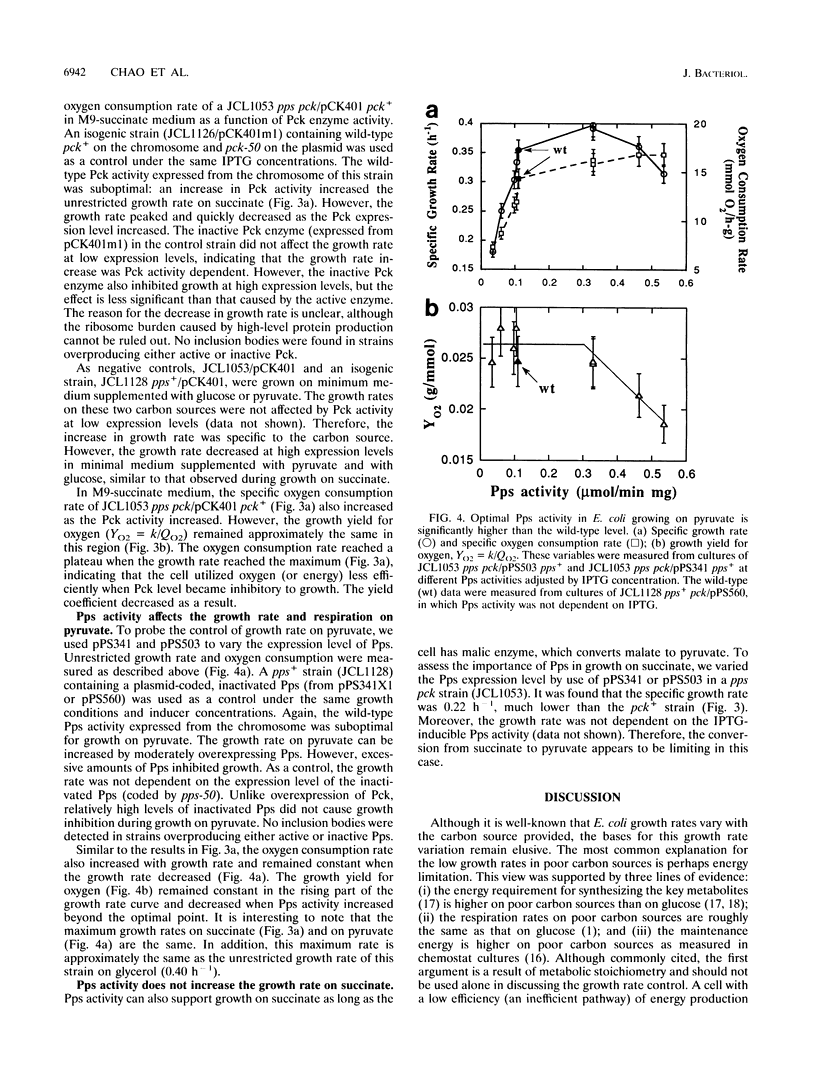
It is well-known that Escherichia coli grows more slowly on gluconeogenic carbon sources than on glucose. This phenomenon has been attributed to either energy or monomer limitation. To investigate this problem further, we varied the expression levels of pck, encoding phosphoenolpyruvate carboxykinase (Pck), and pps, encoding phosphoenolpyruvate synthase (Pps). We found that the growth rates of E. coli in minimal medium supplemented with succinate and with pyruvate are limited by the levels of Pck and Pps, respectively. Optimal overexpression of pck or pps increases the unrestricted growth rates on succinate and on pyruvate, respectively, to the same level attained by the wild-type growth rate on glycerol. Since Pps is needed to supply precursors for biosyntheses, we conclude that E. coli growing on pyruvate is limited by monomer supply. However, because pck is required both for biosyntheses and catabolism for cells growing on succinate, it is possible that growth on succinate is limited by both monomer and energy supplies. The growth yield with respect to oxygen remains approximately constant, even though the overproduction of these enzymes enhances gluconeogenic growth. It appears that the constant yield for oxygen is characteristic of efficient growth on a particular substrate and that the yield is already optimal for wild-type strains. Further increases in either Pck or Pps above the optimal levels become growth inhibitory, and the growth yield for oxygen is reduced, indicating less efficient growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., von Meyenburg K. Are growth rates of Escherichia coli in batch cultures limited by respiration? J Bacteriol. 1980 Oct;144(1):114–123. doi: 10.1128/jb.144.1.114-123.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenhead K., Manian S. S., O'Gara F. Dicarboxylic acid transport in Bradyrhizobium japonicum: use of Rhizobium meliloti dct gene(s) to enhance nitrogen fixation. J Bacteriol. 1988 Jan;170(1):184–189. doi: 10.1128/jb.170.1.184-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Gross L., Joho K. E., McAllister W. T. A modified kanamycin-resistance cassette to facilitate two-codon insertion mutagenesis. Gene. 1992 Feb 1;111(1):143–144. doi: 10.1016/0378-1119(92)90617-x. [DOI] [PubMed] [Google Scholar]
- Chin A. M., Feldheim D. A., Saier M. H., Jr Altered transcriptional patterns affecting several metabolic pathways in strains of Salmonella typhimurium which overexpress the fructose regulon. J Bacteriol. 1989 May;171(5):2424–2434. doi: 10.1128/jb.171.5.2424-2434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A. M., Feucht B. U., Saier M. H., Jr Evidence for regulation of gluconeogenesis by the fructose phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1987 Feb;169(2):897–899. doi: 10.1128/jb.169.2.897-899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. The direct synthesis of phosphoenolpyruvate from pyruvate by Escherichia coli. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):263–280. doi: 10.1098/rspb.1967.0065. [DOI] [PubMed] [Google Scholar]
- Dean A. M., Dykhuizen D. E., Hartl D. L. Fitness as a function of beta-galactosidase activity in Escherichia coli. Genet Res. 1986 Aug;48(1):1–8. doi: 10.1017/s0016672300024587. [DOI] [PubMed] [Google Scholar]
- Degnen G. E., Cox E. C. Conditional mutator gene in Escherichia coli: isolation, mapping, and effector studies. J Bacteriol. 1974 Feb;117(2):477–487. doi: 10.1128/jb.117.2.477-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D. E., Dean A. M., Hartl D. L. Metabolic flux and fitness. Genetics. 1987 Jan;115(1):25–31. doi: 10.1093/genetics/115.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerse R. H., van der Pluijm J., Postma P. W. The repressor of the PEP:fructose phosphotransferase system is required for the transcription of the pps gene of Escherichia coli. Mol Gen Genet. 1989 Aug;218(2):348–352. doi: 10.1007/BF00331288. [DOI] [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Allosteric control by calcium and mechanism of desensitization of phosphoenolpyruvate carboxykinase of Escherichia coli. J Biol Chem. 1980 Feb 25;255(4):1399–1405. [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Genetic and physiological characterization of Escherichia coli mutants deficient in phosphoenolpyruvate carboxykinase activity. J Bacteriol. 1980 Mar;141(3):1115–1121. doi: 10.1128/jb.141.3.1115-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie H. Regulation of transcription of the Escherichia coli phosphoenolpyruvate carboxykinase locus: studies with pck-lacZ operon fusions. J Bacteriol. 1984 Sep;159(3):832–836. doi: 10.1128/jb.159.3.832-836.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Casadaban M. J. Mini-mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol. 1986 Oct;168(1):357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P., Mainzer S. E. Effects of varying the carbon source limiting growth on yield and maintenance characteristics of Escherichia coli in continuous culture. J Bacteriol. 1975 Sep;123(3):1076–1087. doi: 10.1128/jb.123.3.1076-1087.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Pedersen S. Metabolic growth rate control in Escherichia coli may be a consequence of subsaturation of the macromolecular biosynthetic apparatus with substrates and catalytic components. Microbiol Rev. 1990 Jun;54(2):89–100. doi: 10.1128/mr.54.2.89-100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Marr A. G. Growth rate of Escherichia coli. Microbiol Rev. 1991 Jun;55(2):316–333. doi: 10.1128/mr.55.2.316-333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina V., Pontarollo R., Glaeske D., Tabel H., Goldie H. Sequence of the pckA gene of Escherichia coli K-12: relevance to genetic and allosteric regulation and homology of E. coli phosphoenolpyruvate carboxykinase with the enzymes from Trypanosoma brucei and Saccharomyces cerevisiae. J Bacteriol. 1990 Dec;172(12):7151–7156. doi: 10.1128/jb.172.12.7151-7156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niersbach M., Kreuzaler F., Geerse R. H., Postma P. W., Hirsch H. J. Cloning and nucleotide sequence of the Escherichia coli K-12 ppsA gene, encoding PEP synthase. Mol Gen Genet. 1992 Jan;231(2):332–336. doi: 10.1007/BF00279808. [DOI] [PubMed] [Google Scholar]
- Patnaik R., Roof W. D., Young R. F., Liao J. C. Stimulation of glucose catabolism in Escherichia coli by a potential futile cycle. J Bacteriol. 1992 Dec;174(23):7527–7532. doi: 10.1128/jb.174.23.7527-7532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter G. J., Postma P. W., van Dam K. Control of glucose metabolism by enzyme IIGlc of the phosphoenolpyruvate-dependent phosphotransferase system in Escherichia coli. J Bacteriol. 1991 Oct;173(19):6184–6191. doi: 10.1128/jb.173.19.6184-6191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyer J. R., Jeter R. M. Characterization of phosphoenolpyruvate synthase mutants in Salmonella typhimurium. Arch Microbiol. 1989;153(1):26–32. doi: 10.1007/BF00277536. [DOI] [PubMed] [Google Scholar]
- Walsh K., Koshland D. E., Jr Characterization of rate-controlling steps in vivo by use of an adjustable expression vector. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3577–3581. doi: 10.1073/pnas.82.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]



