Abstract
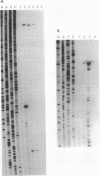
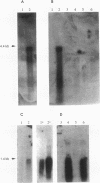
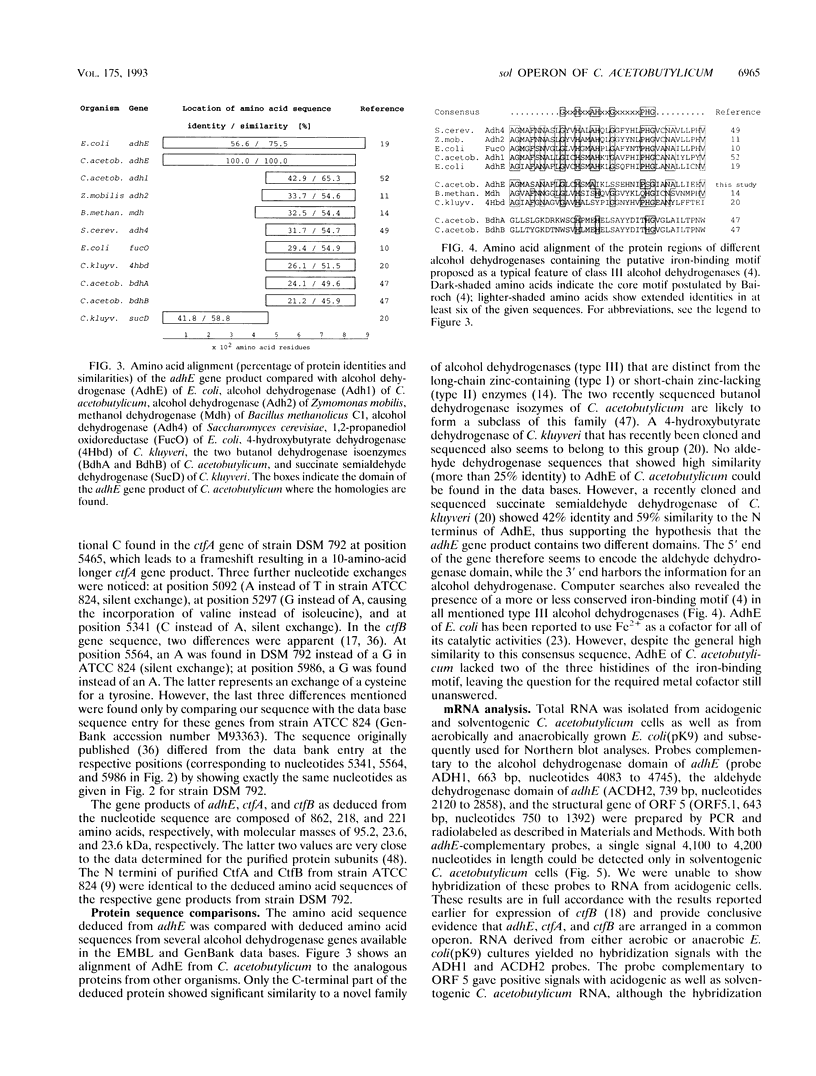
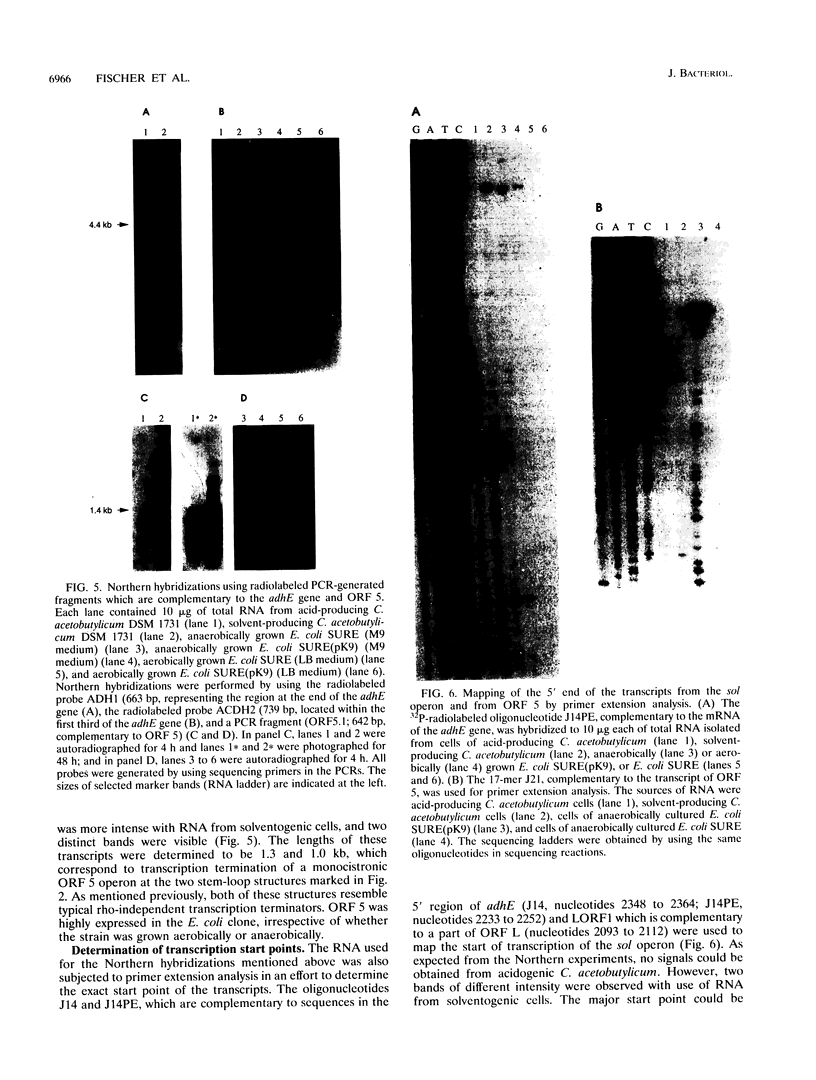
A DNA region of Clostridium acetobutylicum contiguous with the adc operon has been cloned and sequenced. Structural genes encoding the acetoacetyl coenzyme A:acetate/butyrate:coenzyme A transferase (ctfB and ctfA) and an alcohol/aldehyde dehydrogenase (adhE) could be identified. These three genes together with a small open reading frame (ORF) of unknown function (upstream of adhE) formed an operon (sol operon), as shown by mRNA analyses. The complete sol operon was transcriptionally induced or derepressed before the onset of solventogenesis, thus confirming earlier results of Northern hybridizations with a ctfB gene probe (U. Gerischer and P. Dürre, J. Bacteriol. 174:426-433, 1992). Upstream of the sol operon, we identified two putative promoters that were located in regions with possible stem-loop structures formed by several inverted repeats. The distal promoter P1 showed only minor transcription initiation in solventogenic C. acetobutylicum cells but was recognized in Escherichia coli, presumably because of its high similarity to the sigma 70 consensus sequence. The adhE-proximal promoter P2 directed the major transcription start point in solventogenic C. acetobutylicum but was not recognized in E. coli. The clostridial AdhE showed high similarity to a novel family (type III) of alcohol dehydrogenases. Two other ORFs (ORF 5 and ORF 6) were found on the cloned DNA region that showed no significant similarity to sequences in various available data bases. mRNA studies revealed that ORF 5 formed a monocistronic operon and showed increased expression before onset of solventogenesis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl H., Gottwald M., Kuhn A., Rale V., Andersch W., Gottschalk G. Nutritional Factors Affecting the Ratio of Solvents Produced by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):169–172. doi: 10.1128/aem.52.1.169-172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buluwela L., Forster A., Boehm T., Rabbitts T. H. A rapid procedure for colony screening using nylon filters. Nucleic Acids Res. 1989 Jan 11;17(1):452–452. doi: 10.1093/nar/17.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary J. W., Petersen D. J., Papoutsakis E. T., Bennett G. N. Cloning and expression of Clostridium acetobutylicum ATCC 824 acetoacetyl-coenzyme A:acetate/butyrate:coenzyme A-transferase in Escherichia coli. Appl Environ Microbiol. 1990 Jun;56(6):1576–1583. doi: 10.1128/aem.56.6.1576-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Ingram L. O. Similarity of Escherichia coli propanediol oxidoreductase (fucO product) and an unusual alcohol dehydrogenase from Zymomonas mobilis and Saccharomyces cerevisiae. J Bacteriol. 1989 Jul;171(7):3754–3759. doi: 10.1128/jb.171.7.3754-3759.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Sewell G. W., Osman Y. A., Ingram L. O. Cloning and sequencing of the alcohol dehydrogenase II gene from Zymomonas mobilis. J Bacteriol. 1987 Jun;169(6):2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerischer U., Dürre P. Cloning, sequencing, and molecular analysis of the acetoacetate decarboxylase gene region from Clostridium acetobutylicum. J Bacteriol. 1990 Dec;172(12):6907–6918. doi: 10.1128/jb.172.12.6907-6918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerischer U., Dürre P. mRNA analysis of the adc gene region of Clostridium acetobutylicum during the shift to solventogenesis. J Bacteriol. 1992 Jan;174(2):426–433. doi: 10.1128/jb.174.2.426-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlove P. E., Cunningham P. R., Parker J., Clark D. P. Cloning and sequence analysis of the fermentative alcohol-dehydrogenase-encoding gene of Escherichia coli. Gene. 1989 Dec 21;85(1):209–214. doi: 10.1016/0378-1119(89)90483-6. [DOI] [PubMed] [Google Scholar]
- Imamura R., Niki H., Kitaoka M., Yamanaka K., Ogura T., Hiraga S. Characterization of high molecular weights of complexes and polymers of cytoplasmic proteins in Escherichia coli. Res Microbiol. 1992 Oct;143(8):743–753. doi: 10.1016/0923-2508(92)90102-t. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D., Herth W., Knappe J. Ultrastructure and pyruvate formate-lyase radical quenching property of the multienzymic AdhE protein of Escherichia coli. J Biol Chem. 1992 Sep 5;267(25):18073–18079. [PubMed] [Google Scholar]
- Kessler D., Leibrecht I., Knappe J. Pyruvate-formate-lyase-deactivase and acetyl-CoA reductase activities of Escherichia coli reside on a polymeric protein particle encoded by adhE. FEBS Lett. 1991 Apr 9;281(1-2):59–63. doi: 10.1016/0014-5793(91)80358-a. [DOI] [PubMed] [Google Scholar]
- Lorowitz W., Clark D. Escherichia coli mutants with a temperature-sensitive alcohol dehydrogenase. J Bacteriol. 1982 Nov;152(2):935–938. doi: 10.1128/jb.152.2.935-938.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk R., Sergeant A. A simple method for dialysis of small-volume samples. Anal Biochem. 1980 Jul 1;105(2):403–404. doi: 10.1016/0003-2697(80)90477-7. [DOI] [PubMed] [Google Scholar]
- Narberhaus F., Bahl H. Cloning, sequencing, and molecular analysis of the groESL operon of Clostridium acetobutylicum. J Bacteriol. 1992 May;174(10):3282–3289. doi: 10.1128/jb.174.10.3282-3289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F., Giebeler K., Bahl H. Molecular characterization of the dnaK gene region of Clostridium acetobutylicum, including grpE, dnaJ, and a new heat shock gene. J Bacteriol. 1992 May;174(10):3290–3299. doi: 10.1128/jb.174.10.3290-3299.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palosaari N. R., Rogers P. Purification and properties of the inducible coenzyme A-linked butyraldehyde dehydrogenase from Clostridium acetobutylicum. J Bacteriol. 1988 Jul;170(7):2971–2976. doi: 10.1128/jb.170.7.2971-2976.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen D. J., Bennett G. N. Purification of acetoacetate decarboxylase from Clostridium acetobutylicum ATCC 824 and cloning of the acetoacetate decarboxylase gene in Escherichia coli. Appl Environ Microbiol. 1990 Nov;56(11):3491–3498. doi: 10.1128/aem.56.11.3491-3498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen D. J., Cary J. W., Vanderleyden J., Bennett G. N. Sequence and arrangement of genes encoding enzymes of the acetone-production pathway of Clostridium acetobutylicum ATCC824. Gene. 1993 Jan 15;123(1):93–97. doi: 10.1016/0378-1119(93)90545-e. [DOI] [PubMed] [Google Scholar]
- Petersen D. J., Welch R. W., Rudolph F. B., Bennett G. N. Molecular cloning of an alcohol (butanol) dehydrogenase gene cluster from Clostridium acetobutylicum ATCC 824. J Bacteriol. 1991 Mar;173(5):1831–1834. doi: 10.1128/jb.173.5.1831-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P., Palosaari N. Clostridium acetobutylicum Mutants That Produce Butyraldehyde and Altered Quantities of Solvents. Appl Environ Microbiol. 1987 Dec;53(12):2761–2766. doi: 10.1128/aem.53.12.2761-2766.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer U., Dürre P. Possible function of tRNA(Thr)ACG in regulation of solvent formation in Clostridium acetobutylicum. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):147–153. doi: 10.1111/j.1574-6968.1992.tb14033.x. [DOI] [PubMed] [Google Scholar]
- Sauer U., Dürre P. Sequence and molecular characterization of a DNA region encoding a small heat shock protein of Clostridium acetobutylicum. J Bacteriol. 1993 Jun;175(11):3394–3400. doi: 10.1128/jb.175.11.3394-3400.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Kirchniawy F. H., Jungermann K. A. Properties and function of the pyruvate-formate-lyase reaction in clostridiae. Eur J Biochem. 1972 May 23;27(2):282–290. doi: 10.1111/j.1432-1033.1972.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Walter K. A., Bennett G. N., Papoutsakis E. T. Molecular characterization of two Clostridium acetobutylicum ATCC 824 butanol dehydrogenase isozyme genes. J Bacteriol. 1992 Nov;174(22):7149–7158. doi: 10.1128/jb.174.22.7149-7158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. Coenzyme A transferase from Clostridium acetobutylicum ATCC 824 and its role in the uptake of acids. Appl Environ Microbiol. 1989 Feb;55(2):323–329. doi: 10.1128/aem.55.2.323-329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson V. M., Paquin C. E. Homology of Saccharomyces cerevisiae ADH4 to an iron-activated alcohol dehydrogenase from Zymomonas mobilis. Mol Gen Genet. 1987 Sep;209(2):374–381. doi: 10.1007/BF00329668. [DOI] [PubMed] [Google Scholar]
- Wood N. P., Jungermann K. Inactivation of the pyruvate formate lyase of Clostridium butyricum. FEBS Lett. 1972 Oct 15;27(1):49–52. doi: 10.1016/0014-5793(72)80407-1. [DOI] [PubMed] [Google Scholar]
- Young M., Minton N. P., Staudenbauer W. L. Recent advances in the genetics of the clostridia. FEMS Microbiol Rev. 1989 Dec;5(4):301–325. doi: 10.1111/j.1574-6968.1989.tb03402.x. [DOI] [PubMed] [Google Scholar]
- Youngleson J. S., Jones W. A., Jones D. T., Woods D. R. Molecular analysis and nucleotide sequence of the adh1 gene encoding an NADPH-dependent butanol dehydrogenase in the Gram-positive anaerobe Clostridium acetobutylicum. Gene. 1989 May 30;78(2):355–364. doi: 10.1016/0378-1119(89)90238-2. [DOI] [PubMed] [Google Scholar]
- Youngleson Jonathan S., Santangelo Joseph D., Jones David T., Woods David R. Cloning and Expression of a Clostridium acetobutylicum Alcohol Dehydrogenase Gene in Escherichia coli. Appl Environ Microbiol. 1988 Mar;54(3):676–682. doi: 10.1128/aem.54.3.676-682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe H., Jones D. T., Woods D. R. Cloning and expression of Clostridium acetobutylicum endoglucanase, cellobiase and amino acid biosynthesis genes in Escherichia coli. J Gen Microbiol. 1986 May;132(5):1367–1372. doi: 10.1099/00221287-132-5-1367. [DOI] [PubMed] [Google Scholar]
- de Vries G. E., Arfman N., Terpstra P., Dijkhuizen L. Cloning, expression, and sequence analysis of the Bacillus methanolicus C1 methanol dehydrogenase gene. J Bacteriol. 1992 Aug;174(16):5346–5353. doi: 10.1128/jb.174.16.5346-5353.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]